Abstract
Presynaptic inhibitory G protein-coupled receptors play a critical role in regulating transmission at a number of synapses in the central and peripheral nervous system. We generated transgenic mice that express a constitutively active form of an inhibitory Gα subunit to examine the molecular mechanisms underlying the actions of one such receptor, metabotropic glutamate receptor (mGluR) 2, at mossy fiber-CA3 synapses in the hippocampus. mGluR2 participates in at least three types of mossy fiber synaptic plasticity, (i) transient suppression of synaptic transmission, (ii) long-term depression (LTD), and (iii) inhibition of long-term potentiation (LTP), and we find that inhibitory Gα signaling is sufficient to account for the actions of mGluR2 in each. The fact that constitutively active Gαi2 occludes the transient suppression of synaptic transmission by mGluR2, while enhancing LTD, suggests further that these two forms of plasticity are expressed via different mechanisms. In addition, the LTP deficit observed in constitutively active Gαi2-expressing mice suggests that mGluR2 activation may serve as a metaplastic switch to permit the induction of LTD by inhibiting LTP.
Keywords: cAMP, G protein, metabotropic glutamate receptors, long-term depression, long-term potentiation
One important mechanism whereby neuronal activity is controlled is through the action of presynaptic inhibitory G protein-coupled receptors (1, 2). One member of this group is the metabotropic glutamate receptor, mGluR2, which is present at mossy fiber-CA3 pyramidal cell synapses. Here, mGluR2s are localized perisynaptically near presynaptic terminals (3, 4), where they are thought to function as autoreceptors to suppress transmission in response to excess glutamate release. Pharmacological and genetic manipulations have shown that mGluR2s participate in at least three types of synaptic plasticity: (i) a transient suppression of synaptic transmission that occurs in response to receptor activation, (ii) a persistent form of synaptic depression requiring both mGluR2 activation and presynaptic calcium influx (long-term depression, LTD), and (iii) mGluR2-mediated inhibition of long-term potentiation (LTP) (5, 6).
The relationship among these forms of mossy fiber-CA3 synaptic plasticity and the mechanism of mGluR2 involvement in each is not completely clear. Does the involvement of mGluR2 in both transient and persistent synaptic depression reflect a common mechanism, such that the persistent form merely results from a stabilization of the transient form, or does mGluR2 participate in these two processes via independent mechanisms? Similarly, does the inhibition of LTP by group II mGluR activation reflect opposing actions on a common effector pathway for depression and potentiation, or a permissive role for mGluR2 in inducing LTD by blocking LTP? We have begun to address these questions by exploring the signaling pathway downstream of mGluR2 using transgenic mice that express a constitutively active form of Gαi2. We find that the effects of mGluR2 on mossy fiber synaptic plasticity can be accounted for by the actions of inhibitory Gα subunits. However, the fact that transgene expression occludes transient suppression without occluding LTD supports the conclusion that additional biochemical cascades need to be activated along with inhibition of adenylate cyclase for the induction of LTD. Similarly, the fact that transgene expression strongly inhibits LTP without inducing LTD suggests that the participation of inhibitory Gα signaling in LTD may be due, in part, to inhibition of LTP.
Results
Expression of Constitutively Active Gαi2 in tetO-Gαi2, CaMK-tTA Double Transgenic Mice and Inhibition of Adenylate Cyclase.
As is the case with all G protein-coupled receptors, mGluR2 activation causes GDP/GTP exchange in its G protein targets and subsequent dissociation of the active GTP-bound Gα and β/γ components of these heterotrimeric proteins. Activated Gαi/o subunits inhibit particular isoforms of adenylate cyclase (7–9); however, additional actions are mediated through the actions of both Gα and β/γ dimers on other targets (10–16). We generated transgenic mice that express a constitutively active form of Gαi2 to test which of the effects of mGluR2 on mossy fiber synaptic plasticity can be accounted for by signaling mechanisms involving inhibitory Gα subunits.
A constitutively active form of the α subunit of the heterotrimeric G protein, Gi2, has been described previously (17, 18). This form carries a mutation that converts an arginine residue at position 179 in the catalytic site to a cysteine, interfering with the ability of the protein to hydrolyze GTP, and causing it to inhibit adenylate cyclase constitutively. We placed this construct under the control of a tetO promoter and crossed animals carrying this transgene to animals carrying a transgene where expression of the synthetic transactivator, tTA, is driven by the α-calcium/calmodulin kinase II (CaMKII) promoter (19). In progeny that carry both the tetO-Gαi2 and CaMK-tTA transgenes, tTA binds to the tetO promoter and turns on constitutively active Gαi2 expression only in cells where the CaMKII promoter is active.
TetO promoter-driven transgene expression can be suppressed by doxycycline, which prevents binding of tTA to the tetO promoter. To further restrict Gαi2 transgene expression temporally, we maintained mothers and their pups on food containing 40 mg/kg doxycycline until the pups were 10 days old, at which time the animals were shifted to normal food. In animals fed in this manner, hippocampal expression of the Gαi2 transgene RNA was detectable by RT-PCR 5 days after removal from doxycycline (15 days old; Fig. 1B). This was the case even though RNA encoding tTA was detectable by RT-PCR in the same samples at least 2 days before this time. For all of our experiments, we used 15- to 21-day-old mice raised according to this on-doxycycline/off-doxycycline regime.
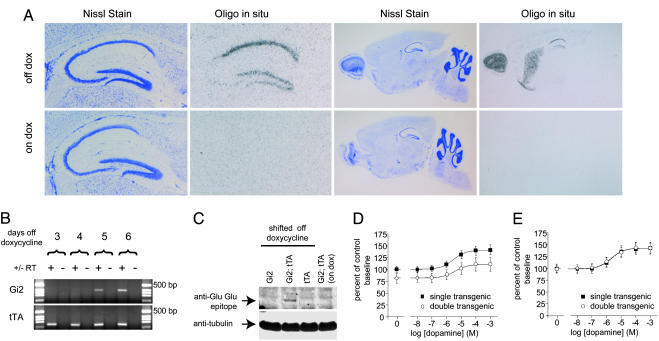
Fig. 1.
Doxycycline-regulated, constitutively active Gαi2 expression in tetO-Gi2, CaMK-tTA double transgenic animals. (A) Constitutively active Gαi2 RNA is expressed in principal cells throughout the hippocampus, cortex, striatum, and olfactory bulb. Shown are Nissl stain of hippocampus (Left) and sagittal (Right) sections of tetO-Gi2, CaMK-tTA double transgenic animals and corresponding oligonucleotide in situ hybridization of the same sections. Animals were raised on doxycycline and either shifted onto normal food at 10 days of age (Upper; off dox) or maintained on doxycycline-containing food (Lower; on dox) until they were killed and prepared for in situ hybridization at 21 days of age. (B) Constitutively active Gαi2 transgene RNA is present in the hippocampi of 15-day-old animals 5 days after removal from doxycycline. Shown are agarose gels of products from RT-PCRs carried out on RNA prepared from littermates killed at 3, 4, 5, and 6 days after switching from doxycycline-containing food to normal food 10 days after birth. (C) Constitutively active Gαi2 protein is expressed in the hippocampi of double transgenic animals and is suppressed by administration of doxycycline. Western blots of hippocampal homogenates prepared from 21-day-old animals raised on doxycycline until 10 days of age (lanes 1–3) or maintained on doxycycline throughout (lane 4) were probed with antibodies to the Glu-Glu epitope present in the transgenic protein (Upper) and tubulin as a loading control (Lower). (D and E) Dopamine-induced adenylate cyclase activity in striatal membranes prepared from 21-day-old double transgenic (open circles) or single transgenic (filled squares) animals either maintained on doxycycline throughout (E) or shifted off doxycycline at 10 days of age (D). Each point represents the mean ± SEM for five (D) or four (E) experiments carried out in triplicate.
Doxycycline-regulated Gαi2 transgene expression is also revealed by oligonucleotide RNA in situ hybridization and Western blotting. In double transgenic animals shifted to normal food, transgene RNA was detected in the cortex, olfactory bulb, striatum, and cell body layers of all three major hippocampal subregions; however, this expression was absent from double transgenic animals maintained on doxycycline (Fig. 1A). Similarly, immunoreactivity to the Glu-Glu epitope tag, included in the transgene, was detected on Western blots of hippocampal homogenates from double transgenic animals shifted to normal food but not from single transgenic control animals or from double transgenic animals maintained on doxycycline (Fig. 1C).
The mutant Gαi2 protein expressed by our transgene was previously shown to constitutively inhibit adenylate cyclase (17). We sought to confirm that this protein exhibited similar activity when expressed in transgenic mice. To do this, we took advantage of the relative abundance of G protein-sensitive adenylate cyclases present in striatal membrane preparations. We observed a significant reduction in adenylate cyclase activity in crude membranes prepared from constitutively active Gαi2-expressing animals compared with nonexpressing control littermates (Fig. 1D) that averaged 14.8% of control across a number of dopamine concentrations (P = 0.0037, two-way ANOVA with repeated measures; n = 5). This difference was not observed between animals of the same genotype when transgene expression was suppressed by doxycycline (Fig. 1E; mean difference = 0.3% of control; P = 0.9636, two-way ANOVA for repeated measures; n = 4). The degree of inhibition of inhibitory Gα protein-sensitive adenylate cyclases in principal cells is likely much higher than the level of inhibition observed here in crude membrane preparations because these preparations include contaminating adenylate cyclase activity from inhibitory Gα protein-insensitive cyclases, as well as cyclase activity from nonprincipal cells where the transgene is not expressed.
Constitutively Active Gαi2 Expression Does Not Alter Synaptic Input–Output Relations or Paired-Pulse Facilitation at Mossy Fiber-CA3 Synapses.
We sought to determine whether constitutively active Gαi2 expression affected basal synaptic transmission at mossy fiber-CA3 synapses by assessing the input–output relationship at a number of different stimulus intensities. Our analysis revealed no significant differences between double transgenic animals and single transgenic controls, indicating that Gαi2 transgene expression did not affect this aspect of basal synaptic transmission (Fig. 2A; single transgenic excitatory postsynaptic current (EPSC) amplitude = 94.8 ± 14.2 pA, n = 14; double transgenic EPSC = 96.1 ± 10.5 pA, n = 14). The single transgenic control animals carried either the CaMK-tTA transgene or the tetO-Gαi2 transgene alone. Because no significant differences were observed between these two groups for this or any of the other physiological measures described, data from these control animals were pooled throughout.
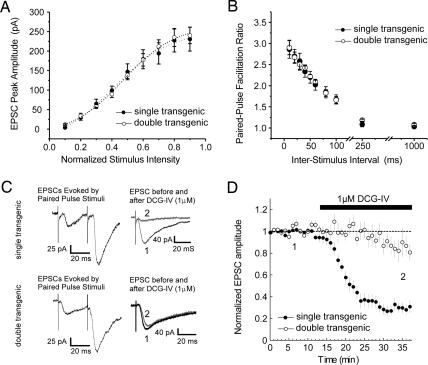
Fig. 2.
Expression of constitutively active Gαi2 does not alter basal synaptic transmission but occludes mGluR2-mediated suppression of transmission. (A) Input–output relation of mossy fiber-evoked EPSC peak amplitude (percent maximum) as a function of normalized stimulus intensity in constitutively active Gαi2-expressing double transgenics (open circles; n = 6) versus pooled single transgenic slices (filled circles; n = 6). (B) Percent paired-pulse facilitation profiles (S2/S1) of mossy fiber-CA3-evoked EPSCs as a function of interstimulus interval in slices from constitutively active Gαi2-expressing (open circles; n = 6) and single transgenic control (filled circles; n = 6) mice. (C) Sample paired-pulse-evoked EPSCs (30-ms interstimulus interval) and group II mGluR-mediated depression of synaptic transmission at mossy fiber-CA3 synapses in a slice from a single transgenic mouse (Upper) and a double transgenic littermate (Lower), illustrating the occlusion of group II mGluR-mediated depression in constitutively active Gαi2-expressing mice. (D) Time course of all experiments comparing the effects of 5 μM DCG-IV (black bar) on mossy fiber-evoked EPSCs in single CA3 pyramidal neurons recorded by whole-cell patch-clamp in slices from double transgenic (open circles; n = 5) versus single transgenic (filled circles; n = 6) mice. In all panels, each point is mean ± SEM of n cells.
As a measure of presynaptic function, we examined paired-pulse facilitation at interstimulus intervals of 10, 20, 30, 40, 50, 75, 100, 250, and 1,000 ms, and observed no differences in mossy fiber-CA3 paired-pulse facilitation profiles between slices from Gαi2 transgene-expressing and control mice. At an interstimulus interval of 20 ms, this facilitation was >2.8 times the initial response, consistent with values observed previously for mossy fiber-CA3 synapses (Fig. 2 B and C).
Constitutively Active Gαi2 Occludes Transient Suppression of Mossy Fiber Synaptic Transmission by Group II mGluR Activation.
If mGluR2-mediated suppression of mossy fiber synaptic transmission results from the activation of inhibitory Gα subunits, then constitutively active Gαi2 expression should affect the ability of group II mGluR agonists to inhibit synaptic transmission at these synapses. To test this possibility, we applied (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) to acute slices from double transgenic and single transgenic control animals, and compared its effects on mossy fiber synaptic transmission. In control slices, 20 min of DCG-IV application produced a 76 ± 4.8% reduction in whole-cell-evoked EPSC amplitude. In contrast, acute slices prepared from constitutively active Gαi2-expressing animals showed little or no response to DCG-IV application (Fig. 2 C and D; 13 ± 9% reduction), indicating that its effects were occluded by the actions of the transgene, and suggesting that the suppression of mossy fiber synaptic transmission by mGluR2 is mediated by inhibitory Gα signaling. This occlusion was observed in double transgenic animals when DCG-IV was applied at concentrations of either 5 or 0.5 μM, and the suppression observed in controls did not persist once the drug was washed out of the slice (Fig. 2D; and see Fig. 4A, which is published as supporting information on the PNAS web site). In addition, we saw no evidence for an effect of transgene expression on the level or distribution of group II mGluR immunoreactivity at mossy fiber terminals (Fig. 4 G and H).
Constitutively Active Gαi2 Enhances LTD at Mossy Fiber-CA3 Synapses.
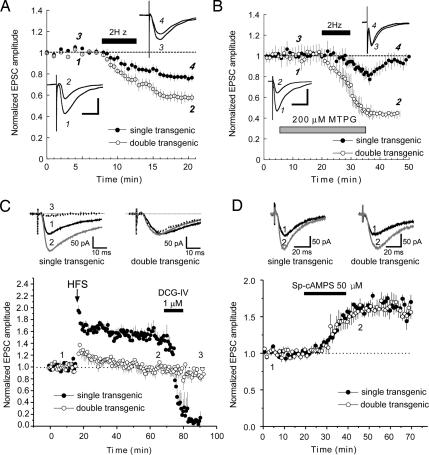
mGluR2 activation has previously been implicated in the induction of LTD at hippocampal mossy fiber-CA3 synapses (20, 21). We therefore sought to determine whether constitutively active Gαi2 expression also affected the induction of this persistent form of synaptic depression. Unlike transient suppression, which was occluded by constitutively active Gαi2, we found that LTD evoked by a 2-Hz, 10-min stimulus train was enhanced ≈2-fold in transgene-expressing animals as compared with controls [Fig. 3A; 58 ± 4% for double transgenics versus 76 ± 3.4% of prestimulus baseline for controls, 20 min post-low-frequency stimuli (LFS); P < 0.05, Student’s t test]. The fact that mossy fiber LTD was not occluded by constitutively active Gαi2 likely reflects the requirement for the combined actions of mGluR2 activation and presynaptic calcium influx for this persistent form of synaptic depression, as compared with transient suppression, which requires only group II mGluR activation (6, 22). The dependence of this LTD enhancement on Gαi2 transgene expression was further confirmed by continued doxycycline administration, which prevented both transgene expression and LTD enhancement (see Fig. 4 Cand D).
Fig. 3.
Stimulus-evoked mossy fiber-CA3 LTD is enhanced, and rescued in the presence of the mGluR II blocker MTPG, in slices from constitutively active Gαi2-expressing mice. (A) Time course of stimulus-evoked LTD (solid bar, 2 Hz for 10 min) of mossy fiber-evoked EPSCs in single CA3 pyramidal neurons recorded by whole-cell patch-clamp in slices from double transgenic mice (open circles; n = 5) versus single transgenic control mice (filled circles; n = 5). (Insets) Representative mossy fiber-evoked EPSCs before (1 and 3) and 15 min after (2 and 4) inducing LTD in single transgenic (upper right) and double transgenic (lower left) slices. (Calibration bars: 25 ms/500 pA.) (B) Time course of experiments comparing stimulus-evoked LTD (black bar; 2 Hz for 10 min) of mossy fiber-evoked EPSCs in single CA3 pyramidal neurons recorded by whole-cell patch-clamp in the presence of 200 μM MTPG (gray bar) in slices from single transgenic control (filled circles; n = 6) versus constitutively active Gαi2-expressing double transgenic mice (open circles; n = 5). (Insets) Representative mossy fiber-evoked EPSCs before (1 and 3) and 15 min after (2 and 4) inducing LTD in MTPG in single transgenic (upper right) and double transgenic (lower left) slices. (Calibration bar: 25 ms/500 pA.) Stimulus-evoked mossy fiber-CA3 LTP is impaired, but SP-cAMPS-induced LTP is normal in constitutively active Gαi2-expressing mice. (C) Time course of experiments comparing theta-burst-evoked LTP (arrow; four theta-burst trains, 10 × 100 Hz/five pulse bursts, 200-ms interburst interval) at mossy fiber-CA3 synapses in slices from double transgenic mice (open circles; n = 7) versus single transgenic mice (filled circles; n = 7). DCG-IV (1 μM; black bar) was bath-applied at the end of each experiment to confirm that mossy fibers were activated. (D) Application of a cell-permeant, hydrolysis-resistant cAMP analogue elicits LTP in both constitutively active Gαi2-expressing and control slices. Shown are time course of experiments where SP-cAMPS (50 μM) was bath-applied for 20 min (black bar) while evoking mossy fiber-CA3 EPSCs every 30 s in hippocampal slices from double transgenic (open circles; n = 6) or single transgenic (filled circles; n = 4) mice. In all panels, each point is mean ± SEM of n slices.
Constitutively Active Gαi2 Rescues the Mossy Fiber LTD Deficit Caused by mGluR Blockade.
The fact that inhibitory Gα signaling appears to be sufficient to account for the actions of mGluR2 in transient suppression raises the possibility that it may also be sufficient to account for the actions of mGluR2 in LTD. To test this possibility, we attempted to elicit mossy fiber LTD in double transgenic and control animals in the presence of the group II/III mGluR antagonist, (RS)-α-methyl-4-tetrazolylphenylglycine (MTPG). In acute slices prepared from control animals, application of 200 μM MTPG blocked LTD normally elicited by 2-Hz, 10-min stimulation (Fig. 3B, filled circles; 99 ± 4.6% of pre-LFS baseline, 20 min post-LFS). By contrast, we did not observe a similar blockade of LTD by MTPG in slices from double transgenic animals (Fig. 3B, open circles; 45 ± 3% of baseline EPSC amplitude, 20 min post-LFS), suggesting that the constitutively active Gαi2 transgene effectively substitutes for mGluR2 activity in mossy fiber LTD induction, and that mGluR2 participates in mossy fiber LTD via the actions of inhibitory Gα subunits. As previously reported, this blockade of mossy fiber LTD by MTPG is reversible, because LTD could be induced in control slices after drug washout (see Fig. 4 E and F). Constitutively active Gαi2 expression restored LTD to levels observed in the absence of the antagonist [Fig. 3B (45 ± 3% of baseline for double transgenics in the presence of MTPG, 20 min post-LFS) compared with Fig. 3A (58 ± 5.7% of baseline for double transgenics in the absence of MTPG)].
Constitutively Active Gαi2 Inhibits Induction of LTP at Mossy Fiber-CA3 Synapses.
Group II mGluR activation has been shown to inhibit stimulus-induced mossy fiber LTP (5, 6). We therefore sought to determine whether this effect of receptor activation was mediated by inhibitory Gα signaling. Theta burst stimulation of mossy fiber afferents resulted in a robust LTP in control animals that was virtually absent in double transgenic animals (Fig. 3C; 151 ± 8.7% 50 min poststimulus for controls compared with 98 ± 7.6% for double transgenics; P < 0.05, Student’s t test). As was the case for the unstimulated slices described in Fig. 2D, application of 1 μM DCG-IV effectively suppressed mossy fiber synaptic transmission in control slices after induction of LTP (Fig. 3C; 14 ± 9.6% of pre-theta-burst tetanus baseline) but had little effect on mossy fiber evoked responses in slices from double transgenic animals (87 ± 10% of pre-theta-burst tetanus baseline). The observation that constitutively active Gαi2 expression mimicked the inhibition of LTP caused by group II mGluR activation suggests that, like transient suppression and LTD, the inhibition of LTP by mGluR2 activation may also be mediated by inhibitory Gα subunits.
Increases in cAMP concentration are thought to play a central role in the induction of mossy fiber LTP (23, 24). If the Gαi2 transgene interferes with potentiation through inhibition of adenylate cyclase and a consequent decrease in [cAMP], then it should be possible to obtain potentiation in double transgenic animals through exogenous application of cAMP. To test this hypothesis, we compared SP-adenosine 3′,5′-monophosphorothioate (SP-cAMPS)-induced potentiation in double transgenic and control animals and found them to be similar in amplitude and time course, supporting the idea that the constitutively active Gαi2 transgene acts via inhibition of adenylate cyclase to reduce cAMP levels and inhibit mossy fiber LTP (Fig. 3D; 162 ± 10% of baseline 50 min after start of drug application for double transgenics compared with 166 ± 22% for controls).
Discussion
We generated transgenic mice that express a constitutively active form of the α subunit of an inhibitory heterotrimeric G protein to identify the molecular mechanisms underlying the involvement of mGluR2 in various forms of mossy fiber synaptic plasticity. Our results suggest that signaling via inhibitory Gα subunits is sufficient to account for the actions of mGluR2 in (i) transient suppression of synaptic transmission, (ii) induction of LTD, and (iii) inhibition of LTP. mGluR2 activation also leads to the release of free β/γ dimers, which regulate several targets, including presynaptic Ca2+ channels (15, 16, 25). However, the actions of this component of heterotrimeric G protein signaling appear to be dispensable in the presence of Gα signaling, for the forms of plasticity examined here.
Our data show that inhibitory Gα signaling is sufficient to account for the participation of mGluR2 in both transient and long-term depression of mossy fiber responses. In addition, the ability of constitutively active Gαi2 to occlude transient but not long-term depression indicates important differences between these two forms of plasticity. If LTD results from stabilization of the same changes that occur during transient suppression, one would expect the continuous suppression observed in the transgenic mice to occlude LTD. The fact that this is not the case suggests that, although these two forms of depression may share a requirement for inhibitory Gα signaling for their induction, they are expressed by distinct mechanisms.
Similarly, the physiological properties observed in the constitutively active Gαi2-expressing mice may also provide insight into the relationship between mossy fiber LTP and LTD. These animals exhibit greatly reduced mossy fiber LTP in conjunction with enhanced LTD, suggesting that mGluR2 and inhibitory Gα signaling may act as a metaplastic switch to permit the induction or expression of LTD, in part through the inhibition of LTP. Alternatively, the reported ability of group II mGluR activation to elicit depotentiation at mossy fiber synapses (5) suggests that the LTP deficit in these animals may reflect constitutive depotentiation as a consequence of the persistent actions of constitutively active Gαi2.
To fully understand the relationship between these different forms of mossy fiber synaptic plasticity and the role of mGluR2 in each, it will be necessary to identify the targets of the mGluR2 signaling pathway acting downstream of inhibitory Gα subunits. Adenylate cyclase I is a likely candidate in this regard, because it is among the adenylate cyclase isoforms sensitive to inhibition by Gi-coupled receptors (26), and because mice homozygous for a knockout mutation of this gene also exhibit impaired mossy fiber LTP (27). Another likely target is the cyclic-AMP-dependent protein kinase (PKA) substrate Rim1α. This multidomain protein is thought to link vesicle docking, via interaction with vesicle-associated, GTP-bound Rab3A, to vesicle priming through interaction with munc13 and syntaxin (28, 29). Rim1α phosphorylation by PKA is associated with certain forms of LTP (30), and, similar to our constitutively active Gαi2-expressing animals, Rim1α knockout mice exhibit both deficient mossy fiber LTP and enhanced LTD (31). However, the fact that DCG-IV still suppresses synaptic transmission in Rim1α knockout animals suggests that, if Rim1α is a target, it is unlikely to be the sole relevant target responsible for mediating all of the effects of mGluR2.
We focused on the role of mGluR2 and inhibitory Gα subunits in mossy fiber synaptic plasticity; however, the relationship examined here may also apply to the actions of other presynaptic inhibitory G protein-coupled receptors. One example is kappa receptors, which bind the opioid peptide dynorphin released by mossy fibers (32). Application of dynorphin causes suppression of synaptic activity and inhibition of LTP similar to that caused by group II mGluR agonists (33, 34), raising the possibility that these receptors may regulate mossy fiber activity through inhibitory Gα activity in a manner similar to that of mGluR2s. Furthermore, adenylate cyclase inhibition by inhibitory Gα signaling may also play a role in another presynaptically expressed form of LTD at Schaffer collateral synapses that results from coordinated adenylate cyclase inhibition (supplied by either mGluR2 or adenosine A1 receptor agonists) and elevation of [cGMP] (35–38). Given the number of presynaptic inhibitory G protein-coupled receptors and their broad distribution throughout the nervous system, it is possible that inhibitory Gα signaling may play a central role in the regulation of synaptic activity at many synapses.
Materials and Methods
Transgenic Animals and Transgene Expression.
The epitope-tagged Gαi2 construct containing the constitutively active mutation R179C (17, 18) was cloned into a tetO promoter containing plasmid pUHD 10-3 (gift from H. Bujard, University of Heidelberg) for generation of transgenic animals by pronuclear injection. The resulting animals were used in conjunction with CaMKIIα-tTA animals (19) to achieve region-restricted, drug-regulated Gαi2 transgene expression. Expression was assessed by oligonucleotide RNA in situ hybridization in fresh-frozen brain sections (39) and by RT-PCR carried out on total hippocampal RNA prepared in TRIzol reagent (GIBCO/BRL) by using the SuperScript First-Strand Synthesis kit (GIBCO/BRL) and standard PCR procedures. Western blots were performed on hippocampal homogenates with antibodies to the Glu-Glu epitope tag (Covance) and β-tubulin (Sigma). For oligonucleotide sequences and further details on methodology, see Supporting Methods, which is published as supporting information on the PNAS web site.
Adenylate Cyclase Assays.
Adenylate cyclase activity was measured in crude striatal membranes from 21-day-old double transgenic and control siblings that were either shifted to normal food at 10 days old or maintained continuously on food containing 40 mg/kg doxycycline. Reaction conditions, Dowex/alumina column chromatography, and analysis were carried out as described in ref. 40. All reactions were carried out in triplicate, and tritiated cAMP was included during purification to monitor recovery. Adenylate cyclase activity in double transgenic and control animals was compared across a range of dopamine concentrations by using two-way ANOVA for repeated measures.
Electrophysiological Recordings.
Transverse slices (400 μm thick) of hippocampi and associated entorhinal cortices were prepared from 15- to 21-day-old animals using a vibratome. Recordings were carried out in a modified Haas-type interface chamber at 33°C on slices continuously perfused with artificial cerebrospinal fluid: 126 mM NaCl, 26 mM NaHCO3, 1.25 mM NaH2PO4, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, and 10 mM d-glucose, gassed with 95% O2/5% CO2 (pH 7.3–7.4) at 3 ml/min. The mossy fiber pathway was stimulated (150-μs square pulses) every 30 s with bipolar stimulating electrodes (Frederick Haer) placed in the dentate gyrus granule cell body layer (stratum granulosum). Baseline stimulus strength (10–100 μA) was adjusted to elicit a response ≈50% of EPSC threshold amplitude for action potential generation. Slices with baseline drift >5% over 15 min were excluded from the analysis.
Blind whole-cell patch-clamp recordings were made at 33°C from pyramidal neurons in CA3 stratum pyramidale in voltage-clamp mode (Axopatch 700B; Axon Instruments). The recording electrode filling solution was as follows: 135 mM Cs-gluconate, 0.5 mM EGTA, 8 mM NaCl, 2 mM Mg-ATP2, 0.3 mM Na-GTP, 10 mM Hepes, and 1 mM QX-314 [N-(2,6-dimethylphenylcarbomoylmethyl)triethylammonium bromide] (pH 7.2–7.3). Stimulating electrodes were placed in or just on the hilar side of the upper blade of the dentate granule cell layer, and mossy fiber EPSCs were identified by rapid rise time (single transgenics, 1.76 ± 0.15 ms, n = 14; double transgenics, 1.67 ± 0.14 ms, n = 14), fast time course (<10 ms), restriction to stratum lucidum, and marked paired-pulse facilitation (>2.8 times at 20-ms interstimulus interval). The group II mGluR agonist DCG-IV (5 μM) was added at the end of experiments to confirm that synaptic events were mossy fiber in origin (41–43). Series resistance was monitored continuously, and experiments were discontinued if it changed by >10%. Whole-cell experiments were performed in the presence of the NMDA antagonist d-2-amino-5-phosphonopentanoic acid (D-AP5) (50 μM) to isolate non-NMDA receptor-mediated mossy fiber LTD and LTP.
LTD was induced by a 10-min train of LFS that consisted of 1,200 × 150 μs duration dc square pulses at a frequency of 2 Hz. LTP was induced by a theta burst stimulation protocol, consisting of 10 bursts (five pulses, 100 Hz each) at a frequency of 5 Hz, repeated four times, 15 s apart. Changes in synaptic strength were normalized to pretreatment baseline in the same slice before averaging across slices.
Statistical Analyses.
Values of LTD and LTP were calculated as change in EPSC amplitude, or field excitatory postsynaptic potential slope, compared with pretetanus baseline. Summary data are mean ± SEM. Group means were compared by using Student’s t test for unpaired data with significance level preset to P < 0.05.
Drug Preparation.
All drugs were stored frozen as stock solutions 100–1,000 times the final concentration and thawed and diluted immediately before addition to perfusate. Final concentrations were as follows: DCG-IV, 5 μM and 0.5 μM (Tocris); MTPG, 200 μM (Tocris); SP-cAMPS, 50 μM (Biolog); D-AP5, 50 μM (Tocris).
Acknowledgments
We thank D. R. Storm and P. E. Castillo for helpful discussions. This work was supported by Whitehall and Alexander von Humboldt Foundation grants, National Institutes of Health Grant R01-NS44421 (to P.K.S.), and National Institutes of Mental Health Conte Center Grant MN50733 (to E.R.K.).
Abbreviations
- CaMKII
calcium/calmodulin kinase II
- DCG-IV
(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- EPSC
excitatory postsynaptic current
- LFS
low-frequency stimuli
- LTD
long-term depression
- LTP
long-term potentiation
- mGluR
metabotropic glutamate receptor
- MTPG
(RS)-α-methyl-4-tetrazolylphenylglycine
- SP-cAMPS
SP-adenosine 3′,5′-monophosphorothioate.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Boehm S., Kubista H. Pharmacol. Rev. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Miller R. J. Annu. Rev. Pharmacol. Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Petralia R. S., Wang Y. X., Niedzielski A. S., Wenthold R. J. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 4.Shigemoto R., Kinoshita A., Wada E., Nomura S., Ohishi H., Takada M., Flor P. J., Neki A., Abe T., Nakanishi S., Mizuno N. J. Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y. L., Huang C. C., Hsu K. S. J. Neurosci. 2001;21:3705–3714. doi: 10.1523/JNEUROSCI.21-11-03705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzounopoulos T., Janz R., Sudhof T. C., Nicoll R. A., Malenka R. C. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 7.Simonds W. F. Trends Pharmacol. Sci. 1999;20:66–73. doi: 10.1016/s0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- 8.Hurley J. H. J. Biol. Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- 9.Chern Y. Cell. Signalling. 2000;12:195–204. doi: 10.1016/s0898-6568(99)00084-4. [DOI] [PubMed] [Google Scholar]
- 10.Mochizuki N., Hibi M., Kanai Y., Insel P. A. FEBS Lett. 1995;373:155–158. doi: 10.1016/0014-5793(95)01031-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen L. T., Gilman A. G., Kozasa T. J. Biol. Chem. 1999;274:26931–26938. doi: 10.1074/jbc.274.38.26931. [DOI] [PubMed] [Google Scholar]
- 12.Corre I., Baumann H., Hermouet S. Oncogene. 1999;18:6335–6342. doi: 10.1038/sj.onc.1203010. [DOI] [PubMed] [Google Scholar]
- 13.Fan X., Brass L. F., Poncz M., Spitz F., Maire P., Manning D. R. J. Biol. Chem. 2000;275:32129–32134. doi: 10.1074/jbc.M004577200. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y. C., Huang J., Ali S., Lowry W., Huang X. Y. Cell. 2000;102:635–646. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 15.Clapham D. E., Neer E. J. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 16.Schwindinger W. F., Robishaw J. D. Oncogene. 2001;20:1653–1660. doi: 10.1038/sj.onc.1204181. [DOI] [PubMed] [Google Scholar]
- 17.Wong Y. H., Federman A., Pace A. M., Zachary I., Evans T., Pouyssegur J., Bourne H. R. Nature. 1991;351:63–65. doi: 10.1038/351063a0. [DOI] [PubMed] [Google Scholar]
- 18.Pace A. M., Faure M., Bourne H. R. Mol. Biol. Cell. 1995;6:1685–1695. doi: 10.1091/mbc.6.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayford M., Bach M. E., Huang Y. Y., Wang L., Hawkins R. D., Kandel E. R. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 20.Yokoi M., Kobayashi K., Manabe T., Takahashi T., Sakaguchi I., Katsuura G., Shigemoto R., Ohishi H., Nomura S., Nakamura K., et al. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K., Manabe T., Takahashi T. Science. 1996;273:648–650. doi: 10.1126/science.273.5275.648. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K., Manabe T., Takahashi T. Eur. J. Neurosci. 1999;11:1633–1638. doi: 10.1046/j.1460-9568.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- 23.Weisskopf M. G., Castillo P. E., Zalutsky R. A., Nicoll R. A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y. Y., Li X. C., Kandel E. R. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 25.Kamiya H., Ozawa S. J. Physiol. (London) 1999;518:497–506. doi: 10.1111/j.1469-7793.1999.0497p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen M. D., Chan G. C., Poser S. W., Storm D. R. J. Biol. Chem. 1996;271:33308–33316. doi: 10.1074/jbc.271.52.33308. [DOI] [PubMed] [Google Scholar]
- 27.Villacres E. C., Wong S. T., Chavkin C., Storm D. R. J. Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betz A., Thakur P., Junge H. J., Ashery U., Rhee J. S., Scheuss V., Rosenmund C., Rettig J., Brose N. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Okamoto M., Schmitz F., Hofmann K., Sudhof T. C. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 30.Lonart G., Schoch S., Kaeser P. S., Larkin C. J., Sudhof T. C., Linden D. J. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- 31.Castillo P. E., Schoch S., Schmitz F., Sudhof T. C., Malenka R. C. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 32.Simmons M. L., Chavkin C. Int. Rev. Neurobiol. 1996;39:145–196. doi: 10.1016/s0074-7742(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 33.Simmons M. L., Terman G. W., Drake C. T., Chavkin C. J. Neurophysiol. 1994;72:1697–1705. doi: 10.1152/jn.1994.72.4.1697. [DOI] [PubMed] [Google Scholar]
- 34.Weisskopf M. G., Zalutsky R. A., Nicoll R. A. Nature. 1993;365:188. doi: 10.1038/365188a0. [DOI] [PubMed] [Google Scholar]
- 35.Reyes-Harde M., Potter B. V., Galione A., Stanton P. K. J. Neurophysiol. 1999;82:1569–1576. doi: 10.1152/jn.1999.82.3.1569. [DOI] [PubMed] [Google Scholar]
- 36.Santschi L., Reyes-Harde M., Stanton P. K. J. Neurophysiol. 1999;82:1577–1589. doi: 10.1152/jn.1999.82.3.1577. [DOI] [PubMed] [Google Scholar]
- 37.Stanton P. K., Heinemann U., Muller W. J. Neurosci. 2001;21:RC167. doi: 10.1523/JNEUROSCI.21-19-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santschi L. A., Zhang X. L., Stanton P. K. J. Neurobiol. 2006;66:205–219. doi: 10.1002/neu.20213. [DOI] [PubMed] [Google Scholar]
- 39.Wisden W., Morris B. J. Situ Hybridization Protocols for the Brain. Amsterdam: Academic; 2002. [Google Scholar]
- 40.Salomon Y. Adv. Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- 41.Calixto E., Thiels E., Klann E., Barrionuevo G. J. Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamiya H., Shinozaki H., Yamamoto C. J. Physiol. (London) 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei S., Pelkey K. A., Topolnik L., Congar P., Lacaille J. C., McBain C. J. J. Neurosci. 2003;23:9786–9795. doi: 10.1523/JNEUROSCI.23-30-09786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]