Abstract
The centrosome functions as the major microtubule-organizing center and plays a vital role in guiding chromosome segregation during mitosis. Centrosome abnormalities are frequently seen in a variety of cancers, suggesting that dysfunction of this organelle may contribute to malignant transformation. In our efforts to identify the protein components of the centrosome and to understand the structure features involved in the assembly and functions of this organelle, we cloned and characterized a centrosome-associated protein called Su48. We found that a coiled coil-containing subdomain of Su48 was both sufficient and required for its centrosome localization. In addition, this structure also modulates Su48 dimerization. Moreover, ectopic expression of Su48 causes abnormal mitosis, and a mutant form of Su48 disrupts the localization of γ-tubulin to the centrosome. Finally, by microinjection of an anti-Su48 antibody, we found that disruption of normal Su48 functions leads to mitotic failure, possibly due to centrosome defects or incomplete cytokinesis. Thus, Su48 represents a previously unrecognized centrosome protein that is essential for cell division. We speculate that Su48 abnormalities may cause aberrant chromosome segregation and may contribute to aneuploidy and malignant transformation.
Keywords: cancer, coiled coil, mitosis
The centrosome functions as the microtubule-organizing center in most animal cells (1–4). This subcellular organelle modulates the formation of the microtubule network, which plays a role in a variety of biological processes, including mitosis, intracellular transportation, cell mobility, and morphogenesis. Centrosomes are reproduced precisely once per cell cycle in coordination with DNA replication. Although each cell typically contains one centrosome in the interphase, the centrosome is duplicated in S phase and becomes the pole of the spindle before the onset of mitosis. Excessive numbers of centrosomes may lead to multipolar spindles and cause abnormal chromosome segregation during mitosis (1–4). Indeed, an increasing body of evidence indicates that aberrant duplication of the centrosomes is associated with various types of tumors (5–9). Abnormal pericentriolar structures are also linked to deregulation of mitosis and transformation (10). Furthermore, a number of centrosome-associated kinases, including the Polo-like kinase and the Aurora-A kinase, have been implicated in the development of aneuploidy and tumors (11–13). These observations support the notions that regulation of the normal function of the centrosome is critical for cell proliferation and that compromising centrosome integrity may contribute to malignancy.
Each centrosome consists of a pair of centrioles surrounded by an electron-dense material known as the pericentriolar material. Microtubule nucleation is mediated by the γ-tubulin ring complexes (γ-TuRCs), which are embedded in the pericentriolar material (14–17). The γ-TuRC consists of γ-tubulin and other subunits including GCP-2, -3, -4, -5, and -6 (18–22). By estimation each centrosome contains hundreds of γ-TuRCs. Whereas a fraction of γ-TuRCs are found diffused in the cytoplasm, γ-TuRCs become more concentrated on the centrosome at the onset of mitosis, where it demonstrates high microtubule-nucleating activity (23). The pericentriolar material apparently contains proteins that can regulate the formation of microtubules as well as duplication of the centrosome.
Because the centrosome is extremely small in size, characterizing its structure and associated components of the complex has been a major challenge. Recent proteomic studies have expanded the list of proteins localized to the centrosome. For example, it was found that the yeast spindle pole body, the counterpart of the mammalian centrosome, contains at least 50 proteins (24). In a mass-spectrometry-based analysis of mammalian centrosomes, Andersen et al. (25) identified 23 previously confirmed centrosome proteins and 41 candidate centrosome proteins. However, it remains to be determined how and whether these proteins are targeted to the centrosome and organized structurally.
In the current study, we cloned and characterized a centrosome-associated protein named Su48. We identified a subdomain of Su48 that contains a coiled-coil motif and is important for its dimerization and localization to the centrosome. Moreover, we determined whether interference with Su48 function affects mitosis. We propose that Su48 is a previously unrecognized centrosomal protein that plays a critical role during cell division.
Results
Cloning and Identification of Su48.
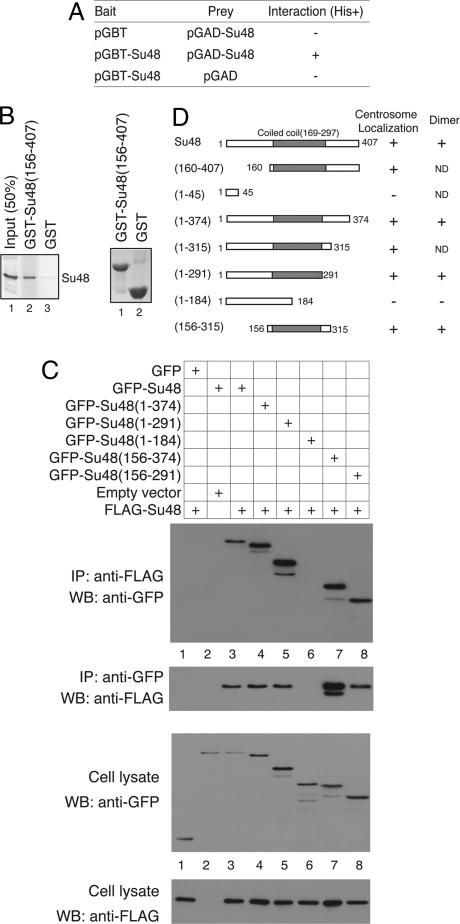
In our previous studies to determine how the Survivin protein functions, we isolated Su48 as one of several proteins that specifically binds to Survivin both in the yeast two-hybrid system and in an in vitro binding assay (4). Su48 was previously cloned in part and deposited in the GenBank database as KIAA0844 (26). As deduced from its primary sequence, the human Su48 is a polypeptide of 407 aa residues, with a putative C2H2-type zinc finger in the N terminus and a coiled-coil structure in the central region. As predicted by the multicoil program (http://multicoil.lcs.mit.edu/cgi-bin/multicoil), the coiled-coil subdomain is likely to mediate formation of dimers. We identified a potential RXXL destruction box in the C terminus of Su48. Furthermore, we noted that Su48 contains putative phosphorylation sites for tyrosine kinase and the cAMP-dependent kinase, respectively. The human Su48 gene was localized to chromosome 10q21, a locus altered in some forms of human cancers [CancerChromosomes search at the National Center for Biotechnology Information web site (www.ncbi.nlm.nih.gov)]. Although Su48 is conserved in human, mouse, rat, and chicken, we have not been able to identify any homologues in lower organisms such as yeast or Caenorhabditis elegans.
We examined the expression patterns of Su48. By using RT-PCR analysis, Su48 can be detected in a variety of rodent tissues, with the highest abundance in the brain (Fig. 1A). In addition, Su48 is also expressed at the early embryonic stages (Fig. 1A). Similarly, the Su48 mRNA can also be found in a number of human cell lines, including HeLa, U2OS, PANC-1, and HBL100 cells but not 293T cells (Fig. 1B).
Fig. 1.
Su48 is expressed in a variety of tissues and cancer cell lines. (A) RT-PCT analysis of Su48 expression. cDNA samples were prepared from various mouse tissues (1, heart; 2, brain; 3, spleen; 4, lung; 5, liver; 6, skeletal muscle; 7, testis; 9–12, whole embryos of day 7, 11,15, and 17, respectively; 13, no cDNA; 14, mouse embryonic day 11 cDNA library). One nanogram of each sample was used. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as control to confirm that the amount of total cDNA used in each reaction was comparable. (B) RT-PCT analysis of Su48 expression in human cell lines. cDNA was prepared from the following human cell lines: PANC-1, 293T, HBL100, HeLa, and U2OS. (C) Su48 protein expression in various cancer cell lines. Endogenous Su48 protein was evaluated using affinity-purified polyclonal anti-Su48 antibody (Upper). To confirm that similar amounts of protein were loaded in each lane, the membrane was reprobed with an anti-α-tubulin antibody (Lower). WB, Western blotting.
Both polyclonal and monoclonal antibodies were developed and characterized (see Figs. 8 and 9, which are published as supporting information on the PNAS web site). The affinity-purified polyclonal antibody that recognizes Su48 specifically was used to determine the endogenous Su48 expression in a number of cell lines (Fig. 1C). Although the Su48 protein level is low or undetectable in many cell lines examined to date, it is considerably high in the pancreatic cancer cell line PANC-1 (Fig. 1C). We evaluated the number of centrosomes in PANC-1 cells and found that as many as 53% of cells have more than two centrosomes per cell. In contrast, <8% of cells harbor excessive centrosomes in HeLa, U2OS, HBL100, or 293T cells. Thus, a correlation may exist between abnormal Su48 expression patterns and cancer cells that have abnormal centrosomes.
Subcellular Localization of Su48.
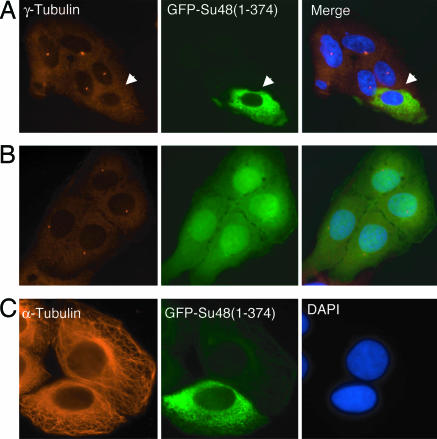
We next investigated the subcellular localization of Su48 by monitoring the GFP-Su48 fusion proteins in transfected U2OS cells. GFP-Su48 forms one or two dot-like structures in each cell. The Su48-containing dots are colocalized with the centrosomal protein γ-tubulin (Fig. 2). Indeed, we found that GFP-Su48 is localized to the centrosome at all stages of the cell cycle (Fig. 2). These observations were confirmed by using FLAG-tagged Su48 (data not shown). Notably, localization of Su48 to the centrosome is not affected by treating the cell with nocodazole, a compound that causes microtubule depolymerization (Fig. 2B). Thus, it appears that Su48 is an integral part of the centrosome and is targeted to the centrosome independently of the microtubule network. We noted that, in cells with high expression levels of exogenous Su48, the protein can also be found in the cytoplasm.
Fig. 2.
Su48 is localized to the centrosome. (A) Fluorescence microscopy images of U2OS cells with transient expression of GFP-Su48 (green). Cells were stained with anti-γ-tubulin antibody (red) and DAPI. The arrows indicate a single centrosome in the G1 stage or a centrosome pair in the S/G2 stage. (B) Su48 is localized to the centrosome in the presence of the microtubule destabilizing agent nocodazole. Images show GFP-Su48 (green) and γ-tubulin (red). (C) Dynamic localization of Su48 on the centrosome. U2OS cells were transfected with GFP-Su48 and synchronized with either a thymidine or nocodazole block. Cells were examined by immunostaining at various stages of the cell cycle. Images show GFP-Su48 (green) and γ-tubulin (red). Note that GFP-Su48 localization on the centrosome pair is asymmetric (arrows) in the S phase. By the G2 stage, GFP-Su48 is distributed evenly on the centrosome pair.
We further examined the subcellular localization of Su48 during the centrosome duplication cycle. To this end, localization of GFP-Su48 was determined in U2OS cells that were synchronized and fixed in the G1, S, or G2 phase of the cell cycle. We found that, after centrosome duplication in the S phase, the distribution of GFP-Su48 on the centrosome pair is asymmetric (Fig. 2C). However, by the G2 stage, the abundance of Su48 in the centrosome pair becomes comparable (Fig. 2C). These observations suggest that a dynamic pattern of Su48 accumulation within the newly synthesized daughter centrosome.
Although a variety of centrosome proteins have been identified, it is not clear whether any common structural motifs are involved in targeting the proteins to the centrosome. We therefore determined the structure required for localization of Su48 to the centrosome. A series of deletion mutants were generated and inspected for the effects on the subcellular localization of the protein. Analysis by using the multicoil program indicates that two subdomains, i.e., amino acid residues 176–207 and 216–254, have the highest probability of forming coiled coils. We found that disruption of these regions, which constitute the cores of the coiled-coil structure, abolishes the centrosomal localization of Su48 (Fig. 3). Notably, the mutant Su48(1–291) retains the ability to localize to the centrosome but forms aggregates around the organelle (Fig. 3). In contrast, deletion of the putative zing-finger structure or the C terminus of Su48 does not affect its localization. More importantly, we have identified a region of Su48 that is sufficient to target the protein to the centrosome (Fig. 3). This region, between amino acid residues 156 and 315, encompasses the coiled-coil motif of Su48. Thus, the current data support the notion that the coiled-coil structure is critical for centrosome localization.
Fig. 3.
Identification of the structure required for centrosome localization of Su48. U2OS cells transfected with a series of GFP-Su48 fusion constructs were stained with the anti-γ-tubulin antibody and DAPI. Images show GFP or GFP-fusion proteins (green), γ-tubulin (red), and DAPI (blue). The arrows indicate the positions of the centrosomes.
Su48 Forms Homodimers.
Because coiled coils are commonly involved in protein–protein interactions, we next investigated whether the structure found in Su48 represents a dimerization motif. We found that Su48 can bind to itself in the yeast two-hybrid system (Fig. 4A). To confirm the interaction, we performed in vitro binding assays using a GST-Su48 fusion protein and Su48 protein produced by in vitro translation. The GST-Su48 fusion protein, but not the GST protein alone, can associate with full-length Su48 (Fig. 4B). This result indicates that the association is direct and is not dependent on the presence of other proteins.
Fig. 4.
Characterization of Su48 dimerization. (A) Self-association of Su48 molecules in yeast two-hybrid system. The yeast strain HF7C was transformed with a combination of “bait” and “prey” plasmid constructs, as indicated. pGBT-Su48, Gal4 DNA-binding domain (DBD)-Su48 fusion; pGBT, Gal4-DBD only; pGAD-Su48, Gal4 transcription-activation domain (AD)-Su48 fusion; pGAD, Gal4-AD only. +, interaction; −, no interaction. (B) Su48 forms dimers in vitro. (Left) Glutathione Sepharose beads loaded with either GST-Su48(156–407) or GST alone were incubated with 35S-labeled full-length Su48. The proteins bound to the beads were separated by SDS/PAGE and visualized by PhosphorImager (lanes 2 and 3). A sample of the labeled Su48 protein (50% of input) was loaded in lane 1. (Right) A duplicate SDS polyacrylamide gel was stained with Coomassie blue R-250 to confirm that comparable amounts of GST or GST-Su48(156–407) protein were used. (C) Identification of the structure involved in Su48 dimerization by coimmunoprecipitation. 293T cells were transfected with a combination of FLAG-Su48, GFP, or various GFP-Su48 fusion constructs as indicated in the uppermost panel. Cell lysates were subjected to immunoprecipitation (IP) followed by Western blot analysis (WB). Immunoblots from top to bottom: panel 1, IP: performed using the anti-FLAG antibody, WB: anti-GFP antibody; panel 2, IP: anti-GFP antibody, WB: anti-FLAG antibody; panel 3, cell lysates analyzed by WB using the anti-GFP antibody. In lane 7, the additional species of FLAG-Su48 probably results from partial protein degradation. Panel 4 shows cell lysates analyzed by WB using the anti-FLAG antibody. (D) Summary of the Su48 localization and dimerization study. Schematic representations of the Su48 deletion mutants used in the studies are shown. ND, not determined.
To examine Su48 dimerization in detail, we performed coimmunoprecipitation analysis and determined whether FLAG-Su48 can associate with GFP-Su48 in 293T cells. Indeed, we found that the GFP-Su48 protein can be coimmunoprecipitated with FLAG-Su48 (Fig. 4C, panel 1). In the reciprocal experiment, the FLAG-Su48 protein also coprecipitates with GFP-Su48 (Fig. 4C, panel 2). Notably, disruption of the core of the coiled-coil structure abolishes the interaction between FLAG-Su48 and GFP-Su48 (Fig. 4C, lane 6). Moreover, the region between amino acid residues 156 and 291 is sufficient to modulate the interaction (Fig. 4C, lane 8). Our results rule out the possibility that the GFP moiety is involved in the interaction (Fig. 4C, lane 1). Taken together, these observations substantiated the idea that the coiled-coil structure of Su48 is involved in formation of homodimers or multimeric assemblies.
Overexpression of Su48 Causes Defects in Mitosis.
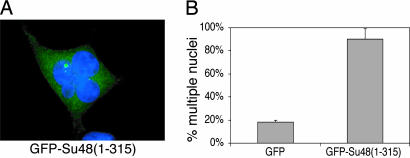
We next investigated whether ectopic expression of Su48 may interfere with mitosis. To this end, 293T cells were transfected with two deletion mutants, Su48(1–315) and Su48(1–374), which contain the coiled-coil domain but lack the C-terminal region. We reasoned that because these species of Su48 can still form dimers and localize to the centrosome through the coiled-coil domain, they may act in a dominant-negative manner. In addition, these mutants can also be expressed at higher levels than the full-length protein, probably as a result of the deletion of the putative destruction boxes in the C-terminal region. Indeed, we found that 90% of cells that have GFP-Su48(1–315) overexpression acquire multiple nuclei, probably as a result of incomplete cell division (Fig. 5). This effect is most profound in 293T cells, perhaps because high levels of expression can be achieved in these cells. The deletion mutant GFP-Su48(1–374) caused similar effects (data not shown).
Fig. 5.
Overexpression of an Su48 deletion mutant causes mitotic defects. (A) Ectopic expression of GFP-Su48(1–315) causes fragmented or multinucleated cells. 293T cells were examined 48 h after transfection with GFP-Su48(1–315). A representative fluorescence microcopy image is shown. Image shows GFP-Su48(1–315) (green) and DAPI (blue). (B) Effect of Su48 overexpression on nucleus morphology in 293T cells. 293T cells were examined 48 h after transfection with GFP-Su48(1–315). Three hundred transfected cells were counted in each experiment. The error bars represent standard deviation calculated from three independent experiments.
We next examined whether the Su48 deletion mutant causes any change in the structure of the centrosome. We specifically inspected the localization of γ-tubulin, as it represents a protein complex involved in a crucial function of the centrosome, namely, microtubule nucleation. We chose U2OS cells for this study because these cells are more adhesive to coverglass and are more suitable for immunostaining. Interestingly, we found that, as a result of GFP-Su48(1–374) overexpression, localization of γ-tubulin to the centrosome was diminished in up to 55% of the cells (Fig. 6A). In contrast, overexpression of the GFP protein alone did not affect γ-tubulin localization (Fig. 6B). Moreover, localization of Centrin, another centrosomal protein, was not disrupted (data not shown). These observations suggest that the mutant form GFP-Su48(1–374) causes displacement of γ-tubulin from the centrosome, which probably indicates changes in the complex structure of this organelle. However, we noted that the organization of the microtubule network was not perturbed (Fig. 6C).
Fig. 6.
Overexpression of a Su48 mutant causes dislocation of γ-tubulin. Immunostaining of U2OS cells transfected with GFP-Su48(1–374) or GFP alone. The figure shows representative images obtained from three experiments. (A) Overexpression of GFP-Su48(1–374) causes displacement of γ-tubulin from the centrosome. The arrow indicates a cell with overexpression of GFP-Su48(1–374) (green), which is diffused throughout the cytosol. Note that γ-tubulin can be detected in nontransfected cells but not in the cell with overexpression of GFP-Su48(1–374). Images show γ-tubulin (red) and DAPI (blue). (B) Overexpression of GFP alone does not affect localization of γ-tubulin. (C) Overexpression of GFP-Su48(1–374) does not affect the organization of microtubule network. Images show GFP-Su48(1–374) (green), α-tubulin (red), and DAPI (blue).
Notably, dislocation of γ-tubulin from the centrosome was only observed in cells with high levels of the Su48 mutant, where the protein is not only found in the centrosome but also has diffused into the cytosol (Fig. 6A). Because GFP-Su48(1–374) can be targeted to the centrosome, it may either block or occupy the docking sites for γ-tubulin on the centrosome. We found that, although the deletion mutant Su48(156–315) can localize to the centrosome, it does not effectively cause γ-tubulin dislocation. Apparently the N terminus or the regions adjacent to the coiled-coil structure are required for dislocating γ-tubulin.
It is also possible that excessive GFP-Su48(1–374) sequesters γ-tubulin into the cytosol and prevents it from binding to the centrosome. To test this possibility, we used coimmunoprecipitation analysis to investigate whether Su48 can associate with γ-tubulin. Our results indicated Su48 does not bind to γ-tubulin (Fig. 9).
Interference with Su48 Function Disrupts Cell Division.
To explore the role of Su48 in mitosis, we performed microinjection in HeLa cells using the Su48 specific monoclonal antibody 24F1 and determined whether interference with Su48 functions by the antibody can affect mitosis. We chose this antibody because it can effectively detect ectopically expressed Su48 in coimmunoprecipitation assays or in immunofluorescence studies, indicating that it can bind to the native form of Su48 protein (Fig. 9).
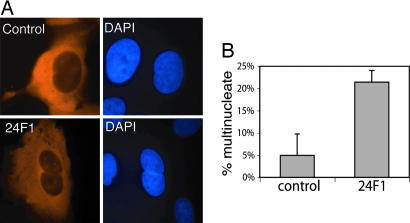
Introduction of the anti-Su48 antibody to HeLa cells increases the percentage of cells that harbor two nuclei within 24 h (Fig. 7). In contrast, the isotope-matched control antibody does not significantly affect the number of binucleated cells (Fig. 7). Microinjection of the disabling monoclonal antibody also caused similar effects in U2OS cells, in which Su48 can be detected at the mRNA level but not at the protein level. However, the 24F1 antibody did not seem to affect the organization of the microtubule network during the limited periods of observation of these studies (data not shown). We also attempted to abrogate Su48 function by the short-interfering RNA technique. However, we have not been able to obtain any short-interfering RNA duplex that can effectively knock down Su48. Nevertheless, the current findings suggest that antibody-induced disruption of endogenous Su48 function causes incomplete mitosis, primarily by blocking cytokinesis.
Fig. 7.
Microinjection of anti-Su48 antibody causes polyploidy. (A) HeLa cells were microinjected with a mouse monoclonal anti-Su48 antibody (24F1) or an isotype-matched control antibody. Twenty-four hours after injection, cells were analyzed by immunostaining with Cy3-conjugated anti-mouse antibody (red). At least 200 cells were injected and analyzed for each sample. (B) Quantification of the effect of anti-Su48 antibody on cell division after microinjection. The percentage of cells with two or more nuclei is shown.
Discussion
Although a variety of centrosomal proteins have been identified, the structures involved in centrosome localization remain to be fully characterized. Here, we have identified a previously unrecognized centrosome-localized protein, Su48, and revealed that a domain containing a coiled-coil structure is both sufficient and required for dimerization and localization of this molecule to the centrosome. Our results also indicate that Su48 plays an essential role in mitosis.
In particular, we have identified a previously unrecognized centrosome-targeting structure, which contains a unique coiled-coil motif. Although coiled coils have been found in a number of centrosome-localized proteins (25), some of these structures probably facilitate formation of protein assemblies but are not necessarily involved in subcellular localization. For example, the centrosome anchor protein AKAP450 and Pericentrin are directed to the centrosome through a conserved 90-aa PACT domain rather than by its long coiled-coil repeats (27). To date, only a few coiled-coil structures have been found to be involved in centrosome localization and, yet, these structures usually vary in length or share low homology in primary sequences. We have noted that the coiled-coil region of TACC4 is similar to that of Su48 in length and can target the protein to the centrosome by association with a related centrosome anchor protein AKAP350 (28). However, the coiled coil of Su48 shares little homology to the comparable region of TACC4 or other centrosomal proteins. Our results clearly demonstrate that the region encompassing the coiled-coil structure of Su48 is sufficient for centrosome localization. Thus, we believe that this region of Su48 represents a previously unrecognized centrosome-targeting motif. It is conceivable that Su48 is guided to the centrosome by binding to other centrosome proteins through this structure.
We found that overexpression of the Su48 coiled-coil structure can lead to structural changes in the centrosome, as reflected by displacement of γ-tubulin. A recent study revealed that Pericentrin is required for anchoring the γ-TuRC to the centrosome through interaction with the γ-TuRC subunits GCP-2 and -3 (29). Although we have not detected interactions of Su48 with Pericentrin, it is possible that excessive Su48 competes with γ-TuRC for the docking site on Pericentrin. Alternatively, ectopic expression of Su48 may disrupt the scaffold in the pericentriolar material formed by Pericentrin. Finally, we recognize the possibility that GFP-Su48, which becomes ubiquitous in the cytosol upon overexpression, may sequester γ-tubulin and prevent it from binding to the centrosome. However, we believe that this scenario is unlikely the case, because our coimmunoprecipitation analysis indicated that Su48 does not directly associate with γ-tubulin. Although it remains to be determined whether overexpression of Su48 affects localization of other proteins to the centrosome, it is clear that overexpression of the coiled coil-containing structure of Su48 causes multinucleated cells, probably by interfering with the normal function of the centrosome.
Our finding that microinjection of Su48 antibody causes binucleated cells indicates an essential role of Su48 function in cell division. The monoclonal anti-Su48 antibody used in this study binds to the N-terminal region that encompasses the putative zinc-finger motif. Although this region is not involved in protein dimerization or centrosome localization, the zinc-finger of Su48 may be essential for an unidentified function within the centrosome. Binding of the antibody after microinjection disables Su48 and causes mitotic defects. Polyploidy of these cells could be the result of failed cytokinesis due to abnormal centrosome function. Indeed, a body of evidence indicates that the centrosome plays a critical role in cytokinesis (30–32). Furthermore, abrogation of Su48 may affect organization of the mitotic spindle, which results in abnormal chromosome separation or incomplete cytokinesis. Although we have not been able to obtain a short-interfering RNA duplex that can effectively diminish Su48 in cells, the effect of endogenous disabling of Su48 by antibody injection is unambiguous. Clearly other aspects of Su48 functions in mitosis may be confirmed by gene-specific mutagenesis in future studies.
The current study raises the possibility that Su48 abnormalities may contribute to aneuploidy and certain aspects of malignant transformation by causing centrosome dysfunction. Centrosome defects may lead to mitotic failure, centrosome supernumerary, and tetraploid phenotypes, which may evolve into global chromosome changes. In particular, tetraploidy or polyploidy may be an intermediate stage in the development of aneuploidy. Because of the importance of the centrosomes in formation of the spindle and separation of the chromosomes during mitosis, cells that adopt multiple centrosomes have the tendency to form multipolar spindles, which cause unequal distribution of the chromosomes to the daughter cells. Both loss of tumor suppressor genes and amplification of protooncogenes may facilitate these cells to survive. The aberration of the centrosomes may increase chromosome instability by gaining or losing chromosomes, thereby contributing to evolution of a transformed phenotype. In agreement with this prediction, we found that Su48 levels are elevated in a variety of cancers, including pancreatic cancer cell lines that harbor amplified centrosomes. To evaluate the role of Su48 in cancer development, it would be beneficial to expand the survey of Su48 expression in a large variety of human cancers. Preliminary studies support the relevance of Su48 expression patterns to human disease.
It should be pointed out that, although centrosome amplification is commonly found in cancers, it is still not clear whether such an abnormality is the cause or consequence of malignant transformation. However, we believe that the centrosomal protein Su48 provides a unique tool to study the consequences of centrosome aberration during mitosis. In particular, the deleted mutant form of Su48 may permit evaluation of the role of centrosome abnormalities relevant to chromosome instability and the transformed phenotype.
In summary, we have identified and characterized a previously unrecognized centrosomal protein, Su48, which is essential for cell division. Most notably, we revealed that a coiled coil-containing domain in Su48 is involved in both dimerization and centrosome localization. On the basis of observations that Su48 is highly expressed in a number of cancer cell lines and that ectopic expression of Su48 can cause centrosome alterations and abnormal mitosis, we speculate that deregulated Su48 may lead to abnormal chromosome segregation and subsequently contribute to aneuploidy and malignant transformation.
Materials and Methods
Cell Culture and Antibodies.
The human osteosarcoma cell line U2OS, cervical cancer cell line HeLa, breast epithelial cell line HBL100, pancreatic cancer cell lines PANC-1, MIYAPAKA2 (MIYA), and PCI43, and the embryonic kidney cell line 293T were maintained in Dulbecco–Vogt-modified Eagle’s medium supplemented with 10% FBS and penicillin–streptomycin antibiotics (Invitrogen). The following antibodies were used: anti-γ-tubulin antibody (GTU-88), anti-α-tubulin (B512) antibody, and anti-FLAG antibody (M2) from Sigma and horseradish peroxidase-conjugated anti-mouse IgG κ light-chain antibody (Invitrogen). Cell cycle synchronization was achieved by a thymidine/nocodazole double-block protocol (see Supporting Methods, which is published as supporting information on the PNAS web site).
Immunofluorescence Microscopy.
293T or U2OS cells were transiently transfected with the wild-type or mutant Su48 construct or empty control plasmid by using FuGene 6 reagent (Roche Applied Science, Indianapolis). Cells were then plated on coverglass and examined by immunostaining 24 or 48 h after transfection. Immunostaining was performed by following a previously published protocol (see ref. 33 and Supporting Methods).
Antibody Production and Microinjection.
GST-Su48(156–407) was used to generate polyclonal antibody in rabbit (see Supporting Methods). The antisera were affinity-purified. His-Su48 was used to immunize mice and make monoclonal antibodies. The hybridoma 24F1 produces an IgG1a that detects FLAG-Su48 in transfected U2OS cells by immunostaining. The monoclonal antibodies were purified with G protein. For Microinjection, HeLa cells were grown on coverslips and injected with antibodies by using glass capillary needles pulled on a micropipette puller. Either 24F1 or an isotype-matched control antibody was injected at a concentration of 5 mg/ml. Cells were fixed and stained with Cy3-conjugated anti-mouse antibody 24 h later. At least 200 cells were injected and counted for each sample. The experiment was repeated twice.
RT-PCR Analysis.
Human cDNA samples were generated by reverse transcription by using total RNA isolated from HeLa, U2OS, PANC-1, HBL100, and 293T cells. Mouse cDNA samples were prepared from mouse tissues or from the mouse embryonic day 11 cDNA library (BD Biosciences Clontech). Details of primers and PCR conditions are provided in Supporting Methods.
Protein Binding Assays.
For in vitro protein binding assay, GST-Su48(156–407) was expressed in bacteria and isolated with glutathione Sepharose beads (Supporting Methods). Full-length Su48 protein was expressed and labeled by using the TnT T7 Quick Coupled Transcription/Translation System (Promega). Glutathione Sepharose beads loaded with GST or GST-Su48 were incubated with in vitro translation product. The protein bound to the beads was resolved by SDS/PAGE and detected by PhosphorImager (Molecular Dynamics). Immunoprecipitation and Western blotting were performed following a previously published protocol (see ref. 33 and Supporting Methods).
Supplementary Material
Acknowledgments
We thank the Sir William Dunn School of Pathology, University of Oxford, for providing certain research funds and facilities. This work was supported by grants from the Abramson Family Cancer Research Institute of the University of Pennsylvania and from the National Institutes of Health (Bethesda).
Abbreviation
- γ-TuRC
γ-tubulin ring complex.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Nigg E. A. Nat. Rev. Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 2.Salisbury J. L., Whitehead C. M., Lingle W. L., Barrett S. L. Biol. Cell. 1999;91:451–460. [PubMed] [Google Scholar]
- 3.Doxsey S. Nat. Rev. Mol. Cell Biol. 2001;2:688–698. doi: 10.1038/35089575. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q., Hirohashi Y., Furuuchi K., Zhao H., Liu Q., Zhang H., Murali R., Berezov A., Du X., Li B., Greene M. I. DNA Cell Biol. 2004;23:475–489. doi: 10.1089/1044549041562276. [DOI] [PubMed] [Google Scholar]
- 5.Lingle W. L., Lutz W. H., Ingle J. N., Maihle N. J., Salisbury J. L. Proc. Natl. Acad. Sci. USA. 1998;95:2950–2955. doi: 10.1073/pnas.95.6.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pihan G. A., Purohit A., Wallace J., Knecht H., Woda B., Quesenberry P., Doxsey S. J. Cancer Res. 1998;58:3974–3985. [PubMed] [Google Scholar]
- 7.Carroll P. E., Okuda M., Horn H. F., Biddinger P., Stambrook P. J., Gleich L. L., Li Y. Q., Tarapore P., Fukasawa K. Oncogene. 1999;18:1935–1944. doi: 10.1038/sj.onc.1202515. [DOI] [PubMed] [Google Scholar]
- 8.Weber R. G., Bridger J. M., Benner A., Weisenberger D., Ehemann V., Reifenberger G., Lichter P. Cytogenet. Cell Genet. 1998;83:266–269. doi: 10.1159/000015168. [DOI] [PubMed] [Google Scholar]
- 9.Sato N., Mizumoto K., Nakamura M., Nakamura K., Kusumoto M., Niiyama H., Ogawa T., Tanaka M. Clin. Cancer Res. 1999;5:963–970. [PubMed] [Google Scholar]
- 10.Lingle W. L., Salisbury J. L. Am. J. Pathol. 1999;155:1941–1951. doi: 10.1016/S0002-9440(10)65513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sen S., Zhou H., White R. A. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff J. R., Anderson L., Zhu Y., Mossie K., Ng L., Souza B., Schryver B., Flanagan P., Clairvoyant F., Ginther C., et al. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H., Kuang J., Zhong L., Kuo W. L., Gray J. W., Sahin A., Brinkley B. R., Sen S. Nat. Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y., Wong M. L., Alberts B., Mitchison T. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]
- 15.Moritz M., Braunfeld M. B., Sedat J. W., Alberts B., Agard D. A. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- 16.Schnackenberg B. J., Khodjakov A., Rieder C. L., Palazzo R. E. Proc. Natl. Acad. Sci. USA. 1998;95:9295–9300. doi: 10.1073/pnas.95.16.9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moritz M., Zheng Y., Alberts B. M., Oegema K. J. Cell Biol. 1998;142:775–786. doi: 10.1083/jcb.142.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy S. M., Urbani L., Stearns T. J. Cell Biol. 1998;141:663–674. doi: 10.1083/jcb.141.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy S. M., Preble A. M., Patel U. K., O’Connell K. L., Dias D. P., Moritz M., Agard D., Stults J. T., Stearns T. Mol. Biol. Cell. 2001;12:3340–3352. doi: 10.1091/mbc.12.11.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fava F., Raynaud-Messina B., Leung-Tack J., Mazzolini L., Li M., Guillemot J. C., Cachot D., Tollon Y., Ferrara P., Wright M. J. Cell Biol. 1999;147:857–868. doi: 10.1083/jcb.147.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunawardane R. N., Martin O. C., Cao K., Zhang L., Dej K., Iwamatsu A., Zheng Y. J. Cell Biol. 2000;151:1513–1524. doi: 10.1083/jcb.151.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oegema K., Wiese C., Martin O. C., Milligan R. A., Iwamatsu A., Mitchison T. J., Zheng Y. J. Cell Biol. 1999;144:721–733. doi: 10.1083/jcb.144.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moudjou M., Bordes N., Paintrand M., Bornens M. J. Cell Sci. 1996;109:875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 24.Wigge P. A., Jensen O. N., Holmes S., Soues S., Mann M., Kilmartin J. V. J. Cell Biol. 1998;141:967–977. doi: 10.1083/jcb.141.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersen J. S., Wilkinson C. J., Mayor T., Mortensen P., Nigg E. A., Mann M. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 26.Nagase T., Ishikawa K., Suyama M., Kikuno R., Hirosawa M., Miyajima N., Tanaka A., Kotani H., Nomura N., Ohara O. DNA Res. 1998;5:355–364. doi: 10.1093/dnares/5.6.355. [DOI] [PubMed] [Google Scholar]
- 27.Gillingham A. K., Munro S. EMBO Rep. 2000;1:524–529. doi: 10.1093/embo-reports/kvd105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steadman B. T., Schmidt P. H., Shanks R. A., Lapierre L. A., Goldenring J. R. J. Biol. Chem. 2002;277:30165–30176. doi: 10.1074/jbc.M201914200. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman W. C., Sillibourne J., Rosa J., Doxsey S. J. Mol. Biol. Cell. 2004;15:3642–3657. doi: 10.1091/mbc.E03-11-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piel M., Nordberg J., Euteneuer U., Bornens M. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- 31.Khodjakov A., Rieder C. L. J. Cell Biol. 2001;153:237–242. doi: 10.1083/jcb.153.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou Y. Y., Rattner J. B. Cell Motil. Cytoskeleton. 2002;51:123–132. doi: 10.1002/cm.10019. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q., Zhang H., Guerrette S., Chen J., Mazurek A., Wilson T., Slupianek A., Skorski T., Fishel R., Greene M. I. Oncogene. 2001;20:4640–4649. doi: 10.1038/sj.onc.1204625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.