Abstract
Netrin-1 is critical for axonal pathfinding which shares similarities with formation of vascular network. Here we report that netrin-1 induction of angiogenesis is mediated by an increase in endothelial nitric oxide (NO•) production, which occurs via a DCC-dependent, ERK1/2-eNOS feed-forward mechanism. Exposure of mature aortic endothelial cells to netrin-1 resulted in a potent, dose-dependent increase in NO• production, detected by electron spin resonance. Scavenging NO• with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) abolished netrin-1 stimulated angiogenesis. Netrin-1-stimulated NO• production or angiogenesis was inhibited by DCC antibody, DCC small interfering RNA (siRNA), specific inhibitors (PD98059, U0126), or siRNAs for MEK1/2. PTIO attenuated ERK1/2 phosphorylation, indicating a feed-forward mechanism. Netrin-1 induced a time-dependent phosphorylation of eNOSs1179, s116 and a rapid dephosphorylation of eNOSt497. Only eNOSs1179 was sensitive to U0126 or PTIO. These data characterized a mechanism whereby netrin-1 promotes angiogenesis, which may broadly relate to cardiovascular, neuronal and cancer physiology.
Keywords: nitric oxide
Netrin-1 is one of the first discovered axon-guiding molecules that are critical for neuronal development (1, 2). It is a secreted protein that is released to circulation after being produced by a variety of cells (3). Upon activation of its attraction-selective receptor deleted in colorectal cancer (DCC) or neogenin, netrin-1 induces axonal outgrowth and crossover through the midline (2, 4); this is at least partially mediated by activation of the mitogen activated protein kinase ERK1/2 (5).
Formation of vascular network shares many similarities with neuronal pathfinding (6). A number of mitogens including VEGF and EGF have been shown to regulate endothelial cell growth and proliferation. Interestingly, netrin-1 is structurally homologous to the endothelial mitogens. It has an N-terminal type IV laminin repeat, followed by three cystein-rich EGF modules and a positively charged C-terminal domain. A recent study demonstrated that netrin-1 stimulates growth of umbilical vein endothelial cells and vascular smooth muscle (7). However, in the presence of the repulsive receptor UNC5B in developing capillaries, netrin-1 induces endothelial filopodial retraction (8). Our current study uniquely studied proliferation and migration of adult mature aortic endothelial cells, and endothelial outgrowth from adult mouse aortic discs to examine the angiogenic effects of netrin-1. Compared to umbilical vein or developing vessels, aorta shares the closest physiology with large adult coronary arteries where therapeutic angiogenesis is beneficial for patients with ischemic coronary artery diseases (9, 10). More importantly, the signaling mechanisms underlying netrin-1 modulation of angiogenesis were identified.
Using the highly specific and sensitive electron spin resonance technology for direct and characteristic measurement of nitric oxide gas radical (NO•), we examined a potential role of NO• in netrin-1 modulation of angiogenesis and the signaling mechanisms underlying netrin-1 stimulation of NO• production. It turns out that DCC is required for netrin-1-induced feed-forward activation of ERK1/2-eNOS involving eNOS phosphorylation at serine 1179 residue, resulting in an increase in endothelial cell NO• production, growth, and migration. This pathway may broadly relate to cardiovascular, neuronal, and cancer physiology.
Results
Netrin-1 Induction of Angiogenesis Is Mediated by NO•.
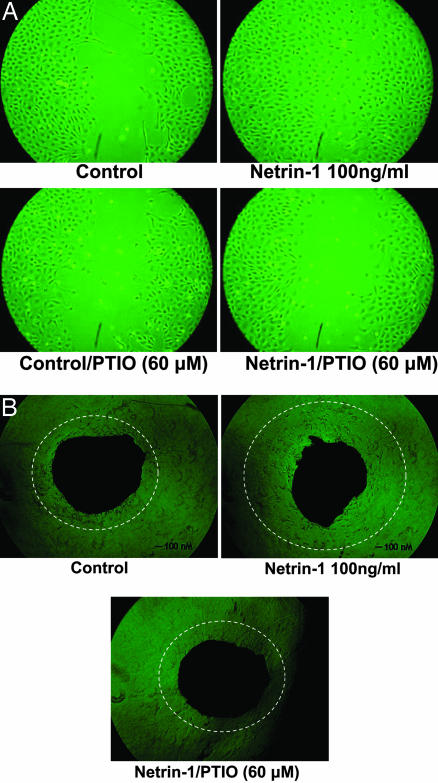
Exposure of aortic endothelial cells to netrin-1 (10, 30, and 100 ng/ml) produced a dose-dependent increase in NO• production as shown by both representative ESR spectra and grouped data from six independent experiments (Fig. 1). Wound proliferation assays indicate that netrin-1 (100 ng/ml, same hereafter) promoted endothelial cell proliferation and migration, resulting in a much faster wound closure of the monolayer (Fig. 2A). This response was completely attenuated by preincubation of cells with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) (60 μmol/liter, 30 min, same hereafter) to scavenge NO• specifically (Fig. 2A). PTIO also prevented wound closure in control cells (Fig. 2A). This finding is consistent with previous observation that basally produced NO• is growth-stimulating. Aortic endothelial outgrowth assay also indicated enhanced formation of capillary-like endothelial network from aortic discs treated with netrin-1 (Fig. 2B). This response was abolished by PTIO (Fig. 2B). The outgrowth assay was performed strictly following studies by Berger et al. (11), where the authors carefully characterized the cells to show that majority is endothelial cells positive for CD31. Taken together, these data strongly suggest that netrin-1 induction of angiogenesis is NO•-dependent.
Fig. 1.
Netrin-1 stimulates endothelial cell nitric oxide (NO•) production. Confluent endothelial cells were incubated with different concentrations of netrin-1 (10, 30, and 100 ng/ml) at 37°C for 60 min in modified Krebs/HEPEs buffer containing the NO•-specific spin trap Fe2+(DETC)2. Cells were then gently collected for analysis of NO• production by using electron spin resonance (ESR). Data are presented as mean ± SEM. (A) Representative ESR spectra. (B) Grouped data from six independent experiments. ANOVA; ∗, P < 0.05; ∗∗, P < 0.01.
Fig. 2.
Netrin-1 induced angiogenesis is nitric oxide (NO•)-dependent. (A) Wound proliferation assay. Proliferating endothelial cells at 95% confluence were scratched gently to make a 1-mm wound, and then immediately exposed to control media or media containing netrin-1 (100 ng/ml). Some cells were pretreated with NO• scavenger PTIO (60 μmol/liter) for 30 min before would creation and addition of netrin-1. Thirty-six hours later, speed of wound closure of endothelial monolayer was captured by using a Nikon digital camera with an inverted microscope. (B) Aortic endothelial outgrowth assay. Freshly isolated mouse aortic discs (1 mm o.d.) were placed in thrombin-coated six-well culture wells, covered with fibrinogen and incubated at 37°C for 1 h to allow formation of a thin fibrin clot layer. Wells were then supplemented with growth media containing 20% FCS, with and without netrin-1 (100 ng/ml) or 30-min PTIO (60 μmol/liter) preincubation. Endothelial cells started to grow out of aortic discs by day 1.5. Three to five days later, a capillary-like endothelial outgrowth network forms around the aortic disk.
Netrin-1 Stimulation of NO• Requires ERK1/2 and DCC.
Mitogen-activated protein kinases ERK1 and -2 are involved in growth signaling of endothelial cells (12). Indeed, DCC-dependent activation of ERK1/2 is required for netrin-1-dependent attractive guidance of axons (5). Likewise, in our experiments, where mature aortic endothelial cells were exposed to netrin-1, ERK1/2 phosphorylation was increased in a time-dependent fashion (Fig. 3A). To examine specific expression of DCC in the aortic endothelial cells, some cell lysates were precleared with a DCC polyclonal antibody before Western blot analysis. As shown in Fig. 3B, DCC protein probed by the specific antibody was present in mature aortic endothelial cells, and the intensity of the target band was markedly diminished after antibody-clearance treatment (EC-1 and EC-2 are cell lysates from two different dishes of endothelial cells, and AbC represents antibody clearance).
Fig. 3.
DCC and ERK1/2 are required for netrin-1 stimulation of nitric oxide (NO•) production. (A) Western blot demonstrating netrin-1 induction of ERK1/2 phosphorylation. Endothelial cells were stimulated with netrin-1 (100 ng/ml) and harvested at different time points for Western blot analysis of ERK1/2 phosphorylation using a phospho-specific antibody. (B) Western blot demonstrating DCC expression in mature aortic endothelial cells. Lysates of endothelial cells from two different dishes (EC-1 and EC-2) were untreated or precleaned with the specific DCC antibody (AbC for antibody clearance treatment) before loading into a SDS/PAGE and analysis with Western blotting. (C and D) Representative spectra and grouped data of ESR analysis of NO• production from endothelial cells pretreated for 30 min with DCC antibody (1 mg/ml) or MEK1/2 inhibitor U0126 (50 μmol/liter) or PD98059 (50 μmol/liter) before netrin-1 stimulation. ESR procedure is described in Experimental Procedures. Data are presented as means ± SEM. ANOVA; ∗, P < 0.01 vs. netrin-1; #, P < 0.01 vs. control.
Endothelial NO• production in response to netrin-1, presented in both representative ESR spectra and grouped data from six independent experiments (Fig. 3 C and D), was attenuated by specific MEK1/2 inhibitor PD98059 (50 μmol/liter, 30-min preincubation), U0126 (50 μmol/liter, 30-min preincubation), or by the antibody neutralizing DCC (1 mg/ml, 30-min preincubation, as used by Keino-Masu et al.; ref. 13). In additional experiments, endothelial cells were transfected with MEK1/2 siRNAs (25 nmol/liter each for MEK1 and MEK2) before netrin-1 stimulation. The siRNAs specifically attenuated MEK1/2 protein expression, but had no effect on the abundance of Akt protein (Fig. 4A). MEK1/2 siRNAs also attenuated netrin-1-induced ERK1/2 phosphorylation (Fig. 4B).
Fig. 4.
A critical role of ERK1/2 in netrin-1 activation of eNOS. (A) Western blots demonstrating that protein expression of MEK1/2, but not Akt, was attenuated with specific MEK1/2 siRNAs (25 nmol/liter each). (B) Western blot demonstrating that netrin-1 induced ERK1/2 phosphorylation was inhibited by specific MEK1/2 siRNAs. (C and D) Representative ESR spectra and grouped data of netrin-1 stimulation of nitric oxide (NO•) from cells transfected with control siRNA (25 nmol/liter) or MEK1/2 siRNAs (25 nmol/liter each). Detailed transfection procedure is included in Experimental Procedures. Data are presented as mean ± SEM. ANOVA; ∗, P < 0.01 vs. control siRNA with netrin-1; #, P < 0.01 vs. control siRNA without stimulation.
Of note, netrin-1 stimulated NO• production in cells transfected with control siRNA (25 nmol/liter) similarly to that was observed in untransfected cells (Figs. 1 and 4 C and D). However, MEK1/2 siRNAs significantly attenuated netrin-1 stimulation of NO• (n = 4, P < 0.05, Fig. 4 C and D), strongly suggesting a critical role of ERK1/2 in netrin-1 activation of eNOS.
To examine more specifically a role of DCC in netrin-1 stimulation of NO•, endothelial cells were transfected with siRNAs specific for DCC (Qiagen) before netrin-1 stimulation. As shown by both representative ESR spectra and grouped data from four independent experiments (Fig. 5A and B), DCC siRNA abolished NO• production in response to netrin-1, clearly implicating a specific role of DCC in mediating netrin-1 activation of eNOS.
Fig. 5.
DCC is specifically required for netrin-1 activation of eNOS. (A) Representative ESR spectra. (B) Grouped data of netrin-1 stimulated nitric oxide (NO•) production from cells transfected with control siRNA (25 nmol/liter) or DCC siRNA (25 nmol/liter). Detailed transfection procedure is included in Experimental Procedures. Data are presented as mean ± SEM. ANOVA; ∗, P < 0.01 vs. control siRNA with netrin-1; #, P < 0.01 vs. control siRNA without stimulation.
Netrin-1 Induction of Angiogenesis Requires DCC-Dependent Feed-Forward Activation of ERK1/2-eNOS: Role of eNOSs1179 Phosphorylation.
Data described so far have demonstrated that netrin-1 induces angiogenesis via NO• production, and the latter occurs via DCC activation of ERK1/2. The next logical step is to examine whether netrin-1-induced angiogenesis is affected by blockade of DCC and ERK1/2, if they were truly upstream of NO•. Intriguingly, pretreatment of endothelial cells for 30 min with DCC antibody (1 mg/ml) or U0126 (50 μmol/liter) extremely potently retarded netrin-1 induced acceleration of endothelial wound closure (Fig. 6A), indicating that DCC and ERK1/2 are both required. However, nonspecific mouse IgG or antibody recognizing neogenin had no effect (Fig. 6B). In addition, whereas control siRNA had no effect on angiogenic responses to netrin-1, DCC siRNA clearly prevented netrin-1-induced acceleration of endothelial cell wound closure (Fig. 6C).
Fig. 6.
DCC and ERK1/2 are required for netrin-1-induced angiogenesis. (A) The pictures are from wound proliferation assay where cells were pretreated with U0126 (50 μmol/liter) or DCC antibody (1 mg/ml) for 30 min before netrin-1 (100 ng/ml, 36 h) stimulation. (B) Wound proliferation assay where cells were pretreated with mouse IgG or neogenin antibody (1 mg/ml) for 30 min before netrin-1 stimulation. (C) Wound proliferation assay where cells were pretransfected with control siRNA (25 nmol/liter) or DCC siRNA (25 nmol/liter) before netrin-1 stimulation.
Furthermore, besides the observations that ERK1/2 is required for netrin-1 stimulation of NO•, NO• itself seems necessary in maintaining ERK1/2 activity. Scavenging NO• with PTIO blocked ERK1/2 phosphorylation in response to netrin-1, as evidenced by representative Western blot and grouped data from four independent experiments, indicating a NO•-dependent feed-forward activation of ERK1/2 (Fig. 7A and B). Although netrin-1 might increase intracellular calcium levels, ERK1/2 activation was not affected by intracellular calcium, as scavenging calcium with BAPTA/AM (10 μmol/liter, 30-min preincubation) had little effect on netrin-1 phosphorylation of ERK1/2.
Fig. 7.
Feed-forward activation of ERK1/2-eNOS by netrin-1. (A) Scavenging nitric oxide (NO•) inhibited ERK1/2 activation. Endothelial cells were pretreated for 30 min with NO• scavenger PTIO (60 μmol/liter) before netrin-1 (100 ng/ml, 20 min) stimulation. Representative Western blot is presented. (B) Grouped densitometric data from Western blot analysis of netrin-1 stimulated ERK1/2 phosphorylation in PTIO-pretreated endothelial cells. (C) Scavenging intracellular calcium with BAPTA/AM (10 μmol/liter, 30-min preincubation) had no effect on netrin-1 stimulation of ERK1/2 phosphorylation. (D) Scavenging NO• with PTIO attenuated ERK1/2-dependent eNOSs1179 phosphorylation.
PTIO-dependent loss of ERK1/2 activation was correlated with a reduction in eNOSs1179 phosphorylation (Fig. 7D), suggesting that ERK1/2-dependent eNOSs1179 phosphorylation is likely responsible for eNOS activation. Netrin-1 stimulation caused a rapid time-dependent increase in eNOSs1179 and eNOSs116 phosphorylations, and a rapid dephosphorylation of eNOSt495 (Fig. 8A–C). Regulation of eNOS phosphorylation at any one of the residues alone is sufficient to activate eNOS in response to agonists (14–16). However, only eNOSs1179 phosphorylation was sensitive to ERK1/2 inhibition by U0126 (Fig. 8), indicating that ERK1/2 activation of eNOS in response to netrin-1 occurs via phosphorylation of the serine 1179 residue. Taken together, these data clearly demonstrate that a DCC-dependent, phosphorylation-requiring ERK1/2-eNOS feed-forward activation is required for netrin-1 induction of endothelial cell growth and migration. This signaling cascade is schematically summarized in Fig. 9.
Fig. 8.
Serine 1179 phosphorylation-dependent activation of eNOS by netrin-1: phosphorylation of eNOS at different serine or threonine residues and their sensitivity to U0126. Endothelial cells were pretreated with U0126 (50 μmol/liter, 30 min) before netrin-1 (100 ng/ml) stimulation. Cells were harvested at different time points for Western blot analysis of eNOSs1179, eNOSs116, and eNOSt497 phosphorylation. (A) Representative Western blots. (B) Grouped densitometric data of Western blot analysis of eNOSs1179 phosphorylation. (C) Grouped densitometric data of Western blot analysis of eNOSs116 phosphorylation. (D) Grouped densitometric data of Western blot analysis of eNOSt497 phosphorylation. Data are presented as mean ± SEM. ANOVA; ∗, P < 0.05 vs. DMSO of corresponding time point; #, P < 0.05 vs. DMSO 0 min.
Fig. 9.
Schematic summary of signaling cascade involved in netrin-1 induction of angiogenesis. Netrin-1 activates DCC to result in activation of ERK1/2 and subsequently endothelial nitric oxide (NO•) production from serine 1179 phosphorylated eNOS. NO• also contributes to ERK1/2 activation, forming a feed-forward cycle. NO• then mediates netrin-1-induced enhancement in endothelial cell growth and migration. Earlier work has shown that proliferating endothelial cell have higher expression of eNOS mRNA via actin cytoskeletal regulation of the mRNA stability. This could form another positive feed-forward mechanism that is growth-stimulating.
Discussion
The current study characterized a mechanism whereby netrin-1 induces angiogenesis. It involves an increased in NO• production that is DCC-dependent, and subsequent to a feed-forward activation of ERK1/2-eNOS in endothelial cells. This signaling cascade may mediate physiological effects of netrin-1 in cell or organ systems other than endothelial cell and endothelium, and may represent a common pathway for cardiovascular, neuronal, and cancer physiology.
It was reported earlier that NO• mediates VEGF induced angiogenesis (17). Fibroblast growth factor 2 also increases NO• production in collateral coronary arteries (18). By reducing caveolin-1 abundance and its inhibitory effect on eNOS, lipid-lowering agent Statins can promote NO•-dependent angiogenesis (19, 20). In addition, Statins up-regulate expression of eNOS (21). Interestingly, endostatin (22), an antiangiogenic agent, exerts its effect via PP2A phosphatase-dependent dephosphorylation of eNOS (23). In keeping with these previous observations, we found that phosphorylation-dependent activation of eNOS and consequent NO• production is critical for angiogenesis in response to the newly characterized pro-angiogenic molecule netrin-1. Utilization of NO• in promoting angiogenesis seems to categorize netrin-1 into the family of potent endothelial mitogens including growth factors and the like.
Our findings support an essential role of DCC in mediating netrin-1 induction of NO• and angiogenesis in mature aortic endothelial cells. The specificity of the antibody used for detecting DCC was established by antibody clearance experiments. The DCC antibody also extremely consistently prevented endothelial NO• production, growth and migration in response to netrin-1 (Figs. 3 C and D and 5). In the earlier study by Lu et al. (8), DCC was found absent in embryonic endothelial cells. Rather, the repulsive receptor UNC5B is present in developing vasculature, mediating netrin-1 induced retraction of filopodia (8). Taken together, these data seem to suggest that mature and developing endothelial cells selectively express different receptors for netrin-1, mediating the dual functions of netrin-1 on endothelial cell growth and migration; this seems similar to the mechanisms of action of netrin-1 in neuronal development, where netrin-1 attracts certain axons while repelling the others depending on selective expression of receptors (13, 24). This finding is also consistent with the observation by Park et al. (7) that netrin-1 is growth-promoting in mature umbilical endothelial cells, although no receptor mechanism was identified in this earlier study.
Using specific pharmacological inhibitors and siRNAs, an intermediate role of ERK1/2 in netrin-1 stimulation of endothelial NO• production was characterized. Furthermore, eNOS phosphorylation at serine 1179 residue seemed to be the downstream effector of ERK1/2 activation. This evidence shows how ERK1/2 can directly regulate eNOS activity independently of transcriptional regulations. We and others have shown that ERK1/2 is involved in transient activation of eNOS by hydrogen peroxide and estrogen (14, 25). It is interesting to speculate that these responses may be partially mediated by eNOSs1179 phosphorylation. ERK1/2 is also downstream of another angiogenic factor VEGF in mediating eNOS activation (26). Of note, our observation that netrin-1 activation of ERK1/2 is growth stimulating is consistent with the only other earlier notion regarding ERK1/2 and netrin-1 that DCC-dependent activation of ERK1/2 mediates netrin-1 induced axonal outgrowth (5).
Interestingly, our findings established a feed-forward mechanism of ERK1/2-eNOS activation. Whereas U0126 inhibited eNOSs1179 phosphorylation, scavenging NO• with PTIO reduced ERK1/2 phosphorylation. Indeed, physiological concentrations of NO• are capable of activating ERK1/2 in endothelial cells (27) and other cell types (28). Fig. 9 illustrates the signaling cascade identified by the current study. Initial activation of ERK1/2 via DCC binding to netrin-1 results in phosphorylation and activation of eNOS to produce NO•, which would in turn contribute to ERK1/2 activation and more NO• production; this results in NO•-dependent augmentation of endothelial cell growth and migration. Interestingly, proliferative endothelial cells have higher expression of eNOS mRNA via actin cytoskeletal regulation of mRNA stability (29, 30), forming the second feed-forward cycle.
In summary, the present study characterized a signaling mechanism whereby netrin-1 stimulates angiogenesis of mature aortic endothelial cells. This pathway may prove pharmacologically promising for therapeutic angiogenesis for patients with ischemic coronary artery disease. It may also represent a common pathway for cardiovascular, neuronal, and cancer physiology that would be important for better understanding of the functional signal transduction at given organ system in the context of general physiology.
Experimental Procedures
Materials.
Purified mouse netrin-1 was purchased from R & D Systems. Polyclonal antibody for DCC was obtained from EMD Calbiochem. Polyclonal antibodies specific for phosphorylated ERK1/2, eNOSs1179, eNOSt497, and native MEK1/2, as well as siRNAs specific for MEK1/2 were obtained from Cell Signaling Technology. DCC siRNAs were purchased from Qiagen. Polyclonal antibodies recognizing phospho-eNOSs116 was obtained from Upstate Biotechnology. Oligofectamine and Opti-MEM for siRNA transfection of endothelial cells were purchased from Invitrogen. Other chemicals were purchased from Sigma in the highest purity.
Endothelial Cell Culture.
Bovine aortic endothelial cells (Cell Systems) were cultured in Media 199 (Invitrogen) containing 10% FCS (reserved lot no. for entire study, Sigma) as described (31).
Wound Proliferation Assay.
Proliferating endothelial cells at 95% confluence were gently scratched by using a 1,000-μl pipette tip to make a 1-mm wound, and then immediately exposed to media containing pharmacological inhibitors and/or, after 30 min, netrin-1. Rate of endothelial cell proliferation and migration, reflected as the speed of wound closure, was captured by using an inverted microscope and a Nikon digital camera 36 h later.
Aortic Endothelial Outgrowth Assay.
Endothelial cell outgrowth from isolated aortic discs were analyzed as described by Berger et al. (11). In brief, six-well cell culture plates were coated with thrombin (0.1 unit/μl). Fresh aortas were isolated from adult C57BL6 mice (The Jackson Laboratory), cleaned of adventitial tissue, and cut into discs of 1-mm o.d. The aortic discs were placed into the six-well plates, and then covered with media containing fibrinogen. After incubation at 37°C for 1 h, a thin layer of fibrin clot has formed. Wells were then supplemented with growth media containing 20% FCS. Endothelial cells started to grow out of aortic discs by day 1.5. Three to five days later, a capillary-like endothelial outgrowth network forms around the aortic disk. Usage of aortas from C57BL6 mice was authorized by the University of Chicago Institutional Animal Care and Use Committee.
Transfection of Endothelial Cells with siRNAs and Western Blot Analysis.
Proliferating endothelial cells were transfected with control siRNA (25 nmol/liter), MEK1/2 siRNAs (25 nmol/liter each, combined) or DCC siRNA (25 nmol/liter) using Oligofectamine for 4 h in Opti-MEM before addition of growth media containing 10% FBS. Forty-eight hours later, cells were either harvested for Western blot analysis of endothelial MEK1/2 expression, or exposed to netrin-1 for subsequent analysis of NO• production, ERK1/2 phosphorylation or wound proliferation assay. Western blot analysis of ERK1/2 or eNOS phosphorylation was performed as described (14).
Electron Spin Resonance Detection of Nitric Oxide Radical.
Bioavailable nitric oxide radical (NO•) in endothelial cells was detected by using electron spin resonance (ESR) as described (31, 32). In brief, endothelial cells were rinsed with modified Krebs/Hepes buffer and incubated with freshly prepared NO•-specific spin trap Fe2+(DETC)2 colloid (0.5 mmol/liter) for 60 min. Gently collected cell suspensions were snap-frozen in liquid nitrogen and loaded into a finger Dewar for analysis with a Miniscope 200 ESR spectrophotometer (Magnettech, Berlin) at the following settings: Bio-field, 3267; field sweep, 100 G; microwave frequency, 9.78 GHz; microwave power, 40 mW; modulation amplitude, 10 G; 4096 points resolution and receiver gain, 900.
Statistical Analysis.
All data are presented as mean ± SEM from four to six independent experiments (different passage of cells used on different days). ANOVA was used to compare means of different experimental groups. Statistical significance is set as P < 0.05.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-077440 and HL-081571, American Heart Association Grant 0435189N, American Diabetes Association Award 7-04-RA-16, and a Career Development Award from the Schweppe Foundation (all to H.C.).
Abbreviations
- DCC
deleted in colorectal cancer
- PTIO
2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Serafini T., Kennedy T. E., Galko M. J., Mirzayan C., Jessell T. M., Tessier-Lavigne M. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy T. E., Serafini T., de la Torre J. R., Tessier-Lavigne M. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 3.Meyerhardt J. A., Caca K., Eckstrand B. C., Hu G., Lengauer C., Banavali S., Look A. T., Fearon E. R. Cell Growth Differ. 1999;10:35–42. [PubMed] [Google Scholar]
- 4.Stein E., Zou Y., Poo M., Tessier-Lavigne M. Science. 2001;291:1976–1982. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- 5.Forcet C., Stein E., Pays L., Corset V., Llambi F., Tessier-Lavigne M., Mehlen P. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P., Tessier-Lavigne M. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- 7.Park K. W., Crouse D., Lee M., Karnik S. K., Sorensen L. K., Murphy K. J., Kuo C. J., Li D. Y. Proc. Natl. Acad. Sci. USA. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X., Le Noble F., Yuan L., Jiang Q., De Lafarge B., Sugiyama D., Breant C., Claes F., De Smet F., Thomas J. L., et al. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 9.Ruel M., Song J., Sellke F. W. Mol. Cell Biochem. 2004;264:119–131. doi: 10.1023/b:mcbi.0000044381.01098.03. [DOI] [PubMed] [Google Scholar]
- 10.Fasol R., Schumacher B., Schlaudraff K., Hauenstein K. H., Seitelberger R. J. Thorac. Cardiovasc. Surg. 1994;107:1432–1439. [PubMed] [Google Scholar]
- 11.Berger A. C., Wang X. Q., Zalatoris A., Cenna J., Watson J. C. Microvasc. Res. 2004;68:179–187. doi: 10.1016/j.mvr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Cai H. Cardiovasc. Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., Tessier-Lavigne M. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- 14.Cai H., Li Z., Davis M. E., Kanner W., Harrison D. G., Dudley S. C., Jr. Mol. Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 15.Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. J. Biol. Chem. 2003;278:14841–14849. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 16.Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. Circ. Res. 2001;88:E68–E75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 17.Papapetropoulos A., Garcia-Cardena G., Madri J. A., Sessa W. C. J. Clin. Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellke F. W., Wang S. Y., Stamler A., Lopez J. J., Li J., Simons M. Am. J. Physiol. 1996;271:H713–H720. doi: 10.1152/ajpheart.1996.271.2.H713. [DOI] [PubMed] [Google Scholar]
- 19.Brouet A., Sonveaux P., Dessy C., Moniotte S., Balligand J. L., Feron O. Circ. Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 20.Sbaa E., Frerart F., Feron O. Trends Cardiovasc. Med. 2005;15:157–162. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Perera O., Perez-Sala D., Navarro-Antolin J., Sanchez-Pascuala R., Hernandez G., Diaz C., Lamas S. J. Clin. Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbich C., Reissner A., Chavakis E., Dernbach E., Haendeler J., Fleming I., Zeiher A. M., Kaszkin M., Dimmeler S. FASEB J. 2002;16:706–708. doi: 10.1096/fj.01-0637fje. [DOI] [PubMed] [Google Scholar]
- 23.Greif D. M., Kou R., Michel T. Biochemistry. 2002;41:15845–15853. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 24.Barallobre M. J., Pascual M., Del Rio J. A., Soriano E. Brain Res. Brain Res. Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Chen D. B., Bird I. M., Zheng J., Magness R. R. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 26.Sonveaux P., Martinive P., DeWever J., Batova Z., Daneau G., Pelat M., Ghisdal P., Gregoire V., Dessy C., Balligand J. L., Feron O. Circ. Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 27.Ridnour L. A., Isenberg J. S., Espey M. G., Thomas D. D., Roberts D. D., Wink D. A. Proc. Natl. Acad. Sci. USA. 2005;102:13147–13152. doi: 10.1073/pnas.0502979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas D. D., Espey M. G., Ridnour L. A., Hofseth L. J., Mancardi D., Harris C. C., Wink D. A. Proc. Natl. Acad. Sci. USA. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnal J. F., Yamin J., Dockery S., Harrison D. G. Am. J. Physiol. 1994;267:C1381–C1388. doi: 10.1152/ajpcell.1994.267.5.C1381. [DOI] [PubMed] [Google Scholar]
- 30.Searles C. D., Ide L., Davis M. E., Cai H., Weber M. Circ. Res. 2004;95:488–495. doi: 10.1161/01.RES.0000138953.21377.80. [DOI] [PubMed] [Google Scholar]
- 31.Chalupsky K., Cai H. Proc. Natl. Acad. Sci. USA. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai H., Li Z., Dikalov S., Holland S. M., Hwang J., Jo H., Dudley S. C., Jr., Harrison D. G. J. Biol. Chem. 2002;277:48311–48317. doi: 10.1074/jbc.M208884200. [DOI] [PubMed] [Google Scholar]