Abstract
Rising levels of atmospheric CO2 are thought to increase C sinks in terrestrial ecosystems. The potential of these sinks to mitigate CO2 emissions, however, may be constrained by nutrients. By using metaanalysis, we found that elevated CO2 only causes accumulation of soil C when N is added at rates well above typical atmospheric N inputs. Similarly, elevated CO2 only enhances N2 fixation, the major natural process providing soil N input, when other nutrients (e.g., phosphorus, molybdenum, and potassium) are added. Hence, soil C sequestration under elevated CO2 is constrained both directly by N availability and indirectly by nutrients needed to support N2 fixation.
Keywords: global climate change, N2 fixation, soil organic matter
Numerous studies have reported a surge in plant growth after an abrupt rise in atmospheric CO2 (1, 2). If increased C assimilation by plants is translated into increased soil organic C content, terrestrial ecosystems might help to mitigate rising anthropogenic CO2 emissions (3). Simulation models project a wide range of responses of soil C sinks to elevated CO2. Some models suggest that low nutrient availability will preclude soil C storage (4, 5), whereas others maintain that soil C can accumulate even when nutrient supplies are low (6). Nitrogen fixation, the main source of natural N input in terrestrial ecosystems (7), has been invoked as a process that can potentially diminish N limitation in nutrient-poor systems. Elevated CO2 has been found to increase N2 fixation (8), which could provide additional N to support C accumulation in soil. However, N2 fixation by plants can be limited by the availability of other nutrients such as molybdenum, phosphorus, and potassium (9).
Until now, empirical evidence to evaluate the effect of nutrient availability on soil C storage under elevated CO2 has been lacking. The effects of nutrient availability and elevated CO2 are difficult to discern in individual experiments because of high spatial variability in soil C and nutrients and the large amount of C in the soil relative to input rates (10, 11). A quantitative integration of results across multiple studies can overcome some of these problems.
In the current study, we summarized the effect of atmospheric CO2 enrichment on soil C by performing a metaanalysis on 80 observations from 41 published and unpublished studies. We also summarized the effect of elevated CO2 on standing root biomass for these studies by using corresponding data for 56 observations on soil C. We divided the studies into three categories of N availability based on N fertilization rates: (i) up to 30 kg·ha−1·yr−1 of N, comparable to maximum atmospheric N depositions in the United States and most of the European Union (12), (ii) between 30 and 150 kg·ha−1·yr−1 of N, typical of extensive agriculture in the United States (13), and (iii) >150 kg·ha−1·yr−1 of N, typical for intensive agriculture in the European Union (13). Similarly, we also evaluated the potential of N2 fixation to supply extra soil N input under increased CO2 concentrations by using 92 observations in 25 published and unpublished studies. We compared studies that received additional non-N nutrients and studies that did not. The databases for soil C, root biomass, N2 fixation, and the results of the metaanalyses can be found in Data Sets 1–7, which are published as supporting information on the PNAS web site.
Results and Discussion
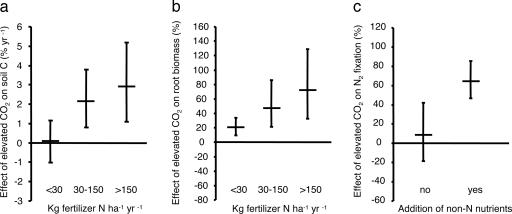
Elevated CO2 had no effect on soil C in ecosystems receiving up to 30 kg·ha−1·yr−1 of N (Fig. 1a). Soil C accumulation became apparent with increasing inputs of N. Elevated CO2 increased soil C by 2.1% per year at N additions between 30 and 150 kg·ha−1·yr−1 and by 2.9% per year at N additions >150 kg·ha−1·yr−1. Whether under natural or planted vegetation, in intact or disturbed soils, or with woody or herbaceous species, elevated CO2 only increased soil C at N additions ≥30 kg·ha−1·yr−1 (Data Set 4). These results, spanning an array of experimental conditions and terrestrial ecosystems, provide powerful evidence that additional N is needed if C is to be stored in soil under elevated CO2.
Fig. 1.
The effect of elevated CO2 on soil C contents, root biomass, and N2 fixation. (a) Change in soil C contents as affected by N fertilization. There is a significant difference between N fertilization classes (P = 0.02). The values for <30, 30–150, and >150 kg·ha−1·yr−1 of N are based on 43, 25, and 12 observations, respectively. (b) Change in root biomass as affected by N fertilization. There is a significant difference between N fertilization classes (P = 0.03). The values for <30, 30–150, and >150 kg·ha−1·yr−1 of N are based on 29, 17, and 10 observations, respectively. (c) Change in N2 fixation as affected by nutrient additions. There is a significant difference between studies that received additional non-N nutrients (43 observations) and studies that did not (49 observations) (P = 0.02). All observations are weighted by experiment duration and number of replicates. All error bars represent 95% confidence intervals.
Why is the soil C response to elevated CO2 restricted by N availability? Because an increase in plant growth under elevated CO2 causes N to accumulate in litter and plant biomass, soil N availability is expected to limit plant growth under elevated CO2 in the long term without the addition of exogenous N (14). By contrast, N fertilization can sustain increases in plant growth and, thus, soil C input under elevated CO2 (15, 16). Indeed, soil N availability limited plant growth under elevated CO2 for the studies contributing to our soil C database; the response of root biomass to elevated CO2 increased with N additions (Fig. 1b). Because new C enters mineral soil mainly through the root system, these results suggest that the effect of N availability on soil C responses to elevated CO2 were caused by differences in soil C input.
When ecosystems under elevated CO2 are subject to N stress, soil microbes might mobilize N through increased decomposition of native soil organic matter (17–19). This so-called priming effect could explain why even in unfertilized experiments, elevated CO2 significantly increases plant biomass (Fig. 1b). Yet, as priming simultaneously increases soil N availability but reduces the soil C reservoir, its potential to accommodate soil C storage is probably small. In fact, the increase in root biomass under elevated CO2 did not lead to C storage in receiving <30 kg·ha−1·yr−1 of N studies (Fig. 1a). Thus, although N limitation alone does not always preclude positive plant growth responses to elevated CO2, the interaction between the C and N cycles in terrestrial ecosystems rapidly constrains the CO2 effect on soil C contents.
Elevated CO2 also can promote plant N uptake by growing fine roots and mycorrhizae (20–22). However, the potential for such redistributions of N to accommodate C sequestration is small because the resulting rise in plant growth will further increase readily available C and, therefore, microbial N demand (23). Hence, mechanisms that increase plant N uptake without a net ecosystem gain of N are likely to be constrained by ecological stoichiometry (24) and, therefore, self-limiting (25). Thus, the potential for C storage under elevated CO2 is highest for ecosystems where plant growth is not limited by N availability. In all other cases, other sources of N are needed to support plant growth and sequestration of soil C.
Nitrogen fixation has been suggested to be one of the other N sources (8). However, our metaanalysis shows that elevated CO2 had no effect on N2 fixation under conditions representative of most of Earth's terrestrial ecosystems: With no fertilizer additions, with intact soils, and with naturally occurring plant communities, the response of N2 fixation to elevated CO2 was indistinguishable from zero (Data Set 6).
Across the entire data set, elevated CO2 increased N2 fixation when other nutrients also were added (Fig. 1c). These results suggest that stimulation of N2 fixation by elevated CO2 is constrained by the availability of nutrients other than N. In all but one case, non-N nutrient additions included both phosphorus and potassium, which are essential for N2 fixation (9). Because non-N nutrients were always added in combination (Data Set 3), we cannot test which elements were especially important in releasing N2 fixation from nutrient limitation. Previous studies have suggested that CO2 stimulation of N2 fixation is restricted by the availability of phosphorus (26, 27) and molybdenum (28). Our findings imply that such nutrient constraints are a general feature of N2 fixation responses to elevated CO2.
We found no evidence that N fertilization suppressed the response of N2 fixation to elevated CO2 (Data Set 6), even though the addition of N fertilizer frequently depresses N2 fixation (29). Because N addition often occurred in combination with additions of other elements (32 of 45 observations), this result may partly reflect the positive effects of additions of other nutrients overwhelming the negative effects of added N. However, in the subset of observations where we could assess the influence of added N in isolation (in the absence of other nutrient supplements), N fertilization had no effect on the response of N2 fixation to elevated CO2 (Data Set 6). Thus, the absence of a response of N2 fixation to elevated CO2 without additions of other nutrients was not an artifact caused by N additions.
Elevated CO2 also increased N2 fixation in experiments with disturbed soils, an effect comparable in magnitude with that observed by adding non-N nutrients (Data Set 6). Soil disturbance likely operates through its direct effect on nutrient availability, because it generally decreases soil organic matter contents and liberates nutrients (30). Planted communities showed a significantly stronger CO2 response for N2 fixation than natural ecosystems. Yet, all studies on natural communities were performed on intact soils, and none of them received non-N nutrient supplements. The CO2 response between natural and planted communities did not differ on intact and unfertilized soil (Data Set 6). Thus, planting per se did not affect the CO2 response of N2 fixation. Together, these results suggest that strong responses of N2 fixation to elevated CO2 depend on the availability of non-N nutrients.
The effect of CO2 on N2 fixation decreased with experiment duration (Data Set 6; P = 0.001). One possible mechanism for this decline is identical to that reducing soil N availability in response to elevated CO2: After an increase in plant growth, micronutrients required for N2 fixation accumulate in litter and plant biomass (31). The resulting decrease in non-N-nutrient availability limits the response of N2 fixation to elevated CO2 or, in some cases, even turns a stimulation into a suppression (28). In addition, light limitation, often absent in short-term experiments, is likely to become more pronounced over time under elevated CO2 (32). Together, the dependency of N2 fixation on supporting nutrients, the lack of a response in natural ecosystems, and the decreasing CO2 response over time strongly imply that the role of N2 fixation in providing additional N needed for C storage under elevated CO2 will be small.
Conclusions
Results presented here show that nutrient limitations of plant growth under elevated CO2 extend to soil C accumulation and N2 fixation. Our analysis thus provides empirical corroboration for the largely untested hypothesis that large C accumulations only occur with increased inputs or reduced losses of N (4, 5, 24) and broadens it to non-N nutrients. Together, these conceptual, theoretical, and empirical approaches suggest a limited potential for rapid C storage in the terrestrial biosphere after an increase in atmospheric CO2.
Methods
Data Collection.
We extracted results for soil C contents, root biomass, and N2 fixation from atmospheric CO2 enrichment studies conducted in the field, in growth chambers, or in glass houses (Data Sets 1–3). We included observations of the effect of elevated CO2 that met several criteria. First, the duration of the experimental CO2 treatment had to be at least 100 days (the approximate length of a growing season in the temperate zone). Second, means and sample sizes had to be available for ambient CO2 treatments (between 300–400 ppmV) and elevated CO2 treatments (450–800 ppmV). Estimates of variance were tabulated when available but were not required for inclusion in the analysis. Third, details of experimental conditions needed to be specified. We only included studies that reported experiment duration, soil sampling depth, plant species, and the type of experimental facility. Studies also needed to indicate N fertilization rates. Most studies applied N additions directly to the soil rather than the canopy. This method of N fertilization is bypassing the possibility of foliar N uptake. Consequently, the lower threshold for N effects on soil C storage and root biomass in our metaanalysis is merely an approximation of atmospheric deposition rates.
Finally, studies needed to indicate whether they involved experiments in pots (i.e., any container with dimensions <1 m) or in ecosystems. We made a distinction between studies on intact and disturbed soils. The latter category included all pot studies, studies on reconstructed soils, and studies that applied tillage during CO2 enrichment.
Because we examined how effects of elevated CO2 varied with experimental conditions, we included separate observations of elevated CO2 effects from a single ecosystem under different experimental treatments (e.g., in multifactorial studies). When studies involved more than one level of CO2 enrichment, we only included results at the level that is approximately twice the ambient CO2 concentration.
To increase sensitivity for detecting small effects on soil C, we included only surface soil samples, with a maximum depth of 30 cm. When studies reported soil C contents for multiple depths, we included results that best represented the 0- to 10-cm soil layer. Our analysis focuses on mineral soils; thus, we excluded measurements on forest litter layers, marshes, and bogs. Because soil C accumulates slowly over time and the effects of CO2 enrichment on soil C are often difficult to discern, we only included measurements after the longest exposure period for each study. Soil C contents were all included in the database as a weight percentage. We converted results reported on an area basis to a weight basis by using soil density data whenever available. When soil density data were not reported, we converted results assuming soil bulk density of 1 g·cm−3 in ambient CO2 and elevated CO2 treatments. We included root biomass data for the whole sampled soil depth. When root biomass data were reported for multiple years, we included data only from the year closest to the year of the corresponding soil C observation.
All forms of biological N2 fixation (i.e., free-living and symbiotic bacteria and cyanobacteria) were included. N2 fixation was determined by acetylene reduction, 15N dilution, or N contents of plant tissue when atmospheric N2 was the only available N source. We explicitly examined the temporal dependence of the N2 fixation response for multiyear studies by using one estimate per treatment combination per year. Although such measurements are not independent, this approach makes it possible to test whether the responses of N2 fixation change through time. Eliminating nonindependence by restricting the data set to one estimate per ecosystem-treatment combination did not substantially alter the results (Data Set 7). Although this analysis did reveal a stronger response of N2 fixation in woody compared with herbaceous vegetation, experiments using woody vegetation were dominated by the use of disturbed soils and the addition of nonnitrogenous fertilizers (19 of 24 observations). In the absence of soil disturbance and non-N nutrient supplements, N2 fixation did not respond to elevated CO2 in either woody or herbaceous plants (Data Set 7).
Metaanalysis.
Data Sets 1–3 were evaluated by using metaanalysis (33). We used the natural log of the response ratio (r = response at elevated CO2/response at ambient CO2) as a metric for the response of N2 fixation and root biomass to elevated CO2. These results are reported as the percentage change under elevated CO2 ((r − 1) × 100). The accumulation of soil C in response to an increase in C input follows a logarithmic pattern over time, yet, in the first 5–10 years, accumulation rates will be approximately linear (34). Because the average duration of CO2 exposure in the metaanalysis was 3.6 years, we assumed linear changes in soil C over time. Accordingly, we used the natural log of the time-adjusted response ratio rt = ((r − 1)/y) + 1 as a response metric, with y as the length of the study in years. We assumed that most of the soil C input occurs during the growing season. Thus, in order to prevent overestimation of annual changes in the soil C pool in short-term studies (<1 year), we used a minimum of y = 1. Results are reported as the percentage change per year under elevated CO2 ((rt − 1) × 100).
Well replicated and long-term studies provide more reliable estimates of ecosystem CO2 responses (10). Thus, we weighted the response metrics by replication and experimental duration by using the function FC = (na × ne)/(na + ne) + (y × y)/(y + y), with na and ne as the number of replicates under ambient and elevated CO2. Using other weighting functions, such as weighting solely by the number of replicates (35) or weighting all studies equally, did not affect the outcome of our analysis (Data Sets 4–7).
The weighting function conventionally used in metaanalyses, i.e., weighting by the inverse of the pooled variance (by using the largest observed variance for missing estimates) yielded similar results. However, this function calculated weights that differed up to three orders of magnitude in size (Data Sets 1–3). By assigning extreme importance to individual observations, average effect sizes were largely determined by a small number of studies. Thus, we favor the alternative weighting functions because they assigned less extreme weights.
We used a mixed model for analysis of all three data sets, based on the assumption that random variation in CO2 responses occurred between studies (14) and bootstrapping (4,999 iterations) to calculate 95% confidence intervals around mean effect sizes for categories of studies. P values for differences between categories of studies and for correlation with experiment duration were calculated by using resampling techniques incorporated in metawin 2.1 (36).
Supplementary Material
Acknowledgments
We thank J. B. West, M. Hoosbeek, S. A. Prior, F. Hagedorn, P. Ambus, P. A. Niklaus, K. Hofmockel, W. Holmes, B. Thomas, F. A. Dijkstra, J. Lichter, R. J. Norby, and W. H. Schlesinger for sharing their data; E. Ainsworth and C. Osenberg for their advice on the statistical procedures; and A. J. Bloom for useful comments on an earlier version of the manuscript. Financial support for this study was provided by the National Science Foundation and the Dutch Ministry of Agriculture, Nature Management, and Fisheries.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Schimel D. S. Glob. Change Biol. 1995;1:77–91. [Google Scholar]
- 2.Mooney H. A., Drake B. G., Luxmoore R. J., Oechel W. C., Pitelka L. F. Bioscience. 1991;41:96–104. [Google Scholar]
- 3.Gifford R. M. Aust. J. Plant Physiol. 1994;21:1–15. [Google Scholar]
- 4.Hungate B. A., Dukes J. S., Shaw R., Luo Y., Field C. B. Science. 2003;302:1512–1513. doi: 10.1126/science.1091390. [DOI] [PubMed] [Google Scholar]
- 5.Rastetter E. B., #x00C5;gren G. I., Shaver G. R. Ecol. Appl. 1997;7:444–460. [Google Scholar]
- 6.Cannell M. G. R., Thornley J. H. M. Glob. Change Biol. 1998;4:431–442. [Google Scholar]
- 7.Galloway J. N., Dentener F. J., Capone D. G., Boyer E. W., Howarth R. W., Seitzinger S. P., Asner G. P., Cleveland C. C., Green P. A., Holland E. A., et al. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- 8.Zanetti S., Hartwig U. A., Luscher A., Hebeisen T., Frehner M., Fischer B. U., Hendrey G. R., Blum H., Nosberber J. Plant Physiol. 1996;112:575–583. doi: 10.1104/pp.112.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitousek P. M., Cassman K., Cleveland C., Crews T., Field C. B., Grimm N. B., Howarth R. W., Marino R., Martinelli L., Rastetter E. B., et al. Biogeochemistry. 2002;57:1–45. [Google Scholar]
- 10.Hungate B. A., Jackson R. B., Field C. B., Chapin F. S., III Plant Soil. 1996;187:135–145. [Google Scholar]
- 11.Schlesinger W. H., Lichter J. Nature. 2001;411:466–469. doi: 10.1038/35078060. [DOI] [PubMed] [Google Scholar]
- 12.Holland E. A., Brasell B. H., Sulzman J., Lamarque J. F. Ecol. Appl. 2005;15:38–57. [Google Scholar]
- 13.FAO Statistical Yearbook. U.N., Rome: Food Agric. Org.; 2004. [Google Scholar]
- 14.Finzi A. C., Delucia E. H., Hamilton J. G., Richter D. D., Schlesinger W. H. Oecologia. 2002;132:567–578. doi: 10.1007/s00442-002-0996-3. [DOI] [PubMed] [Google Scholar]
- 15.Curtis P. S., Wang X. Z. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. [DOI] [PubMed] [Google Scholar]
- 16.Oren R., Ellsworth D. S, Johnsen K. H., Phillips N., Ewers B. E., Maier C., Schafer K. V. R., McCarthy H., Hendrey G., McNulty S. G., et al. Nature. 2001;411:469–472. doi: 10.1038/35078064. [DOI] [PubMed] [Google Scholar]
- 17.Lekkerkerk L. J.A., Van de Geijn S. C., Van Veen J. A. In: Soils and the Greenhouse Effect. Bouwman A., editor. New York: Wiley; 1990. pp. 423–429. [Google Scholar]
- 18.Zak D. R., Pregitzer K. S., Curtis P. S., Teeri J. A., Fogel R., Randlett D. L. Plant Soil. 1993;151:105–117. [Google Scholar]
- 19.Pendall E., Del Grosso S., King J. Y., LeCain D. R., Milchunas D. G., Morgan J. A., Mosier A. R., Ojima D. S., Parton W. A., Tans P. P., White J. W. C. Glob. Biogeochem. Cycles. 2003;17:GB1046. [Google Scholar]
- 20.Zak D. R., Pregitzer K. S., King J. S., Holmes W. E. New Phytol. 2000;147:201–222. [Google Scholar]
- 21.Pregitzer K. S., Zak D. R., Curtis P. S., Kubiske M. E., Teeri J. A., Vogel C. S. New Phytol. 129:579–585. doi: 10.1111/j.1469-8137.1995.tb04295.x. [DOI] [PubMed] [Google Scholar]
- 22.Klironomos J. N., Allen M. F., Rillig M. C., Piotrowski J., Makvandi-Nejad S., Wolfe B. E., Powell J. R. Nature. 2005;433:621–624. doi: 10.1038/nature03268. [DOI] [PubMed] [Google Scholar]
- 23.Diaz S., Grime J. P., Harris J., McPherson E. Nature. 1993;363:616–617. [Google Scholar]
- 24.Sterner R. W., Elser J. J. Ecological Stoichiometry. Princeton: Princeton Univ. Press; 2002. [Google Scholar]
- 25.Luo Y., Su B., Currie W. S., Dukes J. S., Finzi A., Hartwig U., Hungate B., McMurtrie R. E., Oren R., Parton W. J., et al. Bioscience. 2004;54:731–739. [Google Scholar]
- 26.Niklaus P. A., Leadly P. W., Stocklin J., Körner C. Oecologia. 1998;116:67–75. doi: 10.1007/s004420050564. [DOI] [PubMed] [Google Scholar]
- 27.Edwards E. J., McCaffrey S., Evans J. R. New Phytol. 2006;169:157–167. doi: 10.1111/j.1469-8137.2005.01568.x. [DOI] [PubMed] [Google Scholar]
- 28.Hungate B. A., Stiling P. D., Dijkstra P., Johnson D. W., Ketterer M. E., Hymus G. J., Hinkle C. R., Drake B. G. Science. 2004;304:1291. doi: 10.1126/science.1095549. [DOI] [PubMed] [Google Scholar]
- 29.Woodward F. I. New Phytol. 1992;122:239–251. doi: 10.1111/j.1469-8137.1992.tb04228.x. [DOI] [PubMed] [Google Scholar]
- 30.Haas H. J., Evans C. J., Miles E. F. U.S. Department of Agriculture Technical Bulletin. 1957;1164:1–111. [Google Scholar]
- 31.Johnson D. W., Hungate B. A., Dijkstra P., Hymus G., Hinkle C. R., Stiling P., Drake B. G. Ecol. Appl. 2003;13:1388–1399. [Google Scholar]
- 32.Grashoff C., Dijkstra P., Nonhebel S., Schapendonk A. H. C. M., van de Geijn S. C. Glob. Change Biol. 1995;1:417–428. [Google Scholar]
- 33.Hedges L. V., Olkin I. Statistical Methods for Metaanalysis. New York: Academic; 1985. [Google Scholar]
- 34.Schlesinger W. H. Nature. 1990;348:232–234. [Google Scholar]
- 35.Adams D. C., Gurevitch J., Rosenberg M. S. Ecology. 1997;78:1277–1283. [Google Scholar]
- 36.Rosenberg M. S., Adams D. C., Gurevitch J. Statistical Software for Meta-Analsysis. Sunderland, MA: Sinauer; 2000. metawin. Version 2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.