Abstract
IFN-γ-producing CD4+ T cells, although important for protection against acute Toxoplasma gondii infection, can cause gut pathology, which may prove to be detrimental for host survival. Here we show that mice lacking IL-15 gene develop a down-regulated IFN-γ-producing CD4+ T cell response against the parasite, which leads to a reduction in gut necrosis and increased level of survival against infection. Moreover, transfer of immune CD4+ T cells from WT to IL-15−/− mice reversed inhibition of gut pathology and caused mortality equivalent to levels of parental WT mice. Down-regulated CD4+ T cell response in the absence of IL-15, manifested as reduced antigen-specific proliferation, was due to defective priming of the T cell subset by dendritic cells (DCs) of these animals. When stimulated with antigen-pulsed DCs from WT mice, CD4+ T cells from IL-15−/− mice were primed optimally, and robust proliferation of these cells was observed. A defect in the DCs of knockout mice was further confirmed by their reduced ability to produce IL-12 upon stimulation with Toxoplasma lysate antigen. Addition of exogenous IL-15 to DC cultures from knockout mice led to increased IL-12 production by these cells and restored their ability to prime an optimal parasite-specific CD4+ T cell response. To our knowledge, this is the first demonstration of the role of IL-15 in the development of CD4+ T cell immunity against an intracellular pathogen. Furthermore, based on these observations, targeting of IL-15 should have a beneficial effect on individuals suffering from CD4+ T cell-mediated autoimmune diseases.
Keywords: CD4+ T cells, dendritic cells
Cytokines play an important role in protection against an obligate intracellular parasite Toxoplasma gondii (1). Among the cytokines, IFN-γ is central for protection against both acute (2) and chronic infection (3). Mice lacking IFN-γ gene are highly susceptible to T. gondii infection (4, 5), and treatment with anti-IFN-γ antibody can cause reactivation of latent disease (3). In addition to IFN-γ, other cytokines such as IL-12 (6, 7), IL-7 (8), and IL-15 (9, 10), by their ability to stimulate IFN-γ-producing natural killer (NK), CD4, and CD8+ T cells, also have been reported to be involved in host resistance. IFN-γ is primarily secreted during acute infection by NK and CD4+ T cells (11, 12), whereas over the long term, IFN-γ-producing CD8+ T cells serve as major effector cells responsible for keeping chronic infection in a dormant state (3, 13).
IL-12 is produced very early during T. gondii infection (14) and has been reported to play an important role in the induction of CD4+ T cell effectors (15, 16), which are an important source of IFN-γ production during the acute phase of infection (7). Mutant animals lacking IL-12 (17) or normal mice treated with anti IL-12 antibody (6) exhibit severe reduction in IFN-γ response against the parasite and are highly susceptible to infection. Similarly, studies from our laboratory have shown that exogenous IL-7 administration can enhance the IFN-γ-producing cytotoxic T lymphocyte levels in infected animals (8). The importance of another γ-chain cytokine, IL-15, in protective immune response against T. gondii has been demonstrated (18). IL-15 is a pleiotropic cytokine produced by both hematopoietic and nonhematopoietic cells (19). It has been reported to be important for the persistence of CD8+ T cell memory response against infectious diseases (20, 21) and tumor models (22). The cytokine is also considered to be a key regulator of NK cell development, homeostasis, and activity (23, 24). The role of IL-15 in the induction of long-term memory CD8+ T cell response against T. gondii has been demonstrated (18). However, its contribution in the induction of primary immune response during acute T. gondii infection has not been extensively studied. In a recent report with IL-15-deficient mice, Lieberman et al. (25) demonstrated that the lack of IL-15 gene does not affect the outcome of infection in mice challenged i.p. with T. gondii. In the present study, we used IL-15−/− mice to evaluate the importance of this cytokine in the survival and tissue inflammation of animals during per-oral (p.o.) challenge.
Results
IL-15−/− Mice Have Reduced Susceptibility to T. gondii Infection.
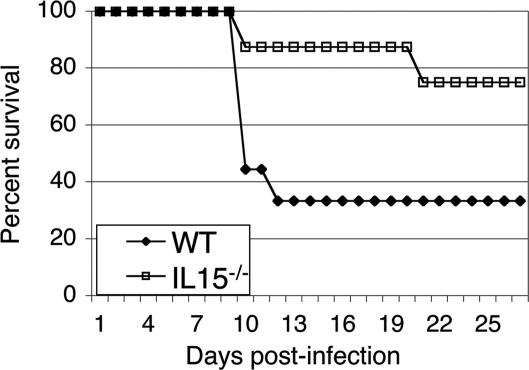
To determine the importance of IL-15 during acute T. gondii infection, knockout and WT mice were challenged orally with 25 cysts of T. gondii. Although 70% of the WT mice succumbed to infection by day 12 postinfection (p.i.) (Fig. 1), mortality in the knockout mice was not observed until day 19 p.i., and only two of eight mice had died when the experiment was terminated. Conversely, when the mice were infected i.p., no difference between WT and knockout mice was observed. Three of six mice from each group died between days 40 and 45 p.i., whereas the remaining animals from both groups survived until the termination of the experiment (data not shown).
Fig. 1.
Survival of IL-15−/− mice against T. gondii infection. IL-15−/− (n = 8) and WT (n = 9) mice were infected orally with 25 cysts of T. gondii. Survival was monitored on a daily basis. The study was performed twice. Data are represented as cumulative percentage of both experiments.
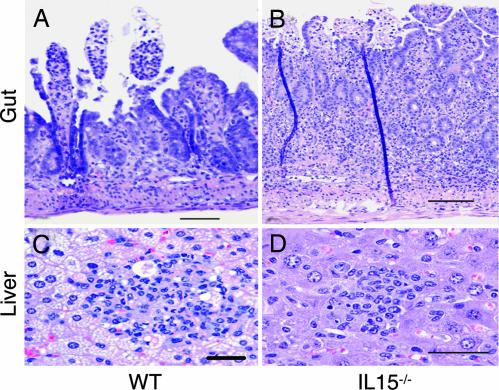
Histopathological analysis of p.o. infected mice was performed at day 10 p.i. Sections of small bowel and liver from infected parental mice showed the patchy epithelial necrosis and scattered hepatic inflammatory nodules (Fig. 2A and B) typical of the hyperimmune response to T. gondii infection (11, 26). IL-15−/− mice showed similar changes, with perhaps more inflammation than the controls, but the affected segments of bowel were minimal and most gut epithelia were intact (Fig. 2C). As expected, liver from WT mice showed extensive fatty changes in hepatocytes (Fig. 2B), whereas changes in IL-15−/− mice were less severe (Fig. 2D).
Fig. 2.
Photomicrographs of representative organs from T. gondii-infected IL-15−/− and WT mice. Mice were killed at day 10 p.i., and organs (liver and gut) were collected. Liver and gut sections were stained with hematoxylin/eosin. (A) WT gut. An example from a patchy segment of the small bowel mucosa shows superficial loss of epithelium and congestion of vessels. (Scale bar: 100 μm.) (B) WT liver. One of numerous parenchymal nodules of inflammatory cells including mononuclear and polymorphonuclear cells. Some necrotic cells are seen with significant fatty change in surrounding hepatocytes. (Scale bar: 50 μm.) (C) Knockout gut. Full thickness inflammation penetrates a small, scattered segment of the small bowel, with comparatively less necrosis and acute inflammation throughout the tissue. Dark vertical lines are artifactual folds of the tissue. (Scale bar: 100 μm.) (D) Knockout liver. Scattered parenchymal nodules are composed predominantly of mononuclear cells with some polymorphonuclear cells, with evidence of scattered hepatocyte necrosis and minimal fatty change. (Scale bar: 50 μm.)
Analysis of Parasite Load.
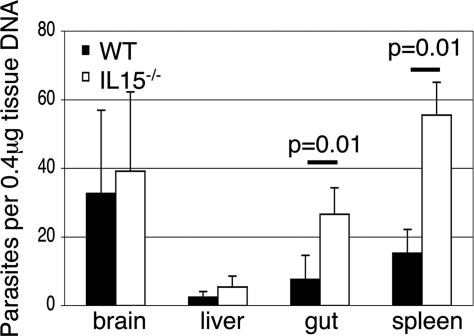
Differences in the parasite burden between the tissues (gut, liver, spleen, and brain) of WT and IL-15−/− mice were analyzed by quantitative PCR at day 10 p.i. Compared with parental WT animals, knockout mice had 3- to 4-fold higher parasite load in the gut and spleen (P = 0.01) (Fig. 3). These observations demonstrate that although knockout mice have elevated parasite load, they survive longer because of reduced inflammatory response to infection.
Fig. 3.
Number of parasites per 0.4 μg of tissue DNA in the tissues of IL-15−/− and parental WT mice. IL-15−/− and WT mice (n = 4) were infected with 25 cysts of T. gondii. At day 10 p.i., tissue DNA was isolated from brain, liver, gut, and spleen. The level of parasite load in the tissues was determined by quantitative PCR. Data are presented as mean ± SD and are representative of two separate experiments.
IFN-γ Response.
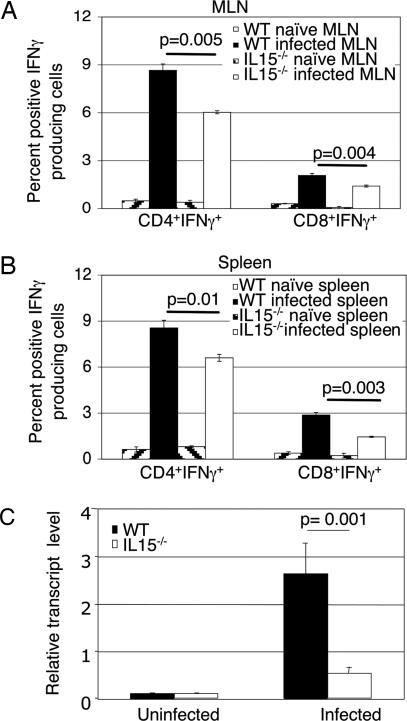
Because T. gondii-mediated inflammatory response primarily depends on IFN-γ-producing CD4+ T cells (11), we evaluated the T cell subset induced in response to infection. IL-15−/− and WT controls were killed at day 10 p.i. Purified CD4+ and CD8+ T cell populations from spleen and mesenteric lymph node (MLN) were analyzed for IFN-γ production by intracellular staining. The percentage of IFN-γ-producing CD4+ T cells in both MLN and spleen were significantly reduced (P = 0.005 and P = 0.01) in the knockout mice (Fig. 4A and B). Similarly, a reduced percentage of IFN-γ-positive CD8+ T cells was observed in the mutant animals. To confirm that reduced gut inflammatory response in the IL-15−/− mice was due to decreased IFN-γ production, the cytokine message in the gut was analyzed by real-time PCR. As shown in Fig. 4C, in comparison with WT animals, guts of knockout mice exhibited significantly lower IFN-γ message in response to infection (P = 0.001).
Fig. 4.
Analysis of IFN-γ-producing T cells from IL-15−/− mice infected with T. gondii. Mice were infected p.o. with T. gondii cysts as mentioned above. MLN (A) and splenocytes (B) were harvested at day 10 p.i., pooled (n = 3), and stimulated in vitro for 4 h. Cells were labeled for CD4+ or CD8+ before intracellular staining for IFN-γ. Data are presented as percentage (mean ± SD) of CD4+ or CD8+ T cells positive for IFN-γ and are pooled from two different experiments. (C) Total gut RNA was isolated from WT and IL-15−/− mice (n = 4) at day 7 p.i. and analyzed for IFN-γ mRNA expression, normalized to β-actin mRNA levels. Relative expression was measured by using the mean from each group and the formula RTL (relative transcript level) = 2−ΔCt × 1000. Statistical bars represent the mean ± SD.
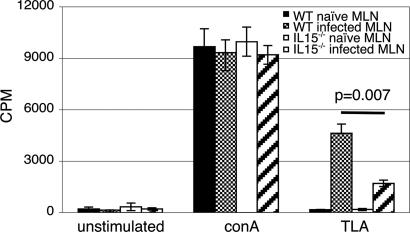
To further establish that knockout mice were unable to induce an optimal CD4+ T cell response, lympho-proliferation assays were performed. At day 10 p.i., purified CD4+ T cells from MLN were stimulated with Toxoplasma lysate antigen (TLA) and proliferation measured. As compared with those of WT mice, CD4+ T cells from knockout animals exhibited significantly less proliferative response to TLA stimulation (P = 0.007) (Fig. 5). No differences in the proliferation between the knockout and WT CD4+ T cells in response to Con A stimulation was observed (Fig. 5). Reduced proliferation of CD4+ T cells from knockout mice could be due to diminished activation of these cells. CD4+ T cells from MLN of both WT and knockout mice were evaluated for activation markers (CD44hi and CD62lo) (27), at day 10 p.i. by FACS analysis. T. gondii infection led to a 3-fold increase in activated CD4+ T cells in the WT mice. Conversely no significant increase in CD4+CD44hiCD62lo population in response to Toxoplasma infection was observed in IL-15−/− mice (data not shown).
Fig. 5.
Antigen-specific proliferation and activation of CD4+ T cells of IL-15−/− mice. Mice (n = 3 per group) were infected orally with 25 cysts, and animals were killed at day 10 p.i. MLNs from each group were harvested and pooled, and CD4+ T cells were isolated by affinity purification. Purified CD4+ T cells (1 × 105 cells per well) were cultured with 1 × 105 irradiated feeder cells and stimulated either with 15 μg/ml TLA or 2 μg/ml Con A. After 72-h incubation, lympho-proliferation was assayed by [3H]thymidine incorporation. The experiment was performed twice with similar results, and the data are representative of one experiment.
Adoptive Transfer of CD4+ T Cells.
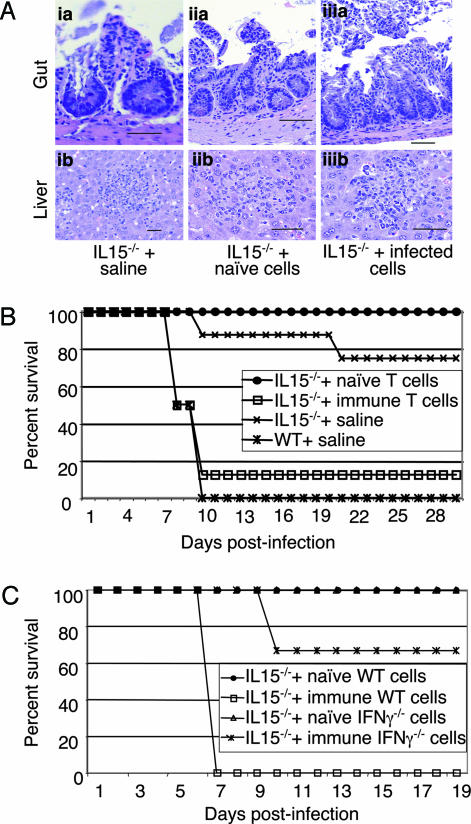
To establish that down-regulated inflammatory response and increased survival of IL-15−/− mice was due to poor CD4+ T cell response, adoptive transfer studies were performed. Purified CD4+ T cells (1 × 107) from infected or naïve WT mice were transferred to knockout animals. Twenty-four hours later the recipients were challenged with 25 cysts of T. gondii. Histopathological analysis on the tissues (liver and gut) of recipient animals was performed at day 10 p.i. Tissues from IL-15 knockout mice infused with CD4+ T cells from naïve WT mice (Fig. 6Aiia and Aiib) resemble the infected controls treated with saline (Fig. 6 Aia and Aib), and both of these animals had mild pathology due to infection. However, when infused with CD4+ T cells from infected WT mice, the knockout animals had much more extensive superficial necrosis of gut epithelia (Fig. 6Aiiia) similar to that observed in the WT controls (Fig. 2A). As expected, WT mice treated with naïve or immune CD4+ T cells developed gut pathology in response to T. gondii infection, similar to the levels of control WT mice injected with saline (data not shown).
Fig. 6.
Adoptive transfer of CD4+ T cells from WT C57BL/6 to IL-15−/− mice. Infected and uninfected mice (10 mice per group) were killed at day 10 p.i., and MLNs were harvested and pooled. CD4+ T cells from the MLN were isolated by affinity purification. Purified CD4+ T cells (1 × 107) isolated from naïve or infected mice were transferred i.v. to IL-15−/− mice (six mice per group). The control mice (nontransferred knockout and WT) were injected with an equal volume of saline. WT mice injected with immune CD4+ T cells were also included as controls. Twenty-four hours posttransfer the recipients were challenged with 25 cysts of T. gondii. At day 10 postchallenge, two of six mice were killed, and histopathological analysis on the tissues (gut and liver) was performed (A). (Aia and Aib) Knockout mice plus saline. (Aia) Gut: An example of one of a few areas of superficial loss of epithelium, also showing a mild increase of inflammatory cells in the lamina propria. (Scale bar: 50 μm.) (Aib) Liver: Scattered parenchymal nodules of mononuclear and polymorphonuclear inflammatory cells are set among hepatic cells with mild fatty change. (Aiia and Aiib) Knockout mice plus naïve CD4+ T cells. (Aaii) Gut: An area of superficial necrosis of epithelium with minimal inflammation. (Scale bar: 100 μm.) (Abii) Liver: An example of one of many nodules made up of mixed inflammatory cells scattered throughout the parenchyma of the liver. (Scale bar: 50 μm.) (Aaiii and Abiii) Knockout mice plus immune CD4+ T cells. (Aaiii) Gut: An example of a segment of small bowel with extensive superficial necrosis with moderate inflammation in the lamina propria. (Scale bar: 100 μm.) (Abiii) Liver: One of many scattered nodules of mixed inflammatory cells with hepatocyte necrosis and minimal fatty change. (B) The remaining four of six mice were monitored for survival on daily basis. Data are pooled from two experiments. (C) Purified CD4+ T cells isolated from infected or uninfected IFN-γ−/− mice were injected to naïve IL-15−/− mice (1 × 107 cells per recipient). Animals (six mice per group) were challenged orally with 25 cysts and monitored for survival. Experiments were performed twice with similar results, and the data are representative of one experiment.
To determine the effect of adoptive transfer on the outcome of infection, recipient animals were monitored for survival. As expected, control WT mice treated with saline exhibited early mortality (days 8–10 p.i), and all of the animals were dead by day 10 p.i. (Fig. 6B). Similarly, as observed earlier, nominal death in the saline-treated knockout mice was observed much later than the WT mice, starting at day 20 p.i. Interestingly, transfer of immune CD4+ T cells from WT mice to IL-15−/− recipients led to their mortality, and >80% recipients died at the same time as WT infected controls. Conversely, transfer of naïve CD4+ T cells from WT to IL-15−/− mice did not increase their mortality to T. gondii infection (Fig. 6B). This group did not even exhibit nominal death observed in saline-treated knockout animals. To determine that inflammatory response in the knockout recipients depended on IFN-γ production of donor CD4+ T cells, adoptive transfer with CD4+ T cells from IFN-γ−/− mice was performed. Unlike the recipients treated with immune CD4+ T cells from WT mice, the majority of knockout mice treated with CD4+ T cells from IFN-γ −/− animals survived the infection, and only two of six mice died until the termination of experiment (Fig. 6C).
Primary CD4+ T Cell Response.
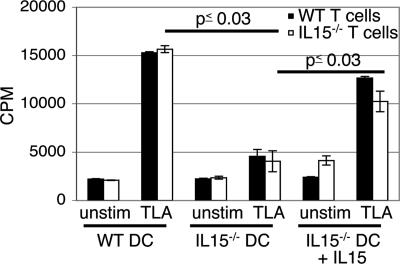
Next, we determined whether down-regulated CD4+ T cell immunity in IL-15−/− mice was due to inadequate priming of this T cell subset. Because dendritic cells (DCs) are known to play a key role in the initiation of immune response (28, 29), we evaluated CD4+ T cells priming against TLA by DCs from IL-15−/− and parental WT mice. DCs from MLN of naïve WT or knockout animals were isolated and pulsed with TLA in an overnight culture. On the following day, purified CD4+ T cells were added, and the proliferative response was subsequently measured. As expected, DCs from the WT mice induced an optimal proliferation of CD4+ T cells from the same strain of mice (Fig. 7). Conversely, CD4+ T cells from IL-15−/− mice showed significantly lower (>3-fold less) proliferation (P = 0.001) when primed with DCs from the same mice. However, when stimulated with antigen-primed DCs of WT mice, CD4+ T cells from knockout animals exhibited an optimal proliferative response, which was almost equivalent to the CD4+ T cells from parental WT mice (Fig. 7). CD4+ T cells from both WT and IL-15−/− mice showed significantly lower proliferation (P = 0.001 and P = 0.03, respectively) when primed with DCs from knockout animals. However, addition of IL-15 to the cultures restored the ability of IL-15−/− DCs to prime CD4+ T cells from both knockout and WT mice (P = 0.001 and P = 0.019, respectively) (Fig. 7).
Fig. 7.
Antigen-specific CD4+ T cell priming of IL-15-deficient mice. DCs and CD4+ T cells from MLN of IL-15−/− and WT mice (six to eight animals per group) were isolated. After overnight incubation of DCs with 20 μg/ml TLA, CD4+ T cells were added. Seventy-two hours later, proliferation was determined by [3H]thymidine incorporation assay. The experiment was performed twice with similar results, and the data are representative of one experiment.
IL-12 Production by DCs.
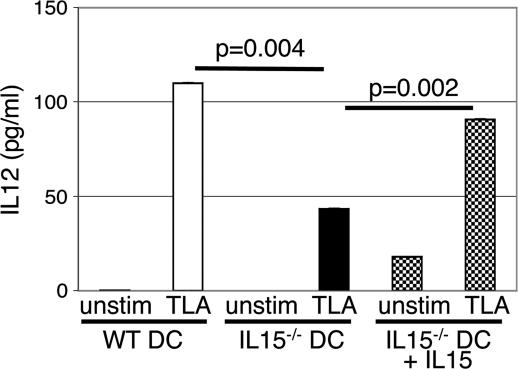
The above observations suggest that down-regulated CD4+ T cell response in IL-15−/− mice appears to be a result of inefficient priming of these cells by DCs. In normal immunocompetent mice, T. gondii infection induces a rapid and large IL-12 production by DCs (14). Moreover, recent studies have demonstrated that IL-15 treatment of DCs results in higher IL-12 production by these cells (28). To establish that DCs from knockout mice are hypo-responsive to T. gondii infection, the level of IL-12 production by DCs from IL-15 knockout mice was evaluated. Purified cells from knockout and WT mice were pulsed with TLA, and IL-12 production in the supernatants was measured by ELISA. As shown in Fig. 8, compared with parental WT mice, DCs from knockout mice released significantly lower (almost 3-fold less) IL-12 upon TLA stimulation (P = 0.004). However, the addition of exogenous IL-15 to cultures led to increase in IL-12 production by knockout DCs (P = 0.002), and cytokine levels released in response TLA stimulation were comparable with cells from WT animals (Fig. 8).
Fig. 8.
IL-15−/− DCs produce less IL-12 in vitro. DCs were isolated from MLNs of IL-15−/− and WT mice (six to eight mice per group) and incubated overnight (5 × 104 cells per well) with 20 μg/ml TLA. After 24-h incubation, IL-12 levels in supernatants were determined by ELISA. The experiment was performed twice with similar results, and the data are representative of one experiment.
Discussion
In the present studies, we demonstrate that mice lacking the IL-15 gene exhibited milder inflammatory reaction in response to oral T. gondii infection. Despite the fact that the parasite levels in the tissues of knockout mice, especially gut, were higher than the WT controls, these animals survived T. gondii infection better than did the parental WT mice. Increased survival of IL-15−/− mice infected p.o. with T. gondii is due to comparatively less gut necrosis in these animals. Analysis of MLN and spleen showed that, as compared with the WT animals, fewer IFN-γ-producing CD4+ T cells in response to T. gondii infection in knockout mice are induced. Suboptimal CD4+ T cell immunity against the parasite in the knockout mice was further manifested by decreased proliferation of these cells in response to antigenic stimulation. Moreover, adoptive transfer of normal immune CD4+ T cells caused increased inflammatory response in the recipients, which led to their death. The lower CD4+ T cell response in the absence of IL-15 was due to defective functioning of DCs as demonstrated by lowered IL-12 secretion by these cells in response to Toxoplasma antigens. Furthermore, addition of IL-15 to DC cultures from knockout mice restored their ability to secrete IL-12 and prime CD4+ T cell response. To our knowledge, this report is the first to demonstrate the role of IL-15 in the induction of primary CD4+ T cell response, and it also emphasizes the importance of this cytokine against acute T. gondii infection.
A recent report by Lieberman et al. (25) has shown that IL-15−/− mice do not exhibit increased susceptibility to i.p. infection. In agreement with these observations, we demonstrate that unlike p.o. challenge, IL-15−/− and WT animals exhibit similar mortality when infection is carried via the i.p. route (data not shown). These findings are very important because difference in the outcome of parasite challenge based on the route of infection has not been clearly demonstrated. The differences can be attributed to the involvement of mucosal immune system during oral infection, which is known to play an important role in protection against many pathogens (30), including T. gondii (31). Our findings suggest that mucosal immune system, in addition to providing protective immunity against oral infection, may play an important role in tailoring the peripheral immune response against T. gondii and other orally acquired pathogens. This unstudied role of mucosal immunity needs to be evaluated.
IL-15 has been described as a proinflammatory cytokine that shares biologic activities with IL-2 (19) and is important for generation of NK cell response (32). The role of IL-15 in the homeostasis of CD8+ T cell memory is well established (33–35). Studies from our laboratory have shown that administration of exogenous IL-15 prolongs CD8+ T cell response against T. gondii (9) and that blockade of this response inhibits the development of long-term CD8+ T cell immunity against the parasite (18). Similarly, the role of IL-15 in the maintenance of memory CD8+ T cell immunity against other infectious disease has been described (20, 21, 36). Because of its proinflammatory properties, the role of IL-15 in a number of autoimmune diseases has been demonstrated (37–39). It inhibits self-tolerance mediated by AICD and facilitates the survival of self-directed memory CD8+ T cells (40). McInnes and colleagues (41) have reported abnormalities of IL-15 in rheumatoid arthritis patients and have suggested that IL-15 precedes TNF-α in the cytokine cascade. IL-15 has also been suggested to have a pathogenic role in other inflammatory diseases, such as sarcoidiosis (42), chronic hepatitis C (43), and ulcerative colitis (44). Antibodies against IL-15 have been used effectively in murine models of autoimmune diseases, including Psoriasis (45). IL-15 has also been shown to be overexpressed in the inflamed mucosa in inflammatory bowel disease patients and responsible for enhancement of local T cell activation, proliferation, and proinflammatory cytokine production (37). It has been suggested that treatment with anti IL-15 agents can serve as potential therapeutic regimen in these patients (46).
As stated earlier, the importance of CD4+ T cell response against T. gondii infection is well documented (2, 3, 47). During the acute phase of the disease, CD4+ T cells are the primary source of host IFN-γ production (48), leading to immunopathology and ultimate death of the animal (11, 26). CD4+ T cells are known to play a primary role in number of autoimmune diseases such as inflammatory bowel disease, Crohn's disease, and experimental autoimmune encephalomyelitis (49–51). However, the importance of IL-15 in the elicitation of primary CD4+ T cell response and subsequent effect on the pathogenesis of these diseases has not been reported.
We demonstrate that in the absence of IL-15, down-regulated CD4+ T cell immune response against T. gondii infection is induced. This reduction is due to impairment of DC response in these animals as a result of which CD4+ T cells are not primed to optimal levels. These findings are supported by earlier reports, which showed that IL-15–IL-15 receptor interaction is critical for early activation of antigen-presenting cells (52). DCs are the primary source of IL-12, which is released during very early stages of infection (14). Our studies demonstrate that DCs from mice lacking IL-15 exhibit decreased IL-12 response to Toxoplasma stimulation and are less effective in priming of CD4+ T cells, which in turn leads to lower inflammatory response in the infected animal. These findings are supported by recent observations demonstrating that priming with IL-15-treated DCs can strongly polarize CD4+ T cells toward production of IFN-γ (28). Although the knockout animals have a higher parasite load in comparison with WT mice, especially in the gut, they survive the infection because of milder pathology in the tissue. All of this is due to suboptimal CD4+ T cell immunity in the absence of IL-15. Direct association of CD4+ T cell defect with increased survival or decreased pathology in IL-15−/− mice is conclusively demonstrated by adoptive transfer of immune CD4+ T cells to these animals. T. gondii infection in these knockout recipients results in gut pathology similar to the WT animals.
Based on previous observations and our current findings, the hypothesis we propose is as follows: T. gondii infection induces a strong activation of DCs, which are important for antigen presentation (29) and induction of CD4+ T cell immune response (29). IL-15 seems to be a critical factor for activation of DCs, and in the absence of this gene, DCs are unable to induce adequate antigen-specific CD4+ T cell levels in the host. Although this subdued DC response inhibits the clearance of the parasite, the inflammatory process at these sites is subdued, thus preventing mortality. Our studies raise some important issues. (i) Is the lower CD8+ T cell response reported in the absence of functional IL-15 (33) due to down-regulated CD4+ T cell immune response in these animals? (ii) Is the suboptimal CD4+ T cell priming by DCs in IL-15−/− mice due to the lower NK cell response described in these animals (33)? NK cells, by their ability to produce IFN-γ, have been recently shown to play an important role in the activation of DCs (53). To our knowledge, this is the first report that demonstrates that IL-15 has a role in priming of CD4+ T cell response. These studies have strong implications for inflammatory or autoimmune diseases where targeting of IL-15 may dampen the severity because of down-regulation of CD4+ T cell response.
Materials and Methods
Mice and Parasites.
Six- to 8-week-old female IL-15−/− (Taconic), IFN-γ−/− (The Jackson Laboratory), and C57BL/6 (WT) (The Jackson Laboratory) mice were maintained in a specific pathogen-free environment in the animal research facility at the Louisiana State University Health Sciences Center. All experiments were performed in accordance with Institutional Animal Care and Use Committee regulations. Mice were challenged p.o. with 20–25 cysts of T. gondii strain 76K, which was maintained by continuous oral passage of cysts through C57BL/6 mice (Charles River Breeding Laboratories). TLA was prepared as described in ref. 10.
Histopathology.
Infected mice from both knockout and WT groups were killed on days 8–10 p.i., and tissues (liver and gut) were collected. The tissues were fixed in buffered 10% formalin, embedded, sectioned, and stained with hematoxylin/eosin for histological examination. Sections were examined and photographed on Kodak T-MAX film with an Olympus Van Ox photomicroscope.
Quantification of Parasite Load.
IL-15−/− and WT mice were infected orally as described above. Quantification of parasite burden on the tissues (spleen, liver, gut, and brain) was performed at day 10 p.i. by quantitative PCR as described in ref. 54.
Activation Markers.
IL-15−/− and WT mice were orally infected with 20–25 cysts of T. gondii as described above. At day 10 p.i., the mice were killed, MLNs were removed, and cell suspensions were prepared. Cells were labeled with anti-CD4+ FITC conjugated, anti-CD44 CyChrome conjugated, and anti-CD62L PE conjugated (eBioscience, San Jose, CA) in PBS-BSA 0.5% for 1 h. Cells were washed several times in buffer, fixed in 1% formaldehyde, and stored at 4°C until acquisition on a FACSVantage system (BD Biosciences).
Cytokine Assay.
IFN-γ production by T cells was evaluated by intracellular staining at day 10 p.i. as described in ref. 18, using the Cytofix/Cytoperm kit (BD Pharmingen).
In Vitro IL-12 Production by DCs.
DCs were isolated from MLN of naïve mice by positive selection by means of magnetic sorting (Miltenyi Biotech) using anti-CDllc beads and according to the manufacturer's instructions. Cells were further purified by flow cytometry, and >96% pure CD11chi cells were plated in 96-well plates at concentrations of 5 × 104 cells per well. Cultures were treated with TLA (20 μg/ml) and recombinant mouse IL-15 (50 ng/ml) (R & D Systems). After overnight incubation, supernatants were collected and assayed for IL-12 production by using cytokine ELISA kit (BioLegend, San Diego) according to the manufacturer's instructions.
Detection of IFN-γ Message.
IFN-γ message in the guts of infected animals was determined by real-time PCR. At day 7 p.i., mice were killed and total tissue RNA was extracted by using TRIzol reagent (Life Technologies). DNA was removed from the extracted RNA according to the standard protocol (55), and RNA was further purified by using the RNeasy spin column (Qiagen). For RT-PCR, 2 μg of RNA was reverse-transcribed by using the SuperScript III first-strand synthesis system (Invitrogen Life Technologies). Amplification was performed by real-time fluorogenic PCR with the SmartMix HM bead (Cepheid, Sunnyvale, CA) on a Cepheid SmartCycler. Each reaction contained one lyophilized SmartMix HM bead, SYBR green I (Cambrex Bio Science Rockland, Rockland, ME), and either 200 nM murine IFN-γ forward and reverse primers (56) or 400 nM β-actin forward and reverse primers (57) (Integrated DNA Technologies, Coralville, IA). Samples were subjected to 40 cycles of 15 s at 95°C and 1 min at 60°C followed by melt curve analysis as described in ref. 56.
Adoptive Transfer of CD4+ T Cells.
CD4+ T cells from WT C57BL/6 or IFN-γ−/− mice infected p.o. with 20 cysts 10 days earlier were transferred to IL-15−/− mice by the i.v. route. Briefly, mesenteric lymphocytes from C57BL/6 mice were collected, and CD4+ T cells were isolated by positive selection via MACS purification, using anti-CD4+ biotinylated antibody and anti-biotin beads (Miltenyi Biotech). Purified CD4+ T cells (1 × 107; >95% pure) were administered i.v. to IL-15−/− mice. Recipients were challenged p.o. with 25 cysts of T. gondii 1 day posttransfer. The animals were monitored for survival on a daily basis.
CD4+ T Cell Proliferation.
CD4+ T cells from C57BL/6 and IL-15−/− mice were isolated from MLN as described above. Antigen-specific proliferation of purified CD4+ T cells was determined by [3H]thymidine incorporation as described in ref. 9.
In a separate series of experiments, in vitro priming of CD4+ T cells by DCs was performed. Purified DCs from MLN of naïve mice were cultured as mentioned above. The next day, 1 × 105 purified CD4+ T cells from pooled MLN were added, cultures were incubated for 4 days, and proliferation of CD4+ T cells was determined.
Acknowledgments
This work was supported by National Institutes of Health Grants AI33325 (to I.A.K.) and AI41930 (to D.J.B.).
Abbreviations
- DC
dendritic cell
- MLN
mesenteric lymph node
- NK
natural killer
- p.i.
postinfection
- p.o.
per-oral(ly)
- TLA
Toxoplasma lysate antigen.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yap G. S., Sher A. Immunobiology. 1999;201:240–247. doi: 10.1016/S0171-2985(99)80064-3. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki Y., Remington J. S. J. Immunol. 1990;144:1954–1956. [PubMed] [Google Scholar]
- 3.Gazzinelli R., Xu Y., Hieny S., Cheever A., Sher A. J. Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 4.Sayles P. C., Johnson L. L. Infect. Immun. 1996;64:3088–3092. doi: 10.1128/iai.64.8.3088-3092.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharton-Kersten T. M., Wynn T. A., Denkers E. Y., Bala S., Grunvald E., Hieny S., Gazzinelli R. T., Sher A. J. Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 6.Khan I. A., Matsuura T., Kasper L. H. Infect. Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Proc. Natl. Acad. Sci. USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasper L. H., Matsuura T., Khan I. A. J. Immunol. 1995;155:4798–4804. [PubMed] [Google Scholar]
- 9.Khan I. A., Casciotti L. J. Immunol. 1999;163:4503–4509. [PubMed] [Google Scholar]
- 10.Khan I. A., Kasper L. H. J. Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 11.Liesenfeld O., Kosek J., Remington J. S., Suzuki Y. J. Exp. Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunter C. A., Subauste C. S., Van Cleave V. H., Remington J. S. Infect. Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan I. A., Ely K. H., Kasper L. H. J. Immunol. 1994;152:1856–1860. [PubMed] [Google Scholar]
- 14.Reis e Sousa C., Hieny S., Scharton-Kersten T., Jankovic D., Charest H., Germain R. N., Sher A. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scharton-Kersten T., Denkers E. Y., Gazzinelli R., Sher A. Res. Immunol. 1995;146:539–545. doi: 10.1016/0923-2494(96)83029-x. [DOI] [PubMed] [Google Scholar]
- 16.Sher A., Collazzo C., Scanga C., Jankovic D., Yap G., Aliberti J. Immunol. Res. 2003;27:521–528. doi: 10.1385/IR:27:2-3:521. [DOI] [PubMed] [Google Scholar]
- 17.Scharton-Kersten T. M., Yap G., Magram J., Sher A. J. Exp. Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan I. A., Moretto M., Wei X. Q., Williams M., Schwartzman J. D., Liew F. Y. J. Exp. Med. 2002;195:1463–1470. doi: 10.1084/jem.20011915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabstein K. H., Eisenman J., Shanebeck K., Rauch C., Srinivasan S., Fung V., Beers C., Richardson J., Schoenborn M. A., Ahdieh M., et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 20.Yajima T., Nishimura H., Sad S., Shen H., Kuwano H., Yoshikai Y. J. Immunol. 2005;174:3590–3597. doi: 10.4049/jimmunol.174.6.3590. [DOI] [PubMed] [Google Scholar]
- 21.Mueller Y. M., Bojczuk P. M., Halstead E. S., Kim A. H., Witek J., Altman J. D., Katsikis P. D. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 22.Schluns K. S., Williams K., Ma A., Zheng X. X., Lefrancois L. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 23.Becknell B., Caligiuri M. A. Adv. Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 24.Waldmann T. A., Tagaya Y. Annu. Rev. Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman L. A., Villegas E. N., Hunter C. A. Infect. Immun. 2004;72:6729–6732. doi: 10.1128/IAI.72.11.6729-6732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan I. A., Schwartzman J. D., Matsuura T., Kasper L. H. Proc. Natl. Acad. Sci. USA. 1997;94:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weninger W., Crowley M. A., Manjunath N., von Andrian U. H. J. Exp. Med. 2001;194:953–966. doi: 10.1084/jem.194.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feili-Hariri M., Falkner D. H., Morel P. A. J. Leukocyte Biol. 2005;78:656–664. doi: 10.1189/jlb.1104631. [DOI] [PubMed] [Google Scholar]
- 29.Foti M., Granucci F., Ricciardi-Castagnoli P. Trends Immunol. 2004;25:650–654. doi: 10.1016/j.it.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Iijima H., Takahashi I., Kiyono H. Rev. Med. Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- 31.Lepage A. C., Buzoni-Gatel D., Bout D. T., Kasper L. H. J. Immunol. 1998;161:4902–4908. [PubMed] [Google Scholar]
- 32.Fehniger T. A., Caligiuri M. A. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 33.Wherry E. J., Becker T. C., Boone D., Kaja M. K., Ma A., Ahmed R. Adv. Exp. Med. Biol. 2002;512:165–175. doi: 10.1007/978-1-4615-0757-4_22. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X., Sun S., Hwang I., Tough D. F., Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 35.Lodolce J. P., Boone D. L., Chai S., Swain R. E., Dassopoulos T., Trettin S., Ma A. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 36.Villinger F., Miller R., Mori K., Mayne A. E., Bostik P., Sundstrom J. B., Sugimoto C., Ansari A. A. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Geboes K., Colpaert S., D'Haens G. R., Rutgeerts P., Ceuppens J. L. J. Immunol. 2000;164:3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 38.Wei X., Orchardson M., Gracie J. A., Leung B. P., Gao B., Guan H., Niedbala W., Paterson G. K., McInnes I. B., Liew F. Y. J. Immunol. 2001;167:277–282. doi: 10.4049/jimmunol.167.1.277. [DOI] [PubMed] [Google Scholar]
- 39.Ruchatz H., Leung B. P., Wei X. Q., McInnes I. B., Liew F. Y. J. Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 40.Waldmann T. A. Trends Mol. Med. 2003;9:517–521. doi: 10.1016/j.molmed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 41.McInnes I. B., Leung B. P., Sturrock R. D., Field M., Liew F. Y. Nat. Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 42.Muro S., Taha R., Tsicopoulos A., Olivenstein R., Tonnel A. B., Christodoulopoulos P., Wallaert B., Hamid Q. J. Allergy Clin. Immunol. 2001;108:970–975. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 43.Jinushi M., Takehara T., Tatsumi T., Kanto T., Groh V., Spies T., Suzuki T., Miyagi T., Hayashi N. J. Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 44.Mention J. J., Ben Ahmed M., Begue B., Barbe U., Verkarre V., Asnafi V., Colombel J. F., Cugnenc P. H., Ruemmele F. M., McIntyre E., et al. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 45.Villadsen L. S., Schuurman J., Beurskens F., Dam T. N., Dagnaes-Hansen F., Skov L., Rygaard J., Voorhorst-Ogink M. M., Gerritsen A. F., van Dijk M. A., et al. J. Clin. Invest. 2003;112:1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldmann T. A. Arthritis Res. Ther. 2004;6:174–177. doi: 10.1186/ar1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casciotti L., Ely K. H., Williams M. E., Khan I. A. Infect. Immun. 2002;70:434–443. doi: 10.1128/IAI.70.2.434-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tato C. M., Villarino A., Caamano J. H., Boothby M., Hunter C. A. J. Immunol. 2003;170:3139–3146. doi: 10.4049/jimmunol.170.6.3139. [DOI] [PubMed] [Google Scholar]
- 49.Connor S. J., Paraskevopoulos N., Newman R., Cuan N., Hampartzoumian T., Lloyd A. R., Grimm M. C. Gut. 2004;53:1287–1294. doi: 10.1136/gut.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawakami N., Odoardi F., Ziemssen T., Bradl M., Ritter T., Neuhaus O., Lassmann H., Wekerle H., Flugel A. J. Immunol. 2005;175:69–81. doi: 10.4049/jimmunol.175.1.69. [DOI] [PubMed] [Google Scholar]
- 51.Silva M. A., Menezes J., Deslandres C., Seidman E. G. Inflamm. Bowel Dis. 2005;11:219–230. doi: 10.1097/01.mib.0000160804.52072.6a. [DOI] [PubMed] [Google Scholar]
- 52.Ohteki T., Suzue K., Maki C., Ota T., Koyasu S. Nat. Immunol. 2001;2:1138–1143. doi: 10.1038/ni729. [DOI] [PubMed] [Google Scholar]
- 53.Adam C., King S., Allgeier T., Braumuller H., Luking C., Mysliwietz J., Kriegeskorte A., Busch D. H., Rocken M., Mocikat R. Blood. 2005;106:338–344. doi: 10.1182/blood-2004-09-3775. [DOI] [PubMed] [Google Scholar]
- 54.Khan I. A., MacLean J. A., Lee F. S., Casciotti L., DeHaan E., Schwartzman J. D., Luster A. D. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- 55.Obar J. J., Crist S. G., Leung E. K., Usherwood E. J. J. Immunol. 2004;173:2705–2714. doi: 10.4049/jimmunol.173.4.2705. [DOI] [PubMed] [Google Scholar]
- 56.Overbergh L., Valckx D., Waer M., Mathieu C. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 57.Lin J. T., Martin S. L., Xia L., Gorham J. D. J. Immunol. 2005;174:5950–5958. doi: 10.4049/jimmunol.174.10.5950. [DOI] [PubMed] [Google Scholar]