Abstract
Forkhead winged-helix transcription factor Foxp3 serves as the dedicated mediator of the genetic program governing CD25+CD4+ regulatory T cell (Tr) development and function in mice. In humans, its role in mediating Tr development has been controversial. Furthermore, the fate of Tr precursors in FOXP3 deficiency has yet to be described. Making use of flow cytometric detection of human FOXP3, we have addressed the relationship between FOXP3 expression and human Tr development. Unlike murine Foxp3− T cells, a small subset of human CD4+ and CD8+ T cells transiently up-regulated FOXP3 upon in vitro stimulation. Induced FOXP3, however, did not alter cell-surface phenotype or suppress T helper 1 cytokine expression. Furthermore, only ex vivo FOXP3+ Tr cells persisted after prolonged culture, suggesting that induced FOXP3 did not activate a Tr developmental program in a significant number of cells. FOXP3 flow cytometry was also used to further characterize several patients exhibiting symptoms of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) with or without FOXP3 mutations. Most patients lacked FOXP3-expressing cells, further solidifying the association between FOXP3 deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Interestingly, one patient bearing a FOXP3 mutation enabling expression of stable FOXP3mut protein exhibited FOXP3mut-expressing cells among a subset of highly activated CD4+ T cells. This observation raises the possibility that the severe autoimmunity in FOXP3 deficiency can be attributed, in part, to aggressive T helper cells that have developed from Tr precursors.
A significant body of evidence has been derived from rodent models demonstrating that, through Foxp3 expression, CD25+CD4+ regulatory T cells (Tr) develop as a separate lineage of CD4+ T cells with a unique and vital function (1–3). Tr have also been identified in humans and have been shown to possess many of the same phenotypic and functional properties as their murine counterparts (4). Mutations of FOXP3 in humans lead to an early-onset, multisystem autoimmune syndrome known as IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) (5–7). Foxp3null and scurfy mice exhibit an analogous autoimmune pathology (8, 9), suggesting that a similar function is served by FOXP3 across phylogeny.
Although it is well established that both murine and human Tr develop as a subset of CD4 single-positive thymocytes (10, 11), the conditions under which Tr arise in peripheral organs is less understood. In mice, no measurable role for Foxp3 has been found in the differentiation or function of non-Tr in response to T cell receptor (TCR) agonists (9). In contrast, human CD25−CD4+ and CD8+ T cells have been shown to increase FOXP3 mRNA and protein levels upon activation, suggesting a cell-intrinsic role for FOXP3 in the regulation of T cell responses in humans (12–14). Furthermore, the existence of IPEX-like individuals that are phenotypically similar to IPEX but lack mutations within the coding region of the FOXP3 gene calls into question the role of FOXP3 as the “master regulator” of human Tr development and function. Thus, two nonmutually exclusive models can be proposed for the role of FOXP3 in regulating immune responses in humans. In the first model, preexisting FOXP3+ Tr are recruited to sites of active immune response where they suppress antigen-specific effector T cells and expand to control the intensity of the response. In the second model, FOXP3−CD4+ T cells responding to neoantigens expressed by target organs or to pathogens give rise to a clonal population consisting of both effector T cells and FOXP3+ Tr, the latter of which may either exist transiently or give rise to long-lived Tr.
Whether the mechanisms of Tr development and function differ in humans and mice is currently an area of significant debate. Recent evidence suggests that the latter model of peripheral Tr development may be more operative in humans than in mice, because some groups have found that, unlike murine cells, stimulation of human CD25−CD4+ T cells results in considerable FOXP3 expression and development of suppressor activity (12, 15). Others have not observed such conversion of naïve T cells into FOXP3-expressing Tr in vitro (16). Determining whether humans generate large numbers of “adaptive” Tr during immune responses, and the mechanisms driving such Tr development, is of substantial basic and practical significance. To address these possibilities and to further examine the relationship between FOXP3 deficiency and IPEX, we investigated FOXP3 expression in ex vivo isolated and activated T cells from normal donors and IPEX patients using our recently developed flow cytometric methodology. Serendipitously, the identification in one patient of activated T cells expressing a loss-of-function mutant FOXP3 suggests the possibility that the severity of IPEX/scurfy autoimmunity may result from an alternative proinflammatory fate of Tr precursors.
Results and Discussion
Flow Cytometric Characterization of Human FOXP3+ Cells.
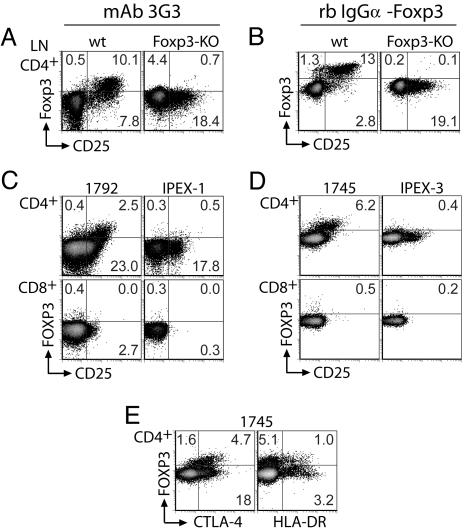
To examine the regulation of FOXP3 expression in individual human T cells, we developed methods for flow cytometric detection of FOXP3 using a novel mouse mAb (3G3) or a digoxigenin-conjugated rabbit polyclonal antibody. Both antibodies detect murine as well as human FOXP3, and their utility for single-cell detection of Foxp3 expression was demonstrated by using normal and Foxp3null mice. Staining of mouse lymph node cells with either antibody revealed Foxp3 expression in the majority of CD25+CD4+ T cells and a small subset of CD25−CD4+ cells (Fig. 1A and B). This Foxp3 expression pattern was similar to that of Foxp3GFP knockin mice (17). Reactivity with Foxp3 was specific, because no staining was observed with either antibody in Foxp3null cells (Fig. 1 A and B). Specificity was further confirmed by mapping of the mAb 3G3 epitope to the amino-terminal portion of FOXP3, a domain unique among all forkhead-family transcription factors (Fig. 5, which is published as supporting information on the PNAS web site). No specific staining was observed in murine CD8+ cells or non-T cells (data not shown).
Fig. 1.
Flow cytometric detection of Foxp3 in murine and human cells. (A and B) Normal or Foxp3-deficient mouse lymph node cells were stained for Foxp3 and cell-surface markers by using digoxigenin-conjugated mAb 3G3 (A) or Foxp3-specific rabbit antibody (B). CD4+ gated lymphocytes are shown. (C–E) Normal (1792 and 1745) or FOXP3-deficient (IPEX) PBMC were stained for FOXP3 and lymphocyte markers by using digoxigenin-conjugated mAb 3G3 (C) or digoxigenin-conjugated Foxp3-specific rabbit antibody (D and E). Both CD4+ and CD8+ gated lymphocytes are shown. Additional IPEX-1 PBMC were not available for subsequent analysis with rabbit antibody. High background staining of Foxp3− cells is a consequence of the three-step staining procedure.
FOXP3 expression profiles in human peripheral blood mononuclear cells (PBMC) were very similar to those observed in murine cells. All CD25highCD4+ cells, previously shown to exhibit potent suppressor function (4), were FOXP3+, whereas only a minority of CD25lowCD4+ and CD25−CD4+ cells exhibited FOXP3 expression. This finding is consistent with the observation that CD25low cells are not suppressive (18) (Fig. 1 C and D). Previous estimates have proposed that the human Tr subset constitutes ≈1–3% of CD4+ T cells. However, the percentage of FOXP3+ cells was found to be closer to 6% in normal donors using our FOXP3-specific rabbit polyclonal antibody. This finding is in complete agreement with recently described flow cytometric detection of human FOXP3 using another novel mAb (14). Similar to Foxp3null mice, patients with FOXP3 mutations affecting mRNA splicing (IPEX-1 and IPEX-3) have no detectable FOXP3+ cells (Fig. 1 C and D and Table 1). Interestingly, CD4+ cells from IPEX patients exhibited a similar proportion of CD25+ cells as normal subjects, suggesting the presence of activated effector T helper (Th) cells despite the administration of immunosuppressants (Fig. 1 C and D and Table 1). FOXP3+CD4+ cells were also enriched in expression of the T cell activation markers CTLA-4 and HLA-DR. In contrast to the correlation seen between high CD25 expression and FOXP3 positivity, however, comparably high expression levels of CTLA-4 and HLA-DR were present on both FOXP3+ and FOXP3− CD4+ T cells (Fig. 1E). In the CD8+ T cell compartment, there were negligible numbers of FOXP3+ cells (compare with the IPEX sample that lacks FOXP3 expression altogether), showing that, in quiescent PBMC, FOXP3-expressing CD8+ cells are rare (Fig. 1 C and D). For reasons likely due to variable epitope accessibility, our 3G3 mAb was somewhat less efficient than the rabbit polyclonal antibody at detecting FOXP3-expressing cells (Fig. 1). However, its utility and specificity for staining FOXP3 in humans is demonstrated here in normal and IPEX patient samples (Fig. 1).
Table 1.
IPEX patients
| Patient | Mutation | Dermatitis | Endocrinopathy type (age at onset) | Other* | Age and treatment when PBMC drawn |
|---|---|---|---|---|---|
| IPEX-1 | 210-210 + 1, GG > AC, splicing Δ | Eczema | IDDM (2 months) | AIHA/ITP ↑IgE | 5 months, FK506/steroids, TPN-dependent |
| IPEX-2-P1 | c.751_753, del GAG, p.ΔE251 | Eczema | IDDM (6 months) and thyroiditis | AIHA ↑IgE | 6 years, intermittent steroids |
| IPEX-2-P2 | c.751_753, del GAG, p.ΔE251 | Eczema | IDDM (6 months) and thyroiditis | ↑IgE | 9 years, FK506 |
| IPEX-3 | g.−6247_−4859 del, splicing Δ | Eczema | None | Food allergies ↑IgE | 4 years, FK506 |
| IPEX-like-1 | N/A | Eczema | IDDM (2 years) and thyroiditis (6 years) | Nephrotic syndrome | 11 years, FK506 |
| IPEX-like-2 | N/A | Eczema | Thyroiditis | Candidiasis ↑IgE | 3 years, azathioprine |
| IPEX-like-3 | N/A | Eczema and alopecia | IDDM (2 years) | None | 3 years |
| IPEX-like-4 | N/A | Exfoliative dermatitis and alopecia | None | Persistent AIHA ↑IgE | 4 months, CsA |
Mutation nomenclature is according to ref. 28. IDDM, insulin-dependent diabetes mellitus; AIHA, autoimmune hemolytic anemia; ITP, immune thrombocytopenia; TPN, total parenteral nutrition; N/A, not available; ↑, high concentration.
*All patients had moderate to severe enteropathy with profuse watery diarrhea.
FOXP3 Expression Is Induced Transiently in Some Human Non-TR CD4+ and CD8+ T Cells upon Activation but Persists only in in Vivo-Generated TR Cells.
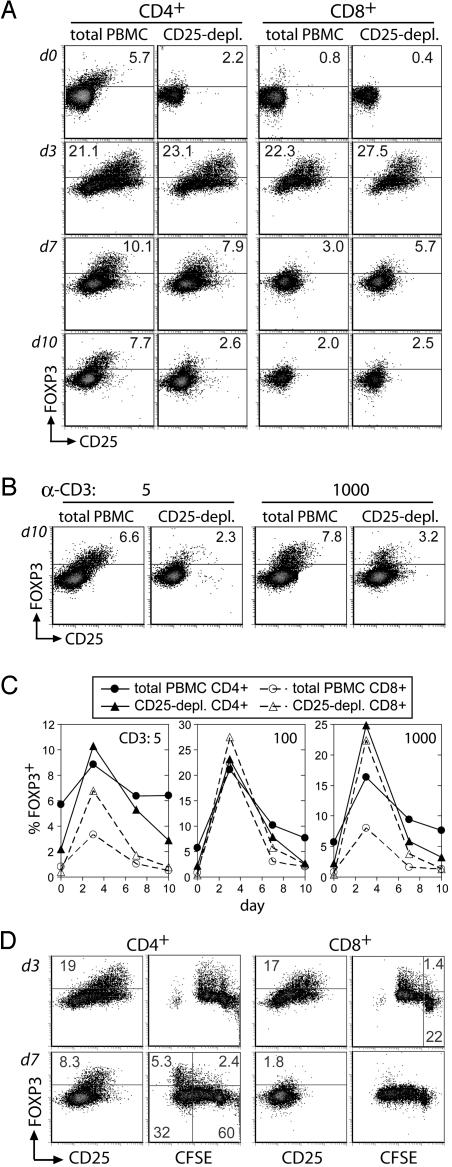
To investigate the degree to which de novo FOXP3 expression might occur in individual human T cells, we examined FOXP3 expression after TCR stimulation. Total or CD25-depleted PBMC were stimulated with varying doses of anti-CD3, and cells were analyzed by flow cytometry on days 3, 7, and 10 of culture. This regimen relies on “presentation” of anti-CD3 antibody to T cells by Fc receptors on antigen-presenting cells, a situation that we feel more closely resembles TCR activation in response to its natural ligands (i.e., peptide/MHC complexes) than plate- or bead-immobilized antibodies. A dramatic increase in the percentage of FOXP3+ cells among both CD4+ and CD8+ T cells was observed after stimulation, with up to 25% of CD4+ cells and 27% of CD8+ cells expressing FOXP3 on day 3 (Fig. 2A and C). The proportion of FOXP3+ T cells diminished progressively over time to near baseline levels by day 10. Interestingly, the relative loss of FOXP3 expression was most dramatic for cell populations that contained fewer FOXP3+ cells before activation (CD8+ cells and CD4+ cells from CD25-depleted PBMC). In contrast, CD4+ cells in cultures of total PBMC retained a FOXP3+CD25+CD4+ subpopulation on day 10 of culture that was strikingly similar to freshly isolated PBMC (Fig. 2 A and B). This pattern of transient FOXP3 expression was observed in cells from four unrelated normal donors and was consistent among monoclonal 3G3, rabbit polyclonal antibody, or monoclonal 259D (14) (data not shown). Furthermore, T cell costimulation was required for FOXP3 induction, because activation of purified T cells with plate-bound anti-CD3 and anti-CD28, but not anti-CD3 alone, promoted similar transient FOXP3 expression (Fig. 6, which is published as supporting information on the PNAS web site).
Fig. 2.
Analysis of FOXP3 expression in activated human CD4+ and CD8+ T cells. (A–C) Total or CD25-depleted PBMC from donor 1745 were stimulated with 5, 100, or 1000 ng/ml anti-CD3. FOXP3 and CD25 expression on CD4+ and CD8+ cells were assessed at days 3, 7, and 10 of culture. Shown are expression profiles for 100 ng/ml anti-CD3 (A), 5 or 1,000 ng/ml anti-CD3 for CD4+ gated cells (B), and the plotted percentage of gated cells expressing FOXP3 (C). FOXP3 was detected with digoxigenin-conjugated FOXP3-specific rabbit antibody. (D) Total PBMC from donor 1745 were labeled with CFSE and stimulated with 100 ng/ml anti-CD3. FOXP3 expression was assessed at days 3 and 7 with digoxigenin-conjugated rabbit antibody. Data are representative of four separate experiments and three normal adult donors.
The substantial size of the FOXP3+ cell population after T cell activation suggests that many of these cells may arise by transient, activation-induced, de novo expression of FOXP3 in non-Tr. However, this is difficult to ascertain because of a preexisting population of FOXP3+CD25−CD4+ T cells potentially capable of in vitro expansion. Indeed, similar experiments with mouse cells revealed a striking enrichment of Foxp3+ cells because of selective outgrowth (Fig. 7, which is published as supporting information on the PNAS web site). To further examine human FOXP3 induction in vitro, PBMC were CFSE-labeled before stimulation with anti-CD3. Cells were evaluated for proliferative responses and FOXP3 expression levels by flow cytometry. On day 3, when increased numbers of FOXP3+ cells were readily observed, FOXP3 expression on both CD4+ and CD8+ cells was not confined to highly divided CFSElow cells (Fig. 3D). Specifically, FOXP3 was found to be expressed in ≈6% of CD8+ T cells that had not yet undergone cell division (Fig. 2D). Thus, unlike murine cells, some human CD8+ and likely CD4+ T cells are capable of de novo FOXP3 induction in vitro. Although FOXP3+ cells on day 7 exhibited a high degree of CFSE dilution, it is likely that most of these cells derived from the efficient proliferation of preexisting Tr because the depletion of CD25+ cells from starting cultures (while not dramatically affecting the degree of FOXP3 induction on day 3) results in a paucity of FOXP3+ cells at later time points (Fig. 2 A–C). Importantly, unlike up-regulation of CD25, only a subset of T cells induced FOXP3 expression, suggesting that FOXP3 induction is stochastic or that some peripheral T cells are poised, i.e., precommitted, to express FOXP3.
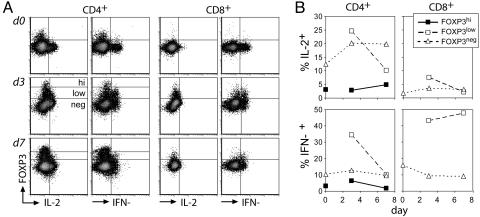
Fig. 3.
Induced FOXP3 does not suppress IL-2 or IFN-γ synthesis. (A) Freshly isolated or stimulated (100 ng/ml anti-CD3) total PBMC from donor 1745 were incubated with PMA, ionomycin, and monensin and stained for FOXP3 (rabbit IgG-digoxigenin), IL-2, IFN-γ, and surface markers as described in Materials and Methods. (B) The percentage of cytokine-expressing cells among FOXP3high, FOXP3low, or FOXP3− cells is plotted. The distinction between high and low FOXP3 expression was not made for CD4+ cells and CD8+ cells on day 0. Data are representative of three separate experiments.
Induced FOXP3 Does Not Suppress Th1 Cytokine Synthesis.
Next we sought to determine whether the induction of FOXP3 resulted in a Tr-like phenotype. Induced FOXP3 did not correlate with altered CD25, glucocorticoid-induced TNF receptor, or CD27 expression (Fig. 2A and data not shown); thus, it was not possible to isolate cells expressing induced FOXP3 for direct suppressor function studies. Ectopic expression of high levels of FOXP3 in naïve human CD4+ T cells suppresses IL-2 and IFN-γ production (16, 19), mirroring the inability of naturally developing Tr to produce these cytokines. Thus, analysis of intracellular cytokine production should serve as an indirect way to assess acquisition of some Tr properties by activated T cells.
To determine whether the induced FOXP3 suppressed these cytokines, cultured cells were examined for FOXP3, IL-2, and IFN-γ expression. As expected, the FOXP3+CD4+ Tr in freshly isolated PBMC did not express either IL-2 or IFN-γ in response to activation on day 0 (Fig. 3). On days 3 and 7 after stimulation, both cytokines were expressed by FOXP3−CD4+ and FOXP3lowCD4+ cells but not by a distinguishable FOXP3high population observed among CD4+ but not CD8+ T cells. The notable lack of Th1 cytokine expression by FOXP3highCD4+ cells suggests that high levels of FOXP3 are required to suppress cytokine synthesis or that this population was derived from preexisting FOXP3+CD4+ Tr present in the starting population (Fig. 3). The latter hypothesis is supported by the observation that the FOXP3highCD4+ population was 20% less (day 3) and 75% less (day 7) abundant in the CD25-depleted vs. undepleted PBMC cultures (data not shown). FOXP3+CD8+ cells demonstrated an expected lack of IL-2 production but efficiently expressed IFN-γ on both day 3 and day 7, indicating that FOXP3 also did not promote a Tr-like transcriptional program in CD8+ T cells. Similar results were obtained with another FOXP3-specific monoclonal 259D using PBMC from a second donor and costaining for TNF-α in addition to IL-2 and IFN-γ (Fig. 8, which is published as supporting information on the PNAS web site). Cells with induced FOXP3 are likely to down-regulate its expression rather than undergo apoptosis, because costaining with the caspase active-site reporter substrate FITC-VAD-FMK identified subsets of apoptotic FOXP3−CD4+ but not FOXP3+CD4+ cells at days 3 and 7 (data not shown). Together, these findings suggest that FOXP3 induced in vitro upon activation of human non-Tr does not promote Tr phenotype development, whereas FOXP3+ Tr generated in vivo persist and maintain their functional characteristics after in vitro expansion.
FOXP3 Expression in IPEX Syndrome.
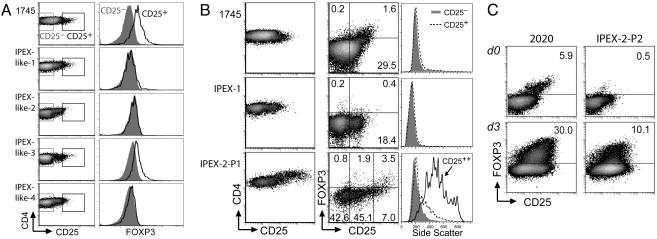
Although FOXP3 mutations have been characterized in more than two-thirds of IPEX patients, we have identified a subgroup of patients exhibiting a similar pattern of autoimmune characteristics but lacking FOXP3 coding or splice-site mutations. In such individuals, identified as IPEX-like (Table 1), FOXP3 deficiency may result from uncharacterized FOXP3 promoter mutations or from mutations in genes required for FOXP3 expression. Alternatively, FOXP3 expression may be intact, and the disease may result from mutations in other genes affecting T cell regulation. To better characterize the etiology of the autoimmune pathology in these IPEX-like patients, PBMC were analyzed for FOXP3 expression with mAb 3G3. Of four IPEX-like patients, three (IPEX-like-1, -2, and -4) lacked FOXP3 expression in the CD25highCD4+ cell population, whereas IPEX-like-3 exhibited moderate FOXP3 expression in CD25highCD4+ cells (Fig. 4A). Thus, we have linked three of four IPEX-like patients who lack FOXP3 coding mutations with FOXP3 deficiency. Promoter mutations that completely or partially attenuate FOXP3 expression are the most likely cause for FOXP3 deficiency in these individuals, the identification of which will significantly advance our understanding of the factors and signals that promote FOXP3 transcription.
Fig. 4.
FOXP3 expression in IPEX. (A and B) Normal (1745), IPEX-like (A), and IPEX (B) PBMC were stained for cell-surface markers and FOXP3 with mAb 3G3. Gated CD4+ cells are shown. Histograms show FOXP3 expression (A) and side scatter (B) on CD4+ cells expressing varying degrees of CD25 as delineated in the adjacent 2D plots. Because the staining procedure results in a decrease in forward scatter, side scatter is a better indicator of cell size. Staining was performed before the development of protocols by using digoxigenin-conjugated rabbit antibody, but additional PBMC from these patients were not available for further study. PBMC shown in A and B were stained in separate experiments. (C) Freshly isolated or simulated (100 ng/ml anti-CD3; 3 days) normal (2020) or IPEX-2-P2 PBMC were stained for FOXP3 with mAb 259D (14).
Among the three IPEX patients, two (IPEX-1 and IPEX-3) have mutations in the 5′ portion of the gene that lead to aberrant mRNA splicing and absence of protein expression. A third IPEX patient (IPEX-2) harboring a single, in-frame amino acid deletion (ΔE251) within the leucine zipper of FOXP3 was also identified. This mutation was of particular interest because it should allow for expression of a full-length, mutant FOXP3 protein. Indeed, ectopic expression of native or mutant FOXP3 in both human fibroblasts and primary CD4+ T cells established that FOXP3ΔE251 protein was stable and could be efficiently detected by flow cytometry (Fig. 9, which is published as supporting information on the PNAS web site). Furthermore, FOXP3ΔE251 was unable to dimerize or to suppress transcription from an IL-2 promoter–luciferase reporter construct, confirming a lack of functional activity (T.R.T., unpublished observations). The presence of the classic IPEX phenotype in this patient strongly argues against FOXP3ΔE251 promoting significant Tr activity. Thus, FOXP3ΔE251 protein should serve as a natural reporter to examine FOXP3 expression in the apparent absence of FOXP3 function, thereby advancing our understanding of the requirements for persistence of Tr precursors as well as the nature of autoimmune effector cells in IPEX. Specifically, the presence of FOXP3ΔE251+ cells in IPEX-2 PBMC would indicate that cells receiving signals that promote FOXP3 expression are able to survive in the absence of FOXP3 function. Such cells could represent those that either (i) attempted Tr development during thymic maturation and migrated to the periphery or (ii) induced FOXP3ΔE251 expression in peripheral tissues, perhaps in response to autoantigens. Alternatively, the lack of a FOXP3ΔE251 population would indicate that FOXP3 function is required for the survival of cells committed to the Tr differentiation pathway.
Two IPEX-2 PBMC samples (P1 and P2) were obtained 3 years apart. The first sample (IPEX-2-P1) was drawn after treatment only with intermittent corticosteroid therapy, before the initiation of other potent immunosuppressants. The second (IPEX-2-P2) was drawn after 2 years of treatment with FK506. Analysis of FOXP3ΔE251 expression in each of these samples revealed intriguing differences. IPEX-2-P1 contained a population of large CD4+ cells expressing very high levels of CD25 (designated CD25++) (Fig. 4 A and B and data not shown). Thirty-three percent of these cells expressed FOXP3ΔE251, but the presence of aggressive systemic autoimmune disease in the patient at the time that the sample was drawn argues against these cells having any significant regulatory function. In contrast, IPEX-2-P2 lacked this population of large CD25++CD4+ cells and possessed a greatly reduced percentage of cells expressing FOXP3ΔE251 (Fig. 4C and data not shown). Despite the paucity of FOXP3ΔE251+ cells in freshly isolated PBMCs, FOXP3ΔE251 expression was induced in 10% of CD4+ IPEX-2-P2 PBMC upon stimulation with anti-CD3 for 3 days, mirroring the kinetics of induction observed in control PBMC. We hypothesize that the large CD25++CD4+ cells found in IPEX-2-P1 are likely to represent aggressive autoreactive effector T cells, some of which also expressed FOXP3ΔE251, and that potent T cell-directed immunosuppression with FK506 resulted in the loss of this population.
In the context of our findings in vitro, two nonmutually exclusive potential mechanisms may explain the presence of FOXP3ΔE251-expressing CD25++CD4+ T cells in IPEX-2-P1. First, Tr precursors that did not receive appropriate signals to continue down a Tr developmental pathway because of lack of functional FOXP3 may have persisted as FOXP3ΔE251-expressing autoreactive effector T cells (i.e., cells bearing TCRs that normally promote thymic Tr development). Alternately, such cells may have arisen from effector T cells that have induced FOXP3ΔE251 expression in response to activation (i.e., cells normally suppressed by Tr). If the FOXP3ΔE251+CD4+ cells arose from non-Tr precursors under conditions similar to those that promote FOXP3 induction in vitro, then a similar population may exist among CD8+ IPEX-2-P1 cells because we have observed FOXP3 induction with equal efficiency in both CD4+ and CD8+ T cells. IPEX-2-P1 CD8+ cells contained a CD25++ subset similar to their CD4+ counterparts, suggesting that some CD8+ T cells were also highly reactive to self antigens (Fig. 10, which is published as supporting information on the PNAS web site); however, the high degree of FOXP3ΔE251 expression found in CD25++CD4+ cells was not observed (Figs. 4B and 10). Thus, signals unique to CD4+ cells appear to promote FOXP3 transcription in FOXP3 deficiency. If FOXP3 does not normally rescue Tr precursors from thymic negative selection, such a signal may derive from the increased TCR affinity Tr typically display for self-peptide/MHC ligands (20, 21). Our recent findings of Tr-specific TCRs expressed in activated CD25+CD4+ T cells from Foxp3null mice support this hypothesis (22).
In conclusion, we have presented the first flow cytometric analysis of human FOXP3 expression in activated human PBMC, demonstrating that FOXP3 induction can be uncoupled from Tr development. Although some FOXP3− T cells up-regulated FOXP3 upon in vitro activation, Th1 cytokine synthesis was not blocked. Furthermore, under conditions that favored the persistence of in vivo-generated Tr, long-lived Tr were not readily derived from activated cells. In vivo, the identification of FOXP3ΔE251+CD25++CD4+ T cells in IPEX-2-P1 suggests that either similar induction can occur in vivo or autoreactive progeny of Tr precursors contribute significantly to the severity of IPEX symptomology. Although these two possibilities are not mutually exclusive, the latter scenario is attractive in that it associates self-reactive TCRs, i.e., those that promote Tr development, with T cells responsible for the multiorgan pathology observed in FOXP3-deficient humans and mice.
Although our findings reveal a lack of functional consequences of transiently induced FOXP3, others have reported de novo generation of FOXP3+ suppressive Tr in more long-term cultures (12, 15). Our findings support the possibility that preexisting Tr, capable of efficient expansion in vitro when in the presence of IL-2-producing T cells, may contribute to the generated Tr population in these experimental systems. Because we have observed a correlation between high FOXP3 expression and repression of Th1 cytokines, sustained expression of high levels of FOXP3 may be required to promote Tr development in vitro. Indeed, our group and others have observed that ectopic expression of only high levels of murine or human FOXP3 results in the acquisition of Tr phenotype and function (J. Fontenot, personal communication) (23). Although our methods for T cell activation did not result in sustained, high-level expression of induced FOXP3, we cannot exclude the possibility that some experimental conditions may promote such expression and subsequent Tr development.
In normal individuals, acute T cell stimulation by high-affinity ligands can occur in response to various forms of neoantigen, including infectious agents, vaccines, alloantigens presented after organ transplantation, and self-antigens in the setting of graft-versus-host disease. Should FOXP3 induction occur in such highly activated T cells, as we have observed in vitro, the degree and longevity of its expression and consequential Tr development is likely to be effected by the maturation state of antigen-presenting dendritic cells (24–26). In mice, similar transient de novo Foxp3 expression has recently been reported for highly activated T cells stimulated in vivo by dendritic cells presenting foreign antigen, whereas only low levels of antigen in the absence of proinflammatory signals resulted in de novo Tr development (27). Our findings support the distinct possibility that transient up-regulation of FOXP3 under proinflammatory conditions may not promote immunosuppressive function in contrast to that mediated by preexisting Tr responding to the same antigens. In aggregate, our data suggest that, despite the capacity for FOXP3 induction after TCR ligation, human T cells require sustained high-level FOXP3 expression for the acquisition of Tr function. Although such conditions may exist for Tr precursors in IPEX, they are not sufficient to elicit suppressor function in the absence of functional FOXP3.
Materials and Methods
Antibodies.
Rabbits and mice were immunized with bacterially expressed recombinant His-tagged full-length murine Foxp3 (gift of Fred Ramsdell; Celltech R&D, Bothell, WA) purified on Ni-NTA-Agarose (Qiagen, Venlo, The Netherlands). Polyclonal antibodies were produced by immunizing rabbits (R&R Rabbitry, Stanwood, WA) every 21 days with 250 μg of His-Foxp3. Hybridoma 3G3 was generated by priming mice with 75 μg of His-Foxp3 followed by three 30-μg boosts before fusion and clone screening by ELISA. Positive clones were subcloned and expanded in GIBCO Hybridoma-SFM. Anti-Foxp3 antibodies were isolated from rabbit antisera or hybridoma supernatant with protein A or protein G Sepharose affinity chromatography (Amersham Pharmacia Biosciences). Antibodies were labeled with digoxigenin-3-O-methylcarbonyl-ε-aminocaproic acid-N-hydroxysuccinimide ester (Roche Diagnostics, Indianapolis).
PBMC Donors.
Normal human PBMC were obtained from volunteer donors by leukopheresis. Participants gave informed consent per guidelines of the Institutional Review Board of the Fred Hutchinson Cancer Research Center. IPEX PBMC were isolated from venous blood for the molecular diagnosis of IPEX syndrome by sequence analysis and flow cytometry after consent of the patients.
T Cell Stimulation.
Total or CD25-depleted (MACS, Miltenyi Biotec) pooled mouse lymph node and spleen cells or human PBMC were cultured at 4 × 106 cells per well (24-well plates) with titrated anti-CD3 (2C11.145 or OKT3) in mouse cell medium (DMEM/10% FBS/50 μM 2-mercaptoethanol/10 mM Hepes/2 mM l-glutamine/1 mM sodium pyruvate/penicillin–streptomycin) or human cell medium (RPMI medium 1640/10% human serum/50 μM 2-mercaptoethanol/12.5 mM Hepes/6 mM l-glutamine/23.8 mM sodium bicarbonate/penicillin–streptomycin).
Flow Cytometry.
For staining with Foxp3-specific rabbit polyclonal IgG, cells were fixed in Cytofix/Cytoperm (BD Biosciences) for 30 min on ice, washed once in DMEM/5% FBS, and frozen at −80°C in DMEM/20% FBS/10% DMSO. Cells were thawed, washed twice in Perm/Wash (PW) (BD Biosciences), and refixed in Cytofix/Cytoperm for 4 min on ice. Cells were washed once with cold DMEM/5% FCS and twice with PBS, resuspended in PBS/500 μg/ml DNase (Roche, Indianapolis)/4 mM MgCl2, and incubated at room temperature for 30–40 min (mouse cells) or 10 min (human cells). Cells were then stained in PW supplemented with 350 mM NaCl (PW500) with either 200 μg/ml normal goat IgG (Jackson ImmunoResearch) (mouse cells) or 5% normal rabbit serum (Jackson ImmunoResearch) (human cells). After 5–10 min, anti-Foxp3 rabbit IgG or digoxigenin-labeled anti-Foxp3 rabbit IgG was added to 10 μg/ml. After three washes in PW500, cells were stained with either 10 μg/ml biotinylated goat anti-rabbit IgG with 200 μg/ml normal goat IgG (mouse cells) or 5 μg/ml biotinylated mouse anti-digoxin mAb with 5% normal mouse serum (human cells) (all Jackson ImmunoResearch reagents). After 20–30 min at room temperature, cells were washed three times with PW stained in PW with allophycocyanin-conjugated streptavidin (BD Biosciences) and other fluorophore-conjugated antibodies specific for cell-surface antigens. After a 20-min incubation at room temperature, cells were washed twice in PW, resuspended in PBS, and analyzed on a FACSCalibur or FACSCanto flow cytometer (BD Biosciences). For staining with digoxigenin-labeled Foxp3-specific mouse mAb (3G3-dig), mouse cells were incubated with 200 μg/ml DNase whereas human cells were neither refixed nor treated with DNase. Normal mouse serum (5%) (Jackson ImmunoResearch) was included during staining with both 3G3-dig and the secondary anti-digoxin reagent. In later experiments, FOXP3 was detected with Alexa Fluor 488-conjugated 259D (14) according to the manufacturer's protocols (BioLegend, San Diego).
For cytokine staining, cells were cultured with PMA (40 ng/ml), ionomycin (1 μg/ml), and monensin (3 μM) for 5 h (day 0 PBMC) or 3 h (cultured PBMC) before fixation and storage at −80°C. Cells were stained with IL-2-FITC, IFN-γ-phycoerythrin (PE), 5% normal mouse serum, and cell-surface markers during incubation with the anti-digoxigenin secondary reagent. Cells were costained with CD4-peridinin chlorophyll protein (SK3), CD8-FITC or CD8-allophycocyanin Cy7 (RPA-T8), CD25-PE or CD25-PECy7 (M-A251), CTLA-4-PE (BNI3), and HLA-DR-PE (G46-6) (BD Biosciences).
Supplementary Material
Acknowledgments
We are grateful to all members of the A.Y.R. laboratory for provocative discussions and to Drs. Frank Ruemmele, Andy Gennery, Lawrence Jung, Robert Hostoffer, and Allison Jones for the referral of patient samples for molecular diagnosis and analysis. This work was supported by grants from Arthritis Foundation and the Lymphoma and Leukemia Society (to M.A.G.), grants from the National Institutes of Health (to A.Y.R., T.R.T., and H.D.O.), a Pfizer Postdoctoral Fellowship in Rheumatology/Immunology (to T.R.T.), the Immunodeficiency Foundation, and the Jeffrey Modell Foundation (H.D.O.). A.Y.R. is a Howard Hughes Medical Institute Investigator.
Abbreviations
- Th
T helper
- IPEX
immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome
- Tr
regulatory T cell
- PBMC
peripheral blood mononuclear cell
- TCR
T cell receptor
- PE
phycoerythrin
- PW
Perm/Wash.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hori S., Nomura T., Sakaguchi S. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 2.Khattri R., Cox T., Yasayko S. A., Ramsdell F. Nat. Immunol. 2003;4:337–342. [PubMed] [Google Scholar]
- 3.Fontenot J. D., Gavin M. A., Rudensky A. Y. Nat. Immunol. 2003;4:330–336. [PubMed] [Google Scholar]
- 4.Baecher-Allan C., Viglietta V., Hafler D. A. Semin. Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Chatila T. A., Blaeser F., Ho N., Lederman H. M., Voulgaropoulos C., Helms C., Bowcock A. M. J. Clin. Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildin R. S., Ramsdell F., Peake J., Faravelli F., Casanova J. L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., et al. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 7.Bennett C. L., Christie J., Ramsdell F., Brunkow M. E., Ferguson P. J., Whitesell L., Kelly T. E., Saulsbury F. T., Chance P. F., Ochs H. D. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Lyon M. F., Peters J., Glenister P. H., Ball S., Wright E. Proc. Natl. Acad. Sci. USA. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot J. D., Rudensky A. Y. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 10.Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 11.Stephens L. A., Mottet C., Mason D., Powrie F. Eur. J. Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 12.Walker M. R., Kasprowicz D. J., Gersuk V. H., Benard A., Van Landeghen M., Buckner J. H., Ziegler S. F. J. Clin. Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan M. E., van Bilsen J. H., Bakker A. M., Heemskerk B., Schilham M. W., Hartgers F. C., Elferink B. G., van der Zanden L., de Vries R. R., Huizinga T. W., et al. Hum. Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Roncador G., Brown P. J., Maestre L., Hue S., Martinez-Torrecuadrada J. L., Ling K. L., Pratap S., Toms C., Fox B. C., Cerundolo V., et al. Eur. J. Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 15.Walker M. R., Carson B. D., Nepom G. T., Ziegler S. F., Buckner J. H. Proc. Natl. Acad. Sci. USA. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yagi H., Nomura T., Nakamura K., Yamazaki S., Kitawaki T., Hori S., Maeda M., Onodera M., Uchiyama T., Fujii S., Sakaguchi S. Int. Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot J. D., Rasmussen J. P., Williams L. M., Dooley J. L., Farr A. G., Rudensky A. Y. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan C., Wolf E., Hafler D. A. Clin. Immunol. 2005;115:10–18. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Oswald-Richter K., Grill S. M., Shariat N., Leelawong M., Sundrud M. S., Haas D. W., Unutmaz D. PLoS Biol. 2004;2:E198. doi: 10.1371/journal.pbio.0020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan M. S., Boesteanu A., Reed A. J., Petrone A. L., Holenbeck A. E., Lerman M. A., Naji A., Caton A. J. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh C. S., Liang Y., Tyznik A. J., Self S. G., Liggitt D., Rudensky A. Y. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh C. S., Zheng Y., Liang Y., Fontenot J. D., Rudensky A. Y. Nat. Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 23.Allan S. E., Passerini L., Bacchetta R., Crellin N., Dai M., Orban P. C., Ziegler S. F., Roncarolo M. G., Levings M. K. J. Clin. Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moseman E. A., Liang X., Dawson A. J., Panoskaltsis-Mortari A., Krieg A. M., Liu Y. J., Blazar B. R., Chen W. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe N., Wang Y. H., Lee H. K., Ito T., Cao W., Liu Y. J. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 26.Gorczynski R. M., Lee L., Boudakov I. Transplantation. 2005;79:1180–1183. [PubMed] [Google Scholar]
- 27.Kretschmer K., Apostolou I., Hawiger D., Khazaie K., Nussenzweig M. C., von Boehmer H. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 28.den Dunnen J. T., Antonarakis S. E. Hum. Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.