Abstract
Nicotinic acid (NA) is commonly used to treat dyslipidemia, but it elicits an adverse effect, termed flushing, which consists of cutaneous vasodilation with associated discomfort. An animal model of NA-induced flushing has been established in mice. As in humans, NA stimulated vasodilation in a dose-dependent manner, was associated with an increase of the vasodilatory prostaglandin (PG) D2 in plasma and could be blocked by pretreatment with aspirin. Two PGD2 receptors have been identified: PGD2 receptor 1 (DP1, also called DP) and PGD2 receptor 2 (DP2, sometimes termed CRTH2). DP2 does not mediate NA-induced vasodilation; the DP2-specific agonist DK-PGD2 (13,14-dihydro-15-keto-PGD2) did not induce cutaneous vasodilation, and DP2−/− mice had a normal vasodilatory response to NA. By contrast, BW245C, a DP1-selective agonist, induced vasodilation in mice, and MK-0524, a DP1-selective antagonist, blocked both PGD2- and NA-induced vasodilation. NA-induced vasodilation was also studied in DP1+/+, DP1+/−, and DP1−/− mice; although NA-induced vasodilation depended almost completely on DP1 in female mice, it depended only partially on DP1 in male mice. The residual NA-induced vasodilation in male DP−/− mice was aspirin-sensitive. Thus, in the mouse, DP1 appears to be an important component involved in NA-induced vasodilation, but other cyclooxygenase-dependent mechanisms also may be involved. A clinical study in healthy men and women demonstrated that treatment with MK-0524 reduced the symptoms of flushing and the increase in skin perfusion after the administration of NA. These studies suggest that DP1 receptor antagonism may be an effective means to suppress NA-induced flushing in humans.
Keywords: aspirin, prostaglandin D2 receptor 1 antagonist, MK-0524, niacin, flushing
Nicotinic acid (NA), sometimes called niacin, is a water-soluble B vitamin that has been used to treat dyslipidemia for almost 50 years (1, 2). Used in high doses, it reduces plasma low-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipoprotein(a) and increases high-density lipoprotein cholesterol and apolipoprotein A-I (3, 4). NA has been shown to have cardiovascular benefit when used alone or in combination with statins (hydroxymethylglutaryl-CoA reductase inhibitors) in several clinical trials (5–7).
Despite these demonstrated beneficial effects, widespread use of NA for dyslipidemia has been limited by symptoms of flushing, which is associated with cutaneous vasodilation of the face, neck, and torso that occurs in nearly all patients (5, 8). Administration of cyclooxygenase (COX) inhibitors, such as aspirin and indomethacin, before ingestion of NA can attenuate the NA-induced cutaneous reactions in most patients (9–13) without affecting its plasma free fatty acid-lowering effect (14). However, doses of aspirin of 325 mg or higher are generally required to significantly block flushing (15), which compromises the utility of this approach for the chronic suppression of flushing. Although the mechanism of action of NA-induced flushing is not completely understood, the sensitivity to COX inhibitors suggests that prostanoids are involved. Indeed, it has been shown that NA greatly increases the plasma levels of prostaglandin (PG) D2 (16), and that the skin is the major source of this vasodilatory prostanoid after NA treatment (17). These results led to the hypothesis that PGD2 generation in the skin drives NA-induced flushing (17). However, because NA treatment also increases other prostanoids (16, 18–20) [for example, PGI2 (16), which is also vasodilatory (21)], the prostanoid(s) responsible for flushing in humans remains to be unequivocally determined.
Two G protein-coupled receptors for PGD2, PGD2 receptor 1 (DP1) (22), also called DP; and PGD2 receptor 2 (DP2) (23, 24), sometimes termed CRTH2 (chemoattractant receptor-homologous molecule expressed on T-helper 2 cells), have been identified. Activation of DP1 by PGD2 leads to the stimulation of adenylate cyclase activity and increased intracellular cAMP levels (22). By contrast, activation of DP2 by PGD2 results in intracellular calcium mobilization (23) and a decrease of intracellular cAMP (25). Thus, in addition to the ambiguity as to whether PGD2 is the key driver of NA-induced flushing, the identity of the relevant PGD2 receptor, DP1, DP2, or both, has not been explored.
This report describes the development of a mouse model of NA-induced vasodilation and its use to demonstrate that this adverse effect is predominantly mediated by means of PGD2 acting through the DP1, rather than the DP2, receptor. A clinical study in humans demonstrated that a selective DP1 antagonist could significantly suppress symptoms of NA-induced flushing, raising the possibility that this approach could be used to increase the tolerability of NA, thereby allowing more patients to access the demonstrated cardiac benefits of this underutilized drug.
Results
Development of a Mouse Model of NA-Induced Vasodilation.
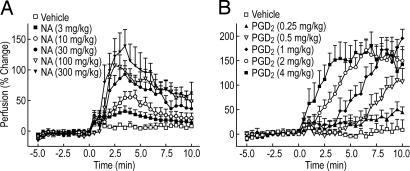
To develop an animal model of NA-induced vasodilation, we used laser Doppler perfusion imaging (LDPI) to examine whether NA would increase cutaneous perfusion (vasodilation) in the mouse ear. As shown in Fig. 1A, male C57BL/6 mice responded to NA treatment with vasodilation in a dose-dependent manner. The response was rapid and reached a maximum within 2–3 min after NA administration; by 10 min, >50% of the effect had abated. NA has been proposed to mediate vasodilation in humans by means of PGD2; thus, we tested whether PGD2 could induce vasodilation in the mouse. When administered s.c., PGD2 induced a rapid vasodilatory response in the ear (Fig. 1B). Significant effects were seen within 2 min with doses of 2 and 4 mg/kg. The vasodilation induced by high doses of PGD2 was greater in magnitude and longer in duration than that elicited by high doses of NA.
Fig. 1.
NA and PGD2 induce cutaneous vasodilation in male C57BL/6 in a dose-dependent manner. Perfusion in mouse ears was measured by LDPI as described in Materials and Methods. (A) NA was dissolved in 5% hydroxypropyl β-cyclodextrin; pH was adjusted to 7.4 with 2 M NaOH and injected at a dose of 0.2 ml per mouse (body weight ≈25 g, n = 4–9) s.c. at 0 min. Plotted values represent the mean ± SEM. (B) PGD2 was dissolved in 5% DMSO and injected at a dose of 0.1 ml per mouse (≈25-g body weight, n = 6–7) s.c. at 0 min. After injection of either NA or PGD2, the perfusion was monitored for an additional 10 min. All plotted values represent the mean ± SEM.
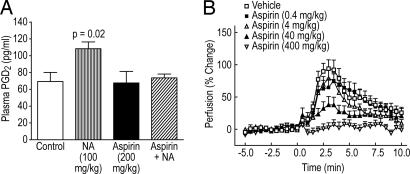
Administration of NA to humans increases plasma PGD2; therefore, we examined whether this increase also occurs in the mouse. NA, when given orally at a dose of 100 mg/kg, increased plasma PGD2 at 2 and 5 min ≈4- (data not shown) and 1.5-fold (Fig. 2A), respectively. This increase was transient; the plasma PGD2 returned to near baseline levels at 30 min (data not shown). Administration of aspirin alone at 200 mg/kg had no effect on plasma PGD2, whereas when given 30 min before the administration of NA, it completely blocked the increase in PGD2 (Fig. 2A). Because pretreatment with aspirin can attenuate NA-induced flushing in humans, we tested whether aspirin could suppress NA-induced vasodilation in mice. Indeed, administration of aspirin 30 min before NA markedly reduced vasodilation in a dose-dependent manner (Fig. 2B).
Fig. 2.
Aspirin inhibits the NA-induced increase in plasma PGD2 level and vasodilation. (A) Male C57BL/6 mice (n = 8) were pretreated by i.p. administration of vehicle or a 200-mg/kg dose of aspirin. Thirty minutes later, mice were treated orally with vehicle or a 100-mg/kg dose of NA. Five minutes later, blood was collected and assayed for PGD2 as described in Materials and Methods. Both NA and aspirin were dissolved in water and neutralized with NaOH. (B) Perfusion in mouse ears was measured by LDPI as described in Materials and Methods. Male C57BL/6 mice (≈25-g body weight, n = 7–16) were pretreated by means of i.p. injection (0.2 ml) with increasing doses of aspirin. Aspirin was dissolved in water. NA was dissolved in 5% hydroxypropyl β-cyclodextrin. Stock solutions of aspirin and NA were neutralized with NaOH before use. All plotted values represent the mean ± SEM.
These results show that, in the mouse, NA increases PGD2 in plasma and induces cutaneous vasodilation in an aspirin-sensitive fashion. Thus, this mouse model appears to be a good representation of NA-induced flushing in humans.
Role of DP1 vs. DP2 in NA-Induced Vasodilation.
Pharmacological studies.
Studies in humans have shown that the plasma concentrations of PGD2, PGI2, and thromboxane A2 (TXA2) are elevated by NA treatment (16–20). Because the magnitude of the reported PGD2 increase far exceeds those reported for PGI2 and TXA2, we focused on the potential role that PGD2 might play in NA-induced vasodilation and examined whether one or both of the two PGD2 receptors, DP1 and DP2, might play a role in vasodilation through use of specific receptor agonists. Administration of BW245C, a high-affinity agonist of human and mouse DP1 that is inactive at DP2 (25, 26), caused a dose-dependent increase of perfusion in the mouse ear (data not shown). Although the vasodilatory effect induced by 2 and 4 mg/kg BW245C was less than that induced by the same dose of PGD2, these results are consistent with the binding affinities of PGD2 and BW245C at mouse DP1 (21 vs. 250 nM) reported in the literature (27). By contrast, DK-PGD2 (13,14-dihydro-15-keto-PGD2), which is a DP2-selective agonist in humans (25) and has a Ki value of 20 nM on mouse DP2 (26), had no effect on cutaneous vascular response at a dose of 2 mg/kg (data not shown). These data suggest that PGD2 causes cutaneous vasodilation in the mouse by acting through DP1, not DP2.
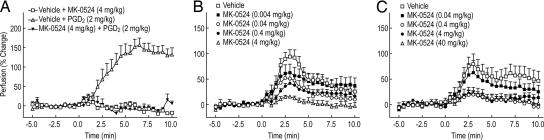
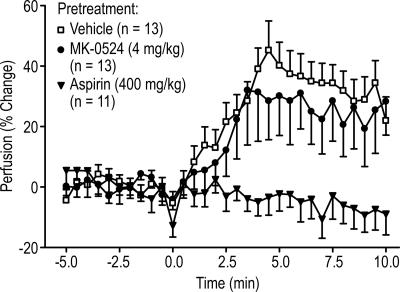
The above experiments suggest a model in mice where NA causes an increase in PGD2, which goes on to activate DP1 and induce vasodilation. To test this hypothesis, we evaluated the effect of MK-0524, a specific antagonist of DP1, on mouse ear vasodilation. As a positive control for DP1 antagonism by MK-0524, we determined that a 30-min preadministration of this compound at a dose of 4 mg/kg completely suppressed PGD2-induced vasodilation (Fig. 3A). MK-0524 also inhibited NA-induced vasodilation in a dose-dependent manner when given i.p. at doses from 0.004 mg/kg to 4 mg/kg 30 min before the administration of NA (Fig. 3B). Although suppression of vasodilation was nearly complete in the presence of the highest dose of MK-0524 (4 mg/kg), there was a small amount of residual NA-induced vasodilation despite a plasma concentration of ≈17 μM (data not shown), which is ≈15,500-fold higher than the IC50 value of MK-0524 in a mouse DP1-mediated cAMP inhibition assay. A similar result was obtained when MK-0524 was given orally; at a dose of 0.04 mg/kg 30 min before the injection of NA, vasodilation was inhibited by ≈30% (estimated by the area under the vasodilation curve from 0 to 10 min), and when higher MK-0524 doses were used (0.4, 4, and 40 mg/kg), an ≈80% reduction was obtained (Fig. 3C). A small amount (20%) of vasodilation was resistant to MK-0524, despite the fact that there was a dose-proportional increase of plasma MK-0524 concentration across the dose range (data not shown).
Fig. 3.
MK-0524, a DP1-selective antagonist, blocks PGD2− and NA-induced cutaneous vasodilation. Perfusion in the ears of male C57BL/6 mice (≈25-g body weight) was measured by LDPI as described in Materials and Methods. (A) Mice (n = 5–11) were pretreated by means of i.p. injection with vehicle or a 4-mg/kg dose of MK-0524 in 5% hydroxypropyl β-cyclodextrin 30 min before the s.c. injection of a 2-mg/kg dose of PGD2 in 5% DMSO at 0 min. (B) Mice (n = 6–16) were pretreated by means of i.p. injection with increasing doses of MK-0524 (0, 0.004, 0.04, 0.4, and 4 mg/kg) in 5% hydroxypropyl β-cyclodextrin 30 min before the s.c. injection of a 100-mg/kg dose of NA in the same vehicle at 0 min. (C) Mice (n = 8–14) were orally pretreated with increasing doses of MK-0524 (0, 0.04, 0.4, 4, and 40 mg/kg) in 0.5% methylcellulose 30 min before the s.c. injection of a 100-mg/kg dose of NA in 5% hydroxypropyl β-cyclodextrin at 0 min. All plotted values represent the mean ± SEM.
These pharmacological results obtained with DP1- and DP2-specific agonists and the DP1-specific antagonist MK-0524 suggest that DP1 is responsible for most, but not all, NA-induced vasodilation in the mouse.
Genetic studies.
To verify the interpretation that DP1, but not DP2, plays an important role in NA-induced vasodilation in the mouse, DP1 null and DP2 null mice were used. DP2−/− (null) and DP2+/+ (WT) (n = 14 each) vasodilated equivalently upon s.c. administration of 100 mg/kg NA (data not shown), confirming that DP2 is not required for NA-induced vasodilation.
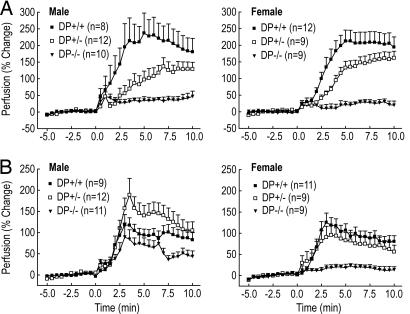
The vasodilatory responses of DP1−/−, DP1+/−, and DP1+/+ littermates to 2 mg/kg PGD2 given s.c. was proportional to DP1 gene dosage (DP+/+ > DP+/− > DP−/−) in both female and male mice, with DP−/− mice devoid of any response (Fig. 4A). By contrast, when DP1−/−, DP1+/−, and DP1+/+ littermates were challenged with 100 mg/kg NA, the vasodilatory responses were different between females and males. In female mice, there was a gene dosage-dependent effect on the average NA-induced vasodilatory response (Fig. 4B). In male mice, however, NA induced more robust average vasodilation in DP+/− than in DP+/+ mice. The complete absence of DP1 in DP1−/− mice resulted in reduced average NA-induced vasodilation, but the effect was significantly more than the almost complete absence of average vasodilation in female DP1−/− females. Although there was very little average NA-induced vasodilation in the female DP1−/− mice (15% of WT), there was NA-induced vasodilation (defined as maximal perfusion >50% above baseline) in some individual mice (3 of 16 in female vs. 14 of 20 in male DP1−/− mice). These findings suggest that DP1 is a key component for NA-induced vasodilation in mice, but that other components can contribute to the process, and that those components more frequently make a contribution in male than in female DP1−/− mice.
Fig. 4.
PGD2-mediated vasodilation depend strictly on DP1 expression in mice, whereas NA-induced vasodilation shows partial dependence on DP1. Vasodilation induced by either PGD2 or NA was measured in DP1+/+, DP1+/−, and DP1−/− mice by LDPI as described in Materials and Methods. At 0 min, mice were s.c.-injected with either 50 μg of PGD2 in 5% DMSO (A) or 2.5 mg of NA in 5% hydroxypropyl β-cyclodextrin (B). Plotted values represent the mean ± SEM.
Residual NA-Induced Vasodilation in Male DP−/− Mice Is Mediated via a COX-Dependent Pathway.
The pharmacological studies with the DP1 antagonist MK-0524 in C57BL/6 mice suggested that ≈20% of the NA-induced vasodilatory response was independent of DP1 (Fig. 3 B and C), and the presence of residual vasodilation in the male DP1−/− mice (Fig. 4B) allowed us to study this process. We therefore tested MK-0524 and aspirin for their effects on NA-induced vasodilation in male DP1−/− mice. MK-0524 was tested to determine whether it displayed DP1-independent effects (for example, by antagonizing a related prostanoid receptor), and aspirin was examined to determine whether the DP1-independent vasodilation was COX-dependent and therefore likely to be mediated by a prostanoid. As shown in Fig. 5, MK-0524 (4 mg/kg) had no effect on residual NA-induced vasodilation in male DP1−/− mice. By contrast, vasodilation was completely blocked by a 400-mg/kg dose of aspirin. These results suggest that NA-induced vasodilation in male DP1−/− mice is mediated via a COX-dependent pathway that cannot be antagonized by MK-0524.
Fig. 5.
NA-induced vasodilation in male DP1−/− mice is mediated via a COX-dependent pathway. Perfusion in the ears of male DP1−/− mice (≈25-g body weight) was measured by LDPI as described in Materials and Methods. Mice were pretreated by means of i.p. injection with vehicle (0.2 ml of 5% hydroxypropyl β-cyclodextrin), MK-0524 (0.5 mg in 0.2 ml per mouse), or aspirin (10 mg in 0.2 ml per mouse) 30 min before the s.c. injection of NA (2.5 mg in 0.2 ml per mouse) at 0 min. MK-0524, aspirin, and NA were dissolved in 5% hydroxypropyl β-cyclodextrin. Stock solutions of aspirin and NA were neutralized with NaOH before use. Plotted values represent the mean ± SEM.
MK-0524 Reduces Flushing Symptoms and Cutaneous Vasodilation in Healthy Subjects Administered NA.
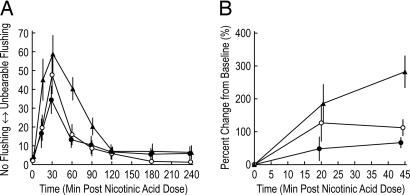
Flushing symptoms in healthy human subjects were measured before and after single 500-mg doses of immediate release (IR) NA given to subjects in a three-period randomized crossover fashion. In each of the periods, subjects received in a placebo-controlled fashion (see Materials and Methods), either with no MK-0524 or with preadministration or coadministration of MK-0524 with IR NA. Fig. 6A shows that flushing symptoms, as reported by the subjects on a visual analog scale, occur rapidly after an oral dose of IR NA. The intensity of flushing symptoms is reduced when 100 mg of MK-0524 is administered 60 min before the dose of IR NA. A comparison of the time-weighted average flushing score between the 60-min pretreatment with 100 mg of MK-0524 and placebo shows an average reduction of 68%; that is, the geometric mean ratio of the time-weighted average MK-0524/placebo scores is 0.32, with a corresponding 90% confidence interval of 0.18, 0.56.
Fig. 6.
MK-0524 reduces NA-induced flushing symptoms and malar skin perfusion in healthy men and women. Each subject received three different treatments in a crossover fashion: placebo to MK-0524 and IR NA (n = 12) (▴), 100 mg of MK-0524 given 60 min before IR NA (n = 12) (•), and 100 mg of MK-0524 given together with IR NA (n = 12) (○). (A) At predose and at specified time points after the dose of 500 mg of IR NA, subjects reported the intensity of flushing symptoms on a visual analog scale. Mean scores (±SEM) are plotted. (B) At predose and 20 and 45 min after the same dose of 500 mg of IR NA, LDPI was performed on a prespecified area on the malar skin, with the same treatments as in A: placebo to MK-0524 (n = 11) (▴), 100 mg of MK-0524 given 60 min before IR NA (n = 12) (•), and 100 mg of MK-0524 given together with IR NA (n = 11) (○). The time points for the LDPI measurements were selected so that they would not interfere with the recording of symptom scores by questionnaire. Mean percentage changes (±SEM) from baseline (defined as predose value) are plotted over time. Note the different time axes in A and B.
LDPI was used to monitor changes in blood flow in the malar skin (Fig. 6B). At 45 min after the administration of IR NA (with no MK-0524 preadministration or coadministration), perfusion increased by 269%. When 100 mg of MK-0524 was given 60 min before IR NA, perfusion was increased to a lesser degree (by 64% from baseline), which corresponds to an ≈76% smaller increase in perfusion compared with placebo to MK-0524. Coadministration of MK-0524 with IR NA was not as effective as preadministration at suppressing vasodilation. No significant differences in flushing symptoms or skin perfusion were observed between the eight men and four women in this study (data not shown).
Discussion
NA-induced flush-like responses have previously been reported in guinea pigs (28, 29) and rats (30). In these studies, the responses were NA dose-dependent, lasted for ≈1 h, and were assessed either by visual examination of the ear redness (29) or by temperature (28, 30). As in humans, these effects in animals were reduced by pretreatment with aspirin or indomethacin (28, 30). In this study, we have established a murine model of NA-induced vasodilation, using LDPI to monitor cutaneous blood flow in the ear. The measurements were straightforward to perform, and the effects were large and reproducible.
We validated the mouse NA-induced vasodilation model by demonstrating that it showed the hallmarks of NA-induced flushing in humans. Specifically, the onset of NA-induced vasodilation occurred within minutes, the intensity was dose-dependent, and it could be inhibited by aspirin (9–13). Like NA in humans, vasodilation in the mouse model occurred at doses that reduce plasma free fatty acids (data not shown), a pharmacodynamic effect of NA that is mediated by its action in adipose tissue (31). These results demonstrate that the mouse can serve as a suitable model for NA-induced flushing.
Several PGs, specifically PGD2 (32, 33), PGI2 (21), and PGE2 (by means of the EP2 and EP4 receptors) (34, 35), can elicit vasodilatory effects. Morrow et al. (17) showed that, within a few minutes of ingestion of NA, circulating PGD2 and its metabolite, 9α,11β-PGF2, were increased several hundred-fold in normal humans. Much larger increases were observed in the superficial venous drainage from a region of skin on which NA had been applied than in arterial blood supplying the skin. In another study by the same group (16), urinary excretion of 2,3-dinor-6-keto PGF1α (a PGI2 metabolite) and PGE-M (a PGE2 metabolite) were increased <2-fold after oral ingestion of 500 mg of NA. These results suggest that PGD2 produced by the skin may be the primary mediator for NA-induced flush in humans. PGD2 is vasodilatory in the mouse, as evidenced by the fact that s.c. injection of PGD2 induced vasodilation in the mouse ear in a time- and dose-dependent manner. Finally, both the NA-stimulated increase in plasma PGD2 and attendant vasodilation were blocked by pretreatment with high doses of aspirin. These results support the hypothesis that NA-induced release of PGD2 contributes significantly to vasodilation.
Two receptors for PGD2, DP1 (22) and DP2 (23, 24), have been identified; therefore, we examined their potential roles in NA-induced vasodilation. Results from studies with BW245C (a DP1-selective agonist) and 13,14-dihydro-15-keto-PGD2 (a DP2-selective agonist) indicate that activation of DP1, but not DP2, is sufficient to produce a vasodilatory response in the mouse ear much like that produced by NA.
The involvement of DP1 in NA-induced vasodilation in mice was further demonstrated by the ability of MK-0524, a DP1-selective antagonist, to suppress this process. MK-0524 has an IC50 value of 1.1 nM in a mouse DP1 functional assay (C.S., G.O., N. Lachance, M. Boyd, C. Berthelette, M. Labelle, L. Li, B. Roy, J. Scheigetz, N. Tsou, et al., unpublished data). Interestingly, despite the fact that sufficient amounts of MK-0524 were present in the circulation to virtually fully antagonize DP1, NA-induced vasodilation was only partially blocked (≈80%), whereas PGD2-induced vasodilation in the mouse was completely blocked. These findings suggest that DP1 is responsible for most, but not all, NA-induced vasodilation in C57BL/6 mice.
To further test the interpretations drawn from the pharmacological experiments, NA-induced vasodilation in DP1 and DP2 null mice on a C57BL/6 background was examined. As expected, NA induced a robust vasodilatory effect in DP2 null mice, and the effect was indistinguishable from that observed with WT mice, confirming that DP2 does not play a role in NA-induced vasodilation. The vasodilatory response to administration of PGD2 to male or female DP1-deficient mice and their WT and heterozygous littermates was also as expected; DP1+/+, DP1+/−, and DP1−/− mice had robust, intermediate, and no vasodilation, respectively. Thus, PGD2-induced vasodilation under our experimental conditions depends completely on DP1. However, when DP1+/+, DP1+/−, and DP1−/− mice were treated with NA, the vasodilatory responses were quite different between females and males. In female DP1+/+, DP1+/−, and DP1−/− mice, NA again induced robust, intermediate, and almost no vasodilation, respectively (only 3 of 16 DP1−/− female mice treated with NA had a vasodilatory response). By contrast, whereas male DP1+/+ mice responded to NA equivalently to females, male DP1+/− had more (rather than less) vasodilation than their DP1+/+ male littermates, and male DP1−/− mice showed a lower, but still substantial, level of NA-induced vasodilation (14 of 20 mice) relative to the male DP1+/+ mice. Thus, there is a significant sexual dimorphism with respect to the ability of NA to induce vasodilation in DP1-deficient mice. The reasons for this behavior are not understood at this time. One speculative explanation might be developmental up-regulation of compensatory mechanisms in male, but less frequently in female, DP1−/+ and DP1−/− mice. The NA-induced DP1-independent vasodilation that can be readily detected in male DP1−/− mice was not suppressed by high levels of MK-0524, suggesting that the ability of this DP1 antagonist to suppress ≈80% of the vasodilation response in WT mice was exclusively due to its effect on DP1. By contrast, the NA-induced DP1-independent vasodilation was suppressed by aspirin, suggesting that it is mediated by a prostanoid (or prostanoids).
Our findings should be interpreted in light of the recent independent work by Benyo et al. (36). These researchers studied NA-induced vasodilation in several mouse strains individually lacking a number of prostanoid receptors that could potentially mediate a vasodilatory response: specifically, DP1−/−, IP−/− (lacking the PGI2 receptor), and EP2−/− and EP4−/− (lacking two of the four known PGE2 receptors). They concluded that NA-induced vasodilation is mediated both by PGD2 acting through DP1 and PGE2 functioning through EP2 and EP4. By contrast, our pharmacological work and our genetic studies in the female DP1−/− mice support a predominant, although not exclusive, role for PGD2 acting through DP1. We speculate that loss of one prostanoid receptor (for example, by genetic ablation) may lead to compensatory changes in another system, and that this compensation may be more common in one sex than another. Thus, application of both genetic and pharmacological studies, as well as pharmacological studies in unique genetic backgrounds, may be required to fully explore the interplay of a number of prostanoids and prostanoid receptors. Specifically, further study with double mutants or use of EP2 and EP4 receptor antagonists in DP1−/− knockout mice may further elucidate the full host of prostanoid/prostanoid receptor systems required for NA-induced vasodilation in the mouse.
The finding that DP1 is a dominant mediator of NA-induced vasodilation in WT mice suggests that DP1 antagonists, such as MK-0524, might find clinical utility in the suppression of NA-induced vasodilation in humans. To address this possibility, a placebo-controlled double-blind crossover study was conducted in healthy human subjects to examine the effects of MK-0524 on flushing symptoms and malar skin perfusion. MK-0524 reduced flushing symptoms and the increase in malar skin perfusion in both males and females when administered before a challenge with IR NA.
Taken together, theses findings support the utility of DP1 receptor antagonists for ameliorating the most common side effect of NA, flushing. Improvement of the tolerability of NA would likely lead to enhanced compliance, thereby allowing more patients to reap the demonstrated cardiovascular benefits of this underutilized drug.
Materials and Methods
Chemicals.
NA, PGD2, and aspirin were obtained from Sigma; BW245C and DK-PGD2 (13,14-dihydro-15-keto-PGD2) were from Cayman Chemical (Ann Arbor, MI); Nembutal sodium solution (50 mg/ml) was from Abbott; hydroxypropyl-β-cyclodextrin was from Cargill (Minneapolis); and dimethyl sulfoxide was from Fisher Scientific. MK-0524 (a DP1-selective antagonist) (C.S., G.O., N. Lachance, M. Boyd, C. Berthelette, M. Labelle, L. Li, B. Roy, J. Scheigetz, N. Tsou, et al., unpublished work) was synthesized at Merck Frosst Canada.
Animals.
Male C57BL/6 mice (14 weeks old) were purchased from Taconic Farms. DP1+/− mice on a C57BL/6–129P2 strain background, originally obtained from Deltagen (San Carlos, CA), were rederived and backcrossed five generations to C57BL/6J at The Jackson Laboratory with marker-assisted backcrossing techniques to yield mice with ≈99% of the chromosomal markers derived from C57BL/6J and with the balance derived from 129P2 and linked to the DP1 locus. Male and female DP1−/−, DP1+/−, and DP1+/+ littermates were generated by The Jackson Laboratory. DP2+/− mice, originally obtained from Deltagen, were rederived and backcrossed to the C57BL/6 strain for six generations at Taconic Farms. Male DP2−/− mice, offspring of homozygous × homozygous breeding, were used in this study. All animals were housed four mice per cage with a 12-h light/12-h dark cycle and had free access to regular chow (TD7012; Harlan Teklad, Madison, WI) and water. All animal experiments were performed in accordance with the Guidelines for Institutional Animal Care and Use Committee at Merck Research Laboratories.
Mouse Ear Vasodilation Measurement by LDPI.
Mice used in vasodilation experiments were at least 15 weeks of age (≈25 g) and were anesthetized with Nembutal (40 mg/kg in 0.3 ml) by means of i.p. injection. Thirty minutes later, each mouse was placed under a LDPI scanner (PeriScan PIM II; Perimed, Stockholm) with its right ear everted to expose the ventral side. Blood flow in an ≈5 × 5-mm section of ear was scanned every 30 s for 5 min to establish the baseline. After the mouse was injected s.c. with vehicle or drug to initiate treatment, the ear was then scanned for another 10 min. When there was a pretreatment, vehicle or the test compound was given orally or i.p. immediately after the injection of Nembutal (≈30 min before treatment). The vehicles used in the study are indicated in the figure legends. The perfusion value at each time point was used to calculate the data. For each animal, the average of the perfusion values of the first 11 scans before the injection of vehicle or drug was used as baseline, and perfusion values of all scans, including −5 to 0 min, were then divided by this average value to yield percentage change relative to the baseline.
Determination of Plasma PGD2 Levels.
Male C57BL/6 mice (≈25 g, n = 8) were pretreated by i.p. administration of vehicle or a 200-mg/kg dose of aspirin. Thirty minutes later, mice were treated orally with vehicle or 100 mg/kg NA. Five minutes later, mice were killed with CO2, and blood was drawn from the vena cava by using a syringe with a 1-inch 25-gauge needle that had been rinsed with 1 M EDTA. Blood samples were kept on ice until completion of the experiment and then were centrifuged at 10,300 × g for 5 min at 4°C. Plasma samples were removed and assayed for PGD2 by using a RIA kit from Amersham Pharmacia Biosciences (TRK890); we followed manufacturer’s suggested procedures except that the final samples were decolored with 10 μl of 30% H2O2 before being counted in a scintillation counter.
Measurement of NA-Induced Flushing Symptoms in Healthy Subjects.
Twelve healthy male (n = 8) and female (n = 4) subjects, 18–45 years of age, with a flushing reaction to NA were randomized to receive the following three treatments in a crossover fashion: (i) treatment A, a single oral dose of placebo to match MK-0524, 60 min before coadministration of 500 mg of NA and a single oral dose of a placebo to match MK-0524; (ii) treatment B, a single oral dose of 100 mg of MK-0524, 60 min before coadministration of 500 mg of NA and a single oral dose of a placebo to match MK-0524; and (iii) treatment C, a single oral dose of placebo to match MK-0524, 60 min before coadministration of 500 mg of NA and a single oral dose of 100 mg of MK-0524. There was a minimum interval of 7 days between treatments. Subject reports of flushing on a 100-mm visual analog scale were obtained −60, 0, 15, 30, 60, 90, 120, 180, and 240 min after administration of NA, as described in ref. 13. Measurements of cutaneous blood flow in the malar skin were also obtained in these subjects by using LDPI at 0, 20, and 45 min after the dose of NA.
Acknowledgments
We thank Tami Crumley, Inge DeLepeleire, Andrea Maes, Larissa Wenning, and Eva Vets for their contributions to the clinical study; Francois Gervais (Merck Frosst Canada) for providing DP2 mice; Stefan Offermanns for communicating unpublished results; and John Paolini and Patricia Detmers for their comments on the manuscript.
Abbreviations
- COX
cyclooxygenase
- PG
prostaglandin
- DP1
PGD2 receptor 1
- DP2
PGD2 receptor 2
- LDPI
laser Doppler perfusion imaging
- NA
nicotinic acid
- IR
immediate release.
Footnotes
Conflict of interest statement: The communicating member, P.S.K., is an employee and officer of Merck & Co., Inc.
References
- 1.Altschul R., Hoffer A., Stephen J. D. Arch. Biochem. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 2.Carlson L. A. J. Int. Med. 2005;258:84–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 4.Kamanna V. S., Kashyap M. L. Curr. Atheroscler. Rep. 2000;2:36–46. doi: 10.1007/s11883-000-0093-1. [DOI] [PubMed] [Google Scholar]
- 5.The Coronary Drug Project Research Group. J. Am. Med. Assoc. 1975;231:360–381. [Google Scholar]
- 6.Brown B. G., Zhao X., Chait A., Fisher L. D., Cheung M. C., Morse J. S., Dowdy A. A., Marino E. K., Bolson E. L., Alaupovic P., et al. N. Engl. J. Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 7.Taylor A. J., Sullenberger L. E., Lee H. J., Lee J. K., Grace K. A. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 8.Knoop R. H., Ginsberg J., Albers J. J., Hoff C., Ogilvie J. T., Warnick. G. R., Burrows E., Retzlaff B., Poole M. Metabolism. 1985;34:642–650. doi: 10.1016/0026-0495(85)90092-7. [DOI] [PubMed] [Google Scholar]
- 9.Svedmyr N., Heggelund A., Aberg G. Acta Pharmacol. Toxicol. 1977;41:397–400. doi: 10.1111/j.1600-0773.1977.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 10.Phillips W. S., Lightman S. L. Lancet. 1981;1:754–756. doi: 10.1016/s0140-6736(81)92627-1. [DOI] [PubMed] [Google Scholar]
- 11.Wilkin J. K., Fortner G., Reinhardt L. A., Flowers O. V., Kilpatrick S. J., Streeter W. C. Clin. Pharmacol. Ther. 1985;38:273–277. doi: 10.1038/clpt.1985.170. [DOI] [PubMed] [Google Scholar]
- 12.Whelan A. M., Price S. O., Fowler S. F., Hainer B. L. J. Fam. Pract. 1992;34:165–168. [PubMed] [Google Scholar]
- 13.Jungnickel P. W., Maloley P. A., Vander Tuin E. L., Peddicord T. E., Campbell J. R. J. Gen. Intern. Med. 1997;12:591–596. doi: 10.1046/j.1525-1497.1997.07118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaijser L., Eklund B., Olsson A. G., Carlson L. Med. Biol. 1979;57:114–117. [PubMed] [Google Scholar]
- 15.Dunn R. T., Ford M. A., Rindone J. P., Kwiecinski F. A. Am. J. Ther. 1995;2:478–480. doi: 10.1097/00045391-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Morrow J. D., Parsons W. G., 3rd, Roberts L. J., 2nd Prostagladins. 1989;38:263–274. doi: 10.1016/0090-6980(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 17.Morrow J. D., Awad J. A., Oates J. A., Roberts L. J., II J. Invest. Dermatol. 1992;98:812–815. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- 18.Olsson A. G., Carlson L. A., Anggard E., Ciabattoni G. Lancet. 1983;322:565–566. doi: 10.1016/s0140-6736(83)90588-3. [DOI] [PubMed] [Google Scholar]
- 19.Nozaki S., Kihara S., Kubo M., Kameda K., Matsuzawa Y., Tarui S. Int. J. Clin. Pharmacol. Ther. Toxicol. 1987;25:643–647. [PubMed] [Google Scholar]
- 20.Saareks V., Ylitalo P., Mucha I., Riutta A. Pharmacol. Toxicol. 2002;90:338–342. doi: 10.1034/j.1600-0773.2002.900608.x. [DOI] [PubMed] [Google Scholar]
- 21.Oliva D., Nicosia S. Pharmacol. Res. Commun. 1987;19:735–765. doi: 10.1016/0031-6989(87)90010-5. [DOI] [PubMed] [Google Scholar]
- 22.Hirata M., Kakizuka A., Aizawa M., Ushikubi F., Narumiya S. Proc. Natl. Acad. Sci. USA. 1994;91:11192–11196. doi: 10.1073/pnas.91.23.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. J. Exp. Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monneret G., Gravel S., Diamond M., Rokach J., Powell W. S. Blood. 2001;98:1942–1948. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 25.Sawyer N., Cauchon E., Chateauneuf A., Cruz R. P., Nicholson D. W., Metters K. M., O’Neill G. P., Gervais F. G. Br. J. Pharmacol. 2002;137:1163–1172. doi: 10.1038/sj.bjp.0704973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hata A. N., Zent R., Breyer M. D., Breyer R. M. J. Pharmacol. Exp. Ther. 2003;306:463–470. doi: 10.1124/jpet.103.050955. [DOI] [PubMed] [Google Scholar]
- 27.Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., Narumiya S. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson R. G., Aberg G., Brattsand R., Ericsson E., Lundholm L. Acta Pharmacol. Toxicol. 1977;41:1–10. doi: 10.1111/j.1600-0773.1977.tb02116.x. [DOI] [PubMed] [Google Scholar]
- 29.Subissi A., Bachi M., Brunori P. J. Pharm. Pharmacol. 1983;35:612–614. doi: 10.1111/j.2042-7158.1983.tb04349.x. [DOI] [PubMed] [Google Scholar]
- 30.Turenne S. D., Seeman M., Ross B. M. Schizophr. Res. 2001;50:191–197. doi: 10.1016/s0920-9964(00)00082-7. [DOI] [PubMed] [Google Scholar]
- 31.Carlson L. A. Acta Med. Scand. 1963;173:719–722. doi: 10.1111/j.0954-6820.1963.tb17457.x. [DOI] [PubMed] [Google Scholar]
- 32.Giles H., Leff P. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 33.Giles H., Leff P., Bolofo M. L., Kelly M. G., Robertson A. D. Br. J. Pharmacol. 1989;96:291–300. doi: 10.1111/j.1476-5381.1989.tb11816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negishi M., Sugimoto Y., Ichikawa A. Biochim. Biophys. Acta. 1995;1259:109–120. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- 35.Coleman R. A., Grix S. P., Head S. A., Louttit J. B., Mallett A., Sheldrick R. L. G. Prostaglandins. 1994;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 36.Benyo Z., Gille A., Kero J., Csiky M., Suchankova M. C., Nusing R., Moers A., Pfeffer K., Offermanns S. J. Clin. Invest. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]