Abstract
Bacterial cytokinesis requires the coordinated assembly of a complex of proteins, collectively known as the divisome, at the incipient division site. DivIB/FtsQ is a conserved component of the divisome in bacteria with cell walls, suggesting that it plays a role in synthesis and/or remodeling of septal peptidoglycan. We demonstrate that the extracytoplasmic region of DivIB comprises three discrete domains that we designate α, β, and γ from the N to C terminus. The α-domain is proximal to the cytoplasmic membrane and coincident with the polypeptide transport-associated domain that was proposed previously to function as a molecular chaperone. The β-domain has a unique 3D fold, with no eukaryotic counterpart, and we show that it interconverts between two discrete conformations via cis–trans isomerization of a Tyr–Pro peptide bond. We propose that this isomerization might modulate protein–protein interactions of the flanking α- and γ-domains. The C-terminal γ-domain is unstructured in the absence of other divisomal proteins, but we show that it is critical for DivIB function.
Keywords: bacterial cytokinesis, divisome, NMR, protein structure
Bacterial cytokinesis requires spatiotemporal coordination of the assembly of a complex of cell division proteins, collectively known as the divisome, at the incipient division site (1, 2). Divisome assembly is initiated by the formation of a circumferential ring of FtsZ (the Z ring) around the inner surface of the cytoplasmic membrane (3, 4). FtsZ, the precursor of eukaryotic tubulin, is a GTPase that most likely provides at least part of the contractile force for septal invagination through dynamic remodeling of the Z ring (5–7). In addition, the Z ring promotes the switch from lateral to preseptal murein synthesis (8), and it provides a scaffold for recruitment of downstream divisomal proteins.
In Escherichia coli the known divisomal proteins are thought to be recruited to the division site in the following hierarchical order: FtsZ←ZipA,FtsA←FtsEX←FtsK←FtsQ←(FtsL, FtsB)←FtsW←FtsI ←FtsN←AmiC, where the arrow indicates that recruitment of a particular protein requires prior assembly of all previously listed components, and parentheses enclose proteins whose recruitment to the divisome is interdependent (2, 9, 10). However, recent studies suggest that some subcomplexes, such as that formed among FtsQ, FtsL, and FtsB, might be preassembled before Z ring formation and trafficked to the developing division site as a preformed complex (2, 11). This latter situation is likely more akin to that in Bacillus subtilis, where the observed interdependent localization of most divisomal proteins argues for a more concerted recruitment to the division site (1, 12).
Whereas the earliest recruits to the division site are either wholly or mostly cytoplasmic (e.g., FtsZ, FtsA, and FtsK), the later recruits are typically bitopic membrane proteins with a short N-terminal cytoplasmic region, a single transmembrane segment, and a larger extracytoplasmic (ec) domain (i.e., FtsQ, FtsL, FtsB, FtsI, and FtsN). Remarkably, the function of these latter proteins has proved enigmatic, with the exception of FtsI, a DD-transpeptidase that incorporates peptide crosslinks into septal murein (13). Thus, a major unrealized goal is determination of the structure and function of each divisomal protein and how they interact and cooperate to form a functional division apparatus. To date, FtsN and ZipA are the only membrane-anchored divisomal proteins for which structural information is available (14–16).
FtsQ is arguably the most enigmatic divisomal protein. First discovered 25 years ago, its role in bacterial cell division remains unclear. It has been proposed that FtsQ provides a structural link between early- and late-assembling divisomal components (17), and recent evidence suggests that its B. subtilis ortholog DivIB might additionally play a role in linking chromosome segregation to asymmetric cell division during sporulation (18). However, the lack of structural information for DivIB or FtsQ has hindered progress in ascertaining their precise role in cell division and the nature of their interactions with other divisomal proteins. In this study we reveal the domain architecture of DivIB and show that the central ec domain has a unique and dynamic 3D fold that might mediate the divisomal interactions of flanking domains.
Results
Domain Architecture of DivIB.
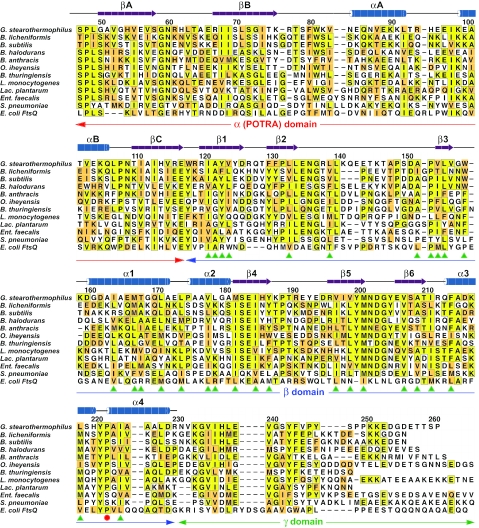
DivIB is a bitopic membrane protein composed of a small N-terminal cytoplasmic region, a single transmembrane segment, and a large ec domain. We focused our studies on the ec domain because the cytoplasmic and transmembrane regions are dispensable for both the cell division function(s) and septal localization of DivIB and FtsQ (19–21). Because thermophilic proteins are often better for structural studies than their mesophilic counterparts, we examined the domain architecture of DivIB from the Gram-positive thermophile Geobacillus stearothermophilus. The amino acid sequence of the ec region of G. stearothermophilus DivIB (residues 47–261; Gste-ecDivIB) is 39% identical to the corresponding region of B. subtilis DivIB (residues 54–263; Bsub-ecDivIB); these regions each have ≈19% sequence identity with the periplasmic region of E. coli FtsQ (Fig. 1).
Fig. 1.
Alignment of the primary structure of Gste-ecDivIB with the ec regions of DivIB from other Gram-positive bacteria as well as the periplasmic region of E. coli FtsQ. The numbering refers to Gste-ecDivIB. The secondary structure of the α (POTRA) domain is a prediction based on multiple sequence alignments (28), whereas the secondary structure of the β-domain was experimentally determined in this study. Sequences were aligned by using clustalw (49) and then manually adjusted to remove gaps in secondary structure elements. Identities are boxed in yellow, and conservative substitutions are shaded orange. The proline that undergoes cis–trans isomerization is marked with a red circle. Buried hydrophobic residues in the Gste-DivIBβ structure are highlighted with green arrowheads. The genus abbreviations refer to Geobacillus (G), Bacillus (B), Oceanobacillus (O), Listeria (L), Lactobacillus (Lac.), Enterococcus (Ent.), Streptococcus (S), and Escherichia (E).
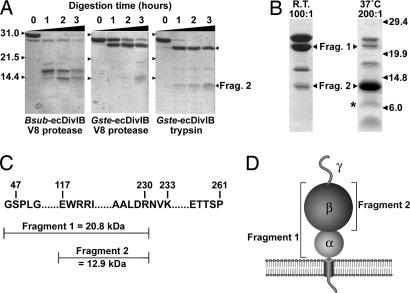
To determine whether the ec region of DivIB contains autonomous subdomains, a 100-fold excess of Gste-ecDivIB and Bsub-ecDivIB was incubated with trypsin or V8 protease, and the progress of the proteolytic reactions was monitored by using SDS/PAGE (Fig. 2A). Although Gste-ecDivIB and Bsub-ecDivIB migrate with apparent masses of ≈30 kDa, the recombinant proteins were confirmed to have the correct mass (24.1 and 23.9 kDa, respectively) by using electrospray mass spectrometry. The anomalous migration behavior of Bsub-ecDivIB was noted previously (22). In all three time courses shown in Fig. 2A, there was rapid appearance of a fragment ≈2–3 kDa smaller than the intact recombinant ecDivIB. This fragment was evident in the Bsub-ecDivIB sample even before protease addition (Fig. 2Ai, lane 1), suggesting that the N and/or C terminus of ecDivIB is highly susceptible to proteolysis. At longer incubation times, proteolysis of both Gste-ecDivIB (Fig. 2A ii and iii) and Bsub-ecDivIB (Fig. 2Ai) produced a fragment with an apparent mass of ≈13–14 kDa. The proteolysis profiles for Gste-ecDivIB and Bsub-ecDivIB were sufficiently similar to indicate they have a similar domain architecture, and consequently we focused on Gste-ecDivIB in subsequent experiments designed to identify the major proteolytic fragments.
Fig. 2.
Domain architecture of DivIB. (A) Coomassie-stained SDS/PAGE gels illustrating proteolysis of Gste-ecDivIB and Bsub-ecDivIB by trypsin or V8 protease at ambient temperature and a DivIB:protease ratio of 100:1. The mass in kDa and running position of the molecular mass standards are indicated to the left of the gels. (B) Coomassie-stained SDS/PAGE gels showing the time course of trypsin proteolysis of Gste-ecDivIB by using the indicated temperatures and DivIB:trypsin ratios. Arrows highlight the two stable fragments that were sequenced by Edman degradation. (C) Summary of the identity of the two stable proteolytic fragments of Gste-ecDivIB as determined by N-terminal sequencing and mass spectrometry. (D) Cartoon of the domain architecture of DivIB showing the two fragments that were resistant to trypsin proteolysis.
Fig. 2B shows the fragments obtained when Gste-ecDivIB was proteolyzed with trypsin for 3 h by using a DivIB:protease ratio of 100:1 at ambient temperature (fragment 1, Fig. 2B Left) or by using a DivIB:protease ratio of 200:1 at 37°C (fragment 2, Fig. 2B Right). Five cycles of N-terminal sequencing indicated that fragment 1 had an intact N terminus (i.e., G47SPLG), whereas the N terminus of fragment 2 (E117WRRI) corresponded to a trypsin cleavage after Arg-116 (Fig. 2C). Mass spectrometry indicated that the C terminus of both fragments was Arg-230.
These results indicate that the ec region of DivIB comprises three discrete domains that we designate α (residues 47–116, 8.0 kDa), β (residues 117–230, 13.2 kDa), and γ (residues 231–261, 3.0 kDa) from the membrane-proximal N terminus to the C terminus (Fig. 2D). Fragment 1 corresponds to α+β, and fragment 2 corresponds to the β-domain. The persistence of β for several hours during trypsin proteolysis suggests that it is structurally autonomous. In contrast, the rapid loss of the γ-domain during trypsin proteolysis and our failure to visualize a 3-kDa fragment corresponding to the size of γ on Tris–tricine SDS/PAGE gels (23) suggest that this domain is unstructured in the absence of other divisomal proteins. A weak band similar in size to that predicted for α is marked with an asterisk in Fig. 2B, but we could not obtain sufficient quantities for mass spectrometry and N-terminal sequencing. Nevertheless, the persistence of the α+β fragment under various proteolysis conditions (Fig. 2) suggests that α is properly folded when placed in context with β. Thus, it seems unlikely that the α-domain requires additional divisomal proteins to stabilize its fold.
3D Structure of DivIB.
The limited solubility of Gste-ecDivIB and Gste-DivIBαβ (residues 47–233) precluded NMR structural studies. This insolubility was not due to oligomerization of DivIB because multiangle laser scattering measurements revealed that Gste-ecDivIB, Gste-DivIBαβ, and Gste-DivIBβ (residues 117–233) are all monomeric at a concentration of ≈50 μM (see Fig. 6, which is published as supporting information on the PNAS web site). These results contrast with the suggestion from bacterial two-hybrid studies that FtsQ self-associates (17, 24). Although it is possible that the transmembrane domain of DivIB promotes self-association in vivo, such a self-association is unlikely to be critical for DivIB function because this region can be replaced with heterologous transmembrane domains without affecting DivIB function (21).
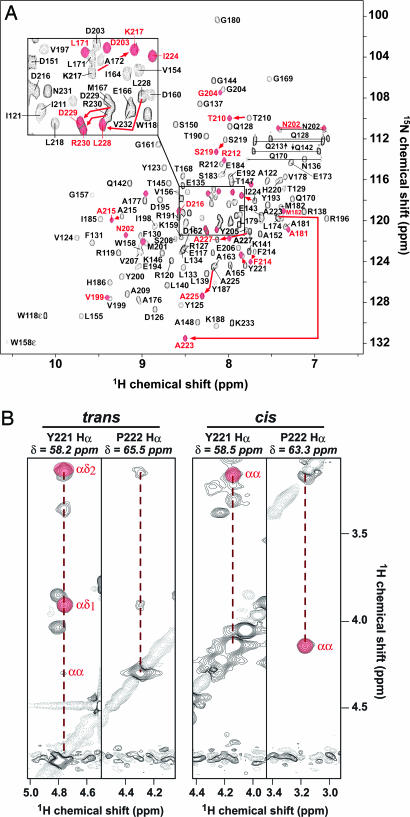
In contrast to Gste-ecDivIB and Gste-DivIBα β, Gste-DivIBβ was soluble at concentrations up to at least 1 mM. Curiously, however, the 2D HSQC spectrum of uniformly 15N-labeled DivIBβ contained more peaks than expected on the basis of its primary structure (Fig. 3A). Careful elimination of proteolytic degradation as a source of the extra NMR peaks in combination with detailed analysis of 3D NOESY spectra (Fig. 3B) revealed that the additional peaks are due to cis–trans isomerization of the Tyr-221–Pro-222 peptide bond located near the C terminus of Gste-DivIBβ.
Fig. 3.
Cis–trans isomerization of Gste-DivIBβ. (A) 2D 1H-15N HSQC spectrum of Gste-DivIBβ. Black peaks correspond to the trans conformer. Red peaks correspond to residues that have significant 1H chemical-shift differences (Δδ ≥ 0.02 ppm) in the cis conformer. Arrows highlight some of the largest chemical-shift differences. (B) Strips from a 13C-edited 3D NOESY-HSQC spectrum of Gste-DivIBβ showing NOEs for the two sets of Hα protons observed for residues Y221 and P222. The 13C chemical shift for each residue is given at the top of each strip. One set of strips shows strong reciprocal Hα-Hα NOEs between these residues, indicative of a cis Tyr–Pro peptide bond (Right). These cross peaks are absent from the alternate strips (Left), which instead show strong NOEs between the Hδ protons of P222 and the Hα proton of Y221, indicative of a trans Tyr–Pro peptide bond.
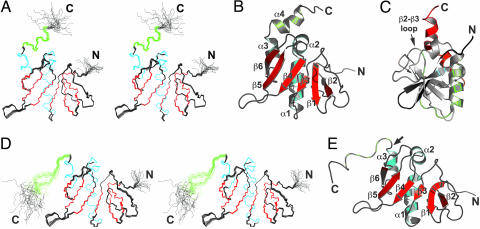
The solution structures of the trans and cis conformers of Gste-DivIBβ are shown in Fig. 4A–C and D and E, respectively, and structural statistics are given in Table 1. As far as we are aware, this is the largest polypeptide subject to prolyl peptide cis–trans isomerization for which the structure of both conformers has been determined by using NMR.
Fig. 4.
Structure of Gste-DivIBβ. (A) Stereoview of the ensemble of 25 Gste-DivIBβ trans conformers overlaid for lowest rms deviation over the backbone atoms of residues 118–229 of the mean coordinate structure. α-Helices and β-strands are colored cyan and red, respectively, except for the C-terminal α-helix, which is green. The structurally disordered N and C termini are labeled. (B) Richardson schematic of the Gste-DivIBβ trans conformer. α-Helices and β-strands are labeled and colored as in A. An arrow indicates the location of the Y221–P222 peptide bond. (C) An alternative view of the Gste-DivIBβ trans conformer with color ramped from dark blue at the N terminus to red at the C terminus. The β-sheet is highly twisted, with the N-terminal strand (β1, blue) and C-terminal strand (β6, orange) oriented almost orthogonally. (D) Stereoview of the ensemble of 25 Gste-DivIBβ cis conformers overlaid for lowest rms deviation over the backbone atoms of residues 118–224 of the mean coordinate structure. α-Helices and β-strands are colored cyan and red, respectively. The region corresponding to the C-terminal helix α4 in the trans conformer is colored green. (E) Richardson schematic of the Gste-DivIBβ cis conformer, with α-helices and β-strands colored as in D. An arrow indicates the location of the Y221–P222 peptide bond. Structures were drawn by using molmol (50) and pymol (51).
Table 1.
Structural statistics for the ensembles of cis and trans Gste-DivIB structures
| trans (1YR1) | cis (2ALJ) | |
|---|---|---|
| Experimental restraints* | ||
| Interproton distance restraints | ||
| Intraresidue | 363 | 324 |
| Sequential | 618 | 556 |
| Medium range (i–j < 5) | 632 | 579 |
| Long range (i–j ≥ 5) | 1,133 | 1,085 |
| Hydrogen-bond restraints† | 88 | 88 |
| Dihedral angle restraints | 200 | 197 |
| Restraints per residue | 25.5 | 23.8 |
| Mean rms deviations from experimental restraints‡ | ||
| NOE distances, Å | 0.01509 ± 0.00027 | 0.01278 ± 0.00034 |
| Dihedral angles, ° | 0.277 ± 0.015 | 0.340 ± 0.024 |
| Mean rms deviations from idealized geometry§ | ||
| Bonds, Å | 0.00216 ± 0.00004 | 0.00214 ± 0.00003 |
| Angles, ° | 0.340 ± 0.005 | 0.336 ± 0.002 |
| Impropers, ° | 0.226 ± 0.004 | 0.202 ± 0.005 |
| Mean energies, kcal mol−1 | ||
| ENOE¶ | 31.3 ± 1.1 | 21.5 ± 1.2 |
| Ecdih¶ | 0.94 ± 0.10 | 1.39 ± 0.20 |
| Ebond | 8.79 ± 0.35 | 8.67 ± 0.27 |
| Eimproper | 7.75 ± 0.29 | 6.21 ± 0.32 |
| Eangle | 60.2 ± 1.6 | 58.7 ± 0.7 |
| Erepel | 41.8 ± 1.5 | 38.9 ± 1.4 |
| rms deviation to mean coordinate structure,‖ Å | ||
| Backbone atoms | 0.17 ± 0.03 | 0.18 ± 0.02 |
| All heavy atoms | 0.66 ± 0.04 | 0.64 ± 0.04 |
| Residues in most favored Ramachandran region, % | 85 | 80 |
*Only structurally relevant restraints, as defined by cyana, are included.
†Two restraints were used per hydrogen bond.
‡All statistics are given as mean ± SD.
§Idealized geometry is defined by the charmm force field in x-plor.
¶Final values of the square-well NOE and dihedral-angle potentials were calculated with force constants of 50 and 200 kcal·mol−1·Å−2, respectively.
‖rms deviation values were calculated over the well defined regions (residues 118–229 for the trans isomer and residues 118–223 for the cis isomer).
The structure of the trans isomer of Gste-DivIBβ, which represents ≈60% of the sample population under the chosen experimental conditions, comprises a six-stranded mixed β-sheet flanked on one side by three α-helices. A fourth helix (green in Fig. 4 A and B) is located C-terminal to the Y221–P222 isomerization site. The β-sheet is highly twisted, with the first and last pair of β-strands oriented almost orthogonally (Fig. 4C). The structure contains a rare left-handed crossover connection between parallel strands β3 and β4 (Fig. 4 A–C). This connection includes helices α1 and α2, which are positioned parallel and perpendicular to β4, respectively. More than 99% of connections between parallel strands in β–α–β motifs are right-handed. In rare instances where left-handed connections do occur, they are often functionally important, as in subtilisin and asparaginase (25), which suggests a possible functional role for the β3–α1–α2–β4 region of DivIBβ.
The structures of the cis and trans conformers of Gste-DivIBβ are very similar except for the C-terminal 11 residues; the mean structures can be superimposed over residues W118–L218 with a backbone rms deviation of 0.8 Å. However, the presence of the cis Y221–P222 peptide bond causes an abrupt reversal in the polypeptide chain direction centered around P222, and the C-terminal α4-helix found in the trans conformer is replaced by a more extended and less well defined structural element in the cis conformer (Fig. 4 D and E). The loss of hydrophobic interactions between residues in α2 and α4 results in a slight reorientation of α2 in the cis conformer (compare B and E in Fig. 4). In contrast with the trans conformer, the N and C termini are well separated and point in opposite directions.
The structural variation between the cis and trans conformers of Gste-DivIBβ is more dramatic than observed for other similarly sized proteins that undergo proline cis–trans isomerization, such as the Itk SH2 domain, the viral coat protein of bacteriophage MS2, and the TB domain of human fibrillin-1 (26). Moreover, in contrast with these proteins, the cis, rather than the trans, isomer presents the most extended, solvent-exposed conformation.
Conservation of the Unique 3D Fold of DivIB.
A search of the Protein Data Bank using dali (27) returned no meaningful structural homologs of Gste-DivIBβ. This result was not entirely surprising because blast searches with DivIB and FtsQ sequences consistently return orthologs of these proteins but no other closely related sequences. Thus, the DivIBβ structure appears to be the first example of this unusual mixed α/β architecture. The 30 buried hydrophobic residues that presumably direct this unique fold (see green arrowheads in Fig. 1) are well conserved in all DivIB/FtsQ sequences despite widely varying levels of overall sequence identity with Gste-DivIBβ. Thus, the unique 3D fold of the β-domain of Gste-DivIB is likely to be conserved in all DivIB/FtsQ proteins.
With the exception of ZapA, there are eukaryotic structural homologs of all bacterial cell division proteins whose structures have been determined, including tubulin (FtsZ), actin (FtsA), and the ubiquitous βαββαβ RNA-binding motif (ZipA and FtsN). DivIB is therefore the first example, to our knowledge, of a membrane-tethered divisomal protein for which there is not a structural counterpart in eukaryotes.
Discussion
The ec regions of DivIB and FtsQ are necessary and sufficient for their divisomal localization and cell division function (19–21), but before this study nothing was known about their molecular architecture. In this study, we demonstrated that the ec region of DivIB comprises three domains that we designated α, β, and γ from N to C terminus. The α- and β-domains are structurally autonomous whereas the γ-domain is proteolytically sensitive and presumably unstructured in the absence of other divisomal proteins. The entire domain architecture of DivIB can now be defined as shown in Fig. 1, and the architecture of FtsQ is expected to be very similar based on sequence homology with DivIB.
Equivalence of the α-Domain and the Polypeptide Transport-Associated (POTRA) Domain.
The α-domain that was experimentally defined in this study corresponds almost exactly to the POTRA domain that was predicted on the basis of bioinformatic analyses to be present in DivIB/FtsQ and in a class of β-barrel outer-membrane proteins involved in transport or assembly of polypeptides (28). This study therefore provides the first experimental evidence, to our knowledge, that the predicted POTRA sequence exists as an autonomously folded protein domain.
The POTRA domain is present in the Toc75 subunit of the chloroplast translocon (29), the Omp85 family of proteins that mediate assembly of outer-membrane proteins (30), and Serratia marcescens hemolysin IB-related proteins that reside in the outer-membrane of Gram-negative bacteria and mediate export of virulence factors (31). Despite their disparate functions, all these proteins are involved in transport or assembly of predominantly unfolded polypeptides. It was therefore proposed that the POTRA domain might function as a chaperone that specifically recognizes secretion- or assembly-competent forms of these polypeptides (28), analogous to the chaperone function of SicP in the bacterial type III secretion system (32).
Is the α-domain of DivIB a chaperone? It seems unlikely that α functions as an intramolecular chaperone for the intrinsically unstable γ-domain because α is insufficient to protect γ from rapid proteolytic degradation in vitro (Fig. 2). We propose instead that α serves as a chaperone for its cognate divisomal partner FtsL. DivIB forms a ternary complex with FtsL and DivIC (2, 11, 33). These latter two proteins are unstructured and intrinsically unstable in vitro in the absence of DivIB (12), and FtsL is rapidly degraded in a divIB-null strain at the nonpermissive temperature (34). Thus, the α-domain of DivIB might serve as a chaperone that stabilizes the unfolded or partially folded form of FtsL, thereby aiding its assembly into the divisome and protecting it from degradation.
Does Isomerization of DivIB Serve as a Molecular Switch?
Almost all reported mutant alleles of DivIB/FtsQ map to the proteolytically sensitive C-terminal γ-domain (22, 35). Consistent with these reports, we found that a B. subtilis divIB-null could be rescued at the nonpermissive temperature by ectopic expression of divIB but not divIBΔγ (Fig. 5A). Deletion of the C-terminal 29–30 residues of FtsQ, which corresponds to the bulk of the γ-domain, abrogates its interaction with all other divisomal proteins in bacterial two-hybrid assays (24), and the deletion mutant fails to recruit FtsL and other downstream divisomal components in vivo (35). Thus, γ is not essential for DivIB/FtsQ localization, but it appears to be the most critical domain for mediating the interaction of DivIB/FtsQ with other divisomal proteins. Surprisingly, but consistent with this hypothesis, the structure of the β-domain is largely devoid of conserved surface residues that might serve as protein interaction epitopes; there is only a single cluster of surface-exposed residues that are well conserved in Gram-positive bacteria (Fig. 5C).
Fig. 5.
Functional analysis of DivIB. (A) B. subtilis strains JH642 (WT), RSA8 (null), RSA9 (ΔdivIB::cat::ermC amyE::divIB-cat; divIB), and RSA29 (ΔdivIB::cat::ermC amyE::divIBΔγ-cat; divIBΔγ) were streaked on LB plates at permissive (30°C) and nonpermissive (48°C) temperatures for the null strain. (B) As for A except the two lower quarters of the plate show variants of RSA9 in which the ectopic copy of divIB contains either a Y224A or P225A mutation. (C) Molecular surface of the trans conformer of Gste-DivIBβ showing conserved solvent-exposed residues. (D) Cartoon illustrating how trans→cis isomerization of the DivIB β-domain might function as a molecular switch that regulates assembly of the DivIB–FtsL–DivIC complex. In this model, FtsL binding activates the switch to the cis conformation. Isomerization to the cis conformation would allow the γ-domain to interact with the C-terminal domains of FtsL and possibly DivIC, and it would permit the α-domain to function as a chaperone for the intrinsically unstable FtsL protein (red arrow).
The ec regions of FtsL and DivIC are predicted to comprise a membrane-proximal coiled-coil region of ≈35 residues followed by a 25- to 30-residue C-terminal region of unknown structure. As with DivIB, all reported mutant alleles of FtsL and DivIC map to the C-terminal region (36, 37). In striking contrast, the coiled-coil domain of B. subtilis FtsL can be extensively mutagenized and replaced with heterologous coiled coils with <35% sequence identity without affecting cell division (37).
Taken together, these data suggest that the interaction among FtsL, DivIC, and DivIB is most likely mediated by their C-terminal regions. Such an interaction would be difficult if the β-domain of DivIB adopted the trans conformation. In this conformation, the N and C termini of the β-domain are in close proximity, and the γ-domain is directed toward the membrane (Fig. 5D Left). The structural data therefore raise a conundrum: if the γ-domain is directed toward membrane, how can it make cognate interactions with the C-terminal regions of FtsL and DivIC, which are predicted to be located ≈55 Å from the membrane if the N-terminal regions of these proteins form extended coiled coils?
One possibility is that cis–trans isomerization of the β-domain serves as a molecular switch that modulates assembly of the FtsL–DivIC–DivIB complex. Interaction of DivIB with FtsL might activate this switch, causing the β-domain to isomerize to the cis conformer. In this conformation (Fig. 5D Right), the γ-domain is located distal to the membrane and is suitably positioned for interaction with the C-terminal domains of FtsL and DivIC. In addition to orienting the γ-domain for interaction with FtsL and/or DivIC, trans→cis isomerization might position the α-domain so it can function as a chaperone for FtsL. Thus, a cis–trans switch might serve two purposes: regulation of the interaction of γ with other divisomal components and modulation of the chaperone activity of α.
Although the cis–trans switch might operate in vivo, it is not essential for vegetative cell division, because we could rescue a B. subtilis divIB-null at the nonpermissive temperature by ectopic expression of divIB containing a P225A mutation (equivalent to P222A in Gste-DivIB), which is expected to abrogate isomerization (Fig. 5B). Similarly, an E. coli ftsQ-null strain can be complemented by a multicopy plasmid expressing an ftsQ(Y227P228→AA) double mutant (N. Goehring, personal communication). However, these experiments do not exclude the possibility that cis–trans isomerization serves as a molecular switch that increases the efficiency of septation or that it plays a role in polar divisions during sporulation in B. subtilis. This hypothesis is intriguing in light of the recent observation that the cdv1 gene of Synechococcus elongatus PCC7942, which encodes a periplasmic cis–trans isomerase, is essential for cell division in this cyanobacterium (38).
Materials and Methods
Production of Recombinant DivIB.
The coding sequence Gste-ecDivIB was identified from a tblastn search of the unfinished G. stearothermophilus genome (www.genome.ou.edu/bstearo.html) with the primary structure of B. subtilis DivIB as the query. This search returned two contigs, 660 and 327, that covered the N- and C-terminal portions, respectively, of Gste-ecDivIB. These contigs enabled design of primers for PCR amplification of Gste-DivIB47–261 from G. stearothermophilus strain NGB101 chromosomal DNA. The corresponding region of B. subtilis divIB (Bsub-DivIB54–263) was PCR-amplified from B. subtilis JH642 chromosomal DNA. PCR primers contained 5′ BamHI and 3′ EcoRI restriction sites for directional cloning of PCR products into pGEX-2T. The resulting plasmids, pSAR15 and pSAR20, encode Gste-DivIB47–261 and Bsub-DivIB54–263, respectively, as in-frame fusions to the C terminus of Schistosoma japonicum GST with a thrombin cleavage site between the gst- and the divIB-coding regions. DNA encoding Gste-DivIBβ was PCR-amplified from pSAR15, then cloned into pGEX-2T to generate plasmid pSAR19.
Gste-DivIB47–261 and Bsub-DivIB54–263 were overproduced by growing BL21 cells harboring pSAR15 or pSAR20 at 37°C in 2× TY medium containing 100 μg ml−1 ampicillin. Cells were induced to express fusion protein at mid-log phase (OD600 ≈ 0.7) by addition of 100 μM IPTG. Cells were harvested 3 h later and lysed by using a French press; then GST-DivIB was purified from the soluble fraction by using glutathione affinity chromatography. DivIB was liberated by incubation of column beads with thrombin for 1.5 h, then purified to >95% purity and simultaneously exchanged into proteolysis buffer (20 mM Tris/150 mM NaCl, pH 8.0) by size-exclusion chromatography on a Superdex 75 column.
DivIB Proteolysis.
Gste-DivIB47–261 and Bsub-DivIB54–263 were preincubated for 15 min at the desired reaction temperature before addition of trypsin or V8 protease (Sigma) from freshly prepared stock solutions (1 mg ml−1). Reactions shown in Fig. 2A contained 1 mg of Gste-DivIB47–261 or Bsub-DivIB54–263 in 600 μl of buffer. Samples (10 μl) were taken before, or at various times after, addition of protease and analyzed by using SDS/PAGE. Preparative digestions (Fig. 2B) contained 2.5 mg of Gste-DivIB47–261 in 600 μl. Reactions proceeded for 3 h, then 200 μl of each reaction mixture was electrophoresed on an SDS/PAGE gel before being blotted onto a poly(vinylidene difluoride) membrane. Bands corresponding to fragments 1 and 2 were excised and submitted for sequencing. The remaining 400 μl of each reaction mixture was lyophilized and resuspended in 200 μl of water containing 0.1% TFA (pH ≈ 3) to deactivate trypsin, and then proteolytic fragments were purified for electrospray mass spectrometry by using HPLC.
Strain Construction.
B. subtilis strain SU321 (ΔdivIB::cat) was converted to erythromycin resistance by using pCm::Er (39) to create strain RSA8 (ΔdivIB::cat::ermC). We verified that RSA8 is resistant to erythromycin, but not chloramphenicol, and displays the temperature-sensitive phenotype of SU321. The WT B. subtilis divIB gene, including its native promoter, was PCR-amplified from B. subtilis strain JH642 and cloned into the HindIII/EcoRI sites of pDG364 to create pSAR50. The promoter and coding region of divIB, minus the γ-domain (divIBΔγ), was amplified by PCR from pSAR50 and inserted into pDG364 to create pSAR69. pSAR50 was also used as the PCR template for introducing specific point mutations. Ectopic copies of B. subtilis divIB were integrated into RSA8 by double crossover at the amyE locus by using pSAR50 or its derivatives, which also integrates a chloramphenicol resistance cassette. Strains RSA9 (ΔdivIB::cat::ermC amyE::divIB-cat) and RSA29 (ΔdivIB::cat::ermC amyE::divIBΔγ-cat) were created by transforming RSA8 with pSAR50 and pSAR69, respectively. Temperature sensitivity was tested by streaking strains on duplicate plates grown at 30°C and 48°C.
Structure Determination.
Uniformly 15N/13C-labeled Gste-DivIBβ was produced as described (40). NMR samples contained ≈1 mM Gste-DivIBβ in 10 mM NaPi, 150 mM NaCl, 10 μM EDTA, 10 μM 4-(2-aminoethyl)benzenesulfonyl fluoride, and 0.02% NaN3 (pH 6.0). Recombinant Gste-DivIBβ contains an N-terminal Gly–Ser extension (a vestige of the thrombin cleavage site), and thus the recombinant protein contains 119 residues.
We previously reported chemical-shift assignments for both conformers of Gste-DivIBβ (40, 41). φ, ψ, and χ1 angle restraints and stereo assignments for β-methylene protons were derived as described (42). The γ- and δ-methyl groups of most Val and Leu residues, respectively, were stereospecifically assigned based on preliminary structures. Slowly exchanging amide protons were identified by reconstituting lyophilized Gste-DivIBβ in 99.96% D2O and recording a series of HSQC spectra over ≈24 h. Hydrogen-bond restraints were applied as reported (43).
Distance restraints were derived from 3D 13C- and 15N-edited NOESY-HSQC spectra (τm = 150 ms) acquired at 35°C on a Varian 600-MHz spectrometer. NOESY spectra were processed with nmrpipe (44), integrated by using xeasy (45), then assigned automatically by using candid (46, 47). candid assigned 85–90% of NOESY crosspeaks, consistent with literature reports (46). Structures were subsequently refined manually. cyana was first used to calculate 600 structures from random starting conformations. The 60 structures with lowest penalty-function values were then refined by means of dynamical simulated annealing by using x-plor (48). The 25 conformers with lowest molecular energies were selected to represent the Gste-DivIBβ structure.
Supplementary Material
Acknowledgments
We thank Liz Harry and Gerry Wake for helpful discussions and Mark Maciejewski for assistance with NMR data acquisition. This study was supported by National Institutes of Health Grant AI048583.
Abbreviations
- ec
extracytoplasmic
- Bsub-ecDivIB
ec region of Bacillus subtilis DivIB
- Gste-DivIBβ
β-domain of Geobacillus stearothermophilus DivIB
- Gste-ecDivIB
ec region of Geobacillus stearothermophilus DivIB
- POTRA
polypeptide transport-associated.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: Coordinates and experimental restraints for the structure of the trans and cis conformers of the DivIB β-domain have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 1YR1 and 2ALJ, respectively).
References
- 1.Errington J., Daniel R. A., Scheffers D. J. Microbiol. Mol. Biol. Rev. 2003;67:52–65. doi: 10.1128/MMBR.67.1.52-65.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goehring N. W., Beckwith J. Curr. Biol. 2005;15:R514–R526. doi: 10.1016/j.cub.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 3.Lutkenhaus J., Addinall S. G. Annu. Rev. Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 4.Margolin W. Nat. Rev. Mol. Cell Biol. 2005;6:862–871. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Löwe J., Amos L. A. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 6.Stricker J., Maddox P., Salmon E. D., Erickson H. P. Proc. Natl. Acad. Sci. USA. 2002;99:3171–3175. doi: 10.1073/pnas.052595099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romberg L., Levin P. A. Annu. Rev. Microbiol. 2003;57:125–154. doi: 10.1146/annurev.micro.57.012903.074300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Pedro M. A., Quintela J. C., Holtje J. V., Schwarz H. J. Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buddelmeijer N., Beckwith J. Curr. Opin. Microbiol. 2002;5:553–557. doi: 10.1016/s1369-5274(02)00374-0. [DOI] [PubMed] [Google Scholar]
- 10.Weiss D. S. Mol. Microbiol. 2004;54:588–597. doi: 10.1111/j.1365-2958.2004.04283.x. [DOI] [PubMed] [Google Scholar]
- 11.Buddelmeijer N., Beckwith J. Mol. Microbiol. 2004;52:1315–1327. doi: 10.1111/j.1365-2958.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 12.Robson S. A., Michie K. A., Mackay J. P., Harry E., King G. F. Mol. Microbiol. 2002;44:663–674. doi: 10.1046/j.1365-2958.2002.02920.x. [DOI] [PubMed] [Google Scholar]
- 13.Goffin C., Ghuysen J. M. Microbiol. Mol. Biol. Rev. 1998;62:1079–1093. doi: 10.1128/mmbr.62.4.1079-1093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mosyak L., Zhang Y., Glasfeld E., Haney S., Stahl M., Seehra J., Somers W. S. EMBO J. 2000;19:3179–3791. doi: 10.1093/emboj/19.13.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moy F. J., Glasfeld E., Mosyak L., Powers R. Biochemistry. 2000;39:9146–9156. doi: 10.1021/bi0009690. [DOI] [PubMed] [Google Scholar]
- 16.Yang J. C., Van Den Ent F., Neuhaus D., Brevier J., Lowe J. Mol. Microbiol. 2004;52:651–660. doi: 10.1111/j.1365-2958.2004.03991.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Lallo G., Fagioli M., Barionovi D., Ghelardini P., Paolozzi L. Microbiology. 2003;149:3353–3359. doi: 10.1099/mic.0.26580-0. [DOI] [PubMed] [Google Scholar]
- 18.Real G., Autret S., Harry E. J., Errington J., Henriques A. O. Mol. Microbiol. 2005;55:349–367. doi: 10.1111/j.1365-2958.2004.04399.x. [DOI] [PubMed] [Google Scholar]
- 19.Dai K., Xu Y., Lutkenhaus J. J. Bacteriol. 1996;178:1328–1334. doi: 10.1128/jb.178.5.1328-1334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J. C., Weiss D. S., Ghigo J.-M., Beckwith J. J. Bacteriol. 1999;181:521–530. doi: 10.1128/jb.181.2.521-530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katis V. L., Wake R. G. J. Bacteriol. 1999;181:2710–2718. doi: 10.1128/jb.181.9.2710-2718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harry E. J., Stewart B. J., Wake R. G. Mol. Microbiol. 1993;7:611–621. doi: 10.1111/j.1365-2958.1993.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 23.Schagger H., von Jagow G. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 24.Karimova G., Dautin N., Ladant D. J. Bacteriol. 2005;187:2233–2243. doi: 10.1128/JB.187.7.2233-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller M., Rao J. K., Wlodawer A., Gribskov M. R. FEBS Lett. 1993;328:275–279. doi: 10.1016/0014-5793(93)80943-o. [DOI] [PubMed] [Google Scholar]
- 26.Andreotti A. H. Biochemistry. 2003;42:9515–9524. doi: 10.1021/bi0350710. [DOI] [PubMed] [Google Scholar]
- 27.Holm L., Sander C. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Pulido L., Devos D., Genevrois S., Vicente M., Valencia A. Trends Biochem. Sci. 2003;28:523–526. doi: 10.1016/j.tibs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis P., Soll J. Biochim. Biophys. Acta. 2001;1541:64–79. doi: 10.1016/s0167-4889(01)00147-1. [DOI] [PubMed] [Google Scholar]
- 30.Voulhoux R., Bos M. P., Geurtsen J., Mols M., Tommassen J. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 31.Hertle R. Curr. Protein Pept. Sci. 2000;1:75–89. doi: 10.2174/1389203003381423. [DOI] [PubMed] [Google Scholar]
- 32.Stebbins C. E., Galán J. E. Nature. 2001;414:77–81. doi: 10.1038/35102073. [DOI] [PubMed] [Google Scholar]
- 33.Noirclerc-Savoye M., Le Gouellec A., Morlot C., Dideberg O., Vernet T., Zapun A. Mol. Microbiol. 2005;55:413–424. doi: 10.1111/j.1365-2958.2004.04408.x. [DOI] [PubMed] [Google Scholar]
- 34.Daniel R. A., Errington J. Mol. Microbiol. 2000;36:278–289. doi: 10.1046/j.1365-2958.2000.01857.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen J. C., Minev M., Beckwith J. J. Bacteriol. 2002;184:695–705. doi: 10.1128/JB.184.3.695-705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levin P. A., Losick R. J. Bacteriol. 1994;176:1451–1459. doi: 10.1128/jb.176.5.1451-1459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sievers J., Errington J. J. Bacteriol. 2000;182:5572–5579. doi: 10.1128/jb.182.19.5572-5579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyagishima S. Y., Wolk C. P., Osteryoung K. W. Mol. Microbiol. 2005;56:126–143. doi: 10.1111/j.1365-2958.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 39.Steinmetz M., Richter R. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 40.Robson S. A., Gorbatyuk V. Y., Maciejewski M. W., King G. F. J. Biomol. NMR. 2005;31:261–262. doi: 10.1007/s10858-005-0178-9. [DOI] [PubMed] [Google Scholar]
- 41.Robson S. A., King G. F. J. Biomol. NMR. 2005;33:135. doi: 10.1007/s10858-005-2590-6. (lett.) [DOI] [PubMed] [Google Scholar]
- 42.Rowland S. L., Burkholder W. F., Cunningham K. A., Maciejewski M. W., Grossman A. D., King G. F. Mol. Cell. 2004;13:689–701. doi: 10.1016/s1097-2765(04)00084-x. [DOI] [PubMed] [Google Scholar]
- 43.King G. F., Shih Y.-L., Maciejewski M. W., Bains N. P. S., Pan B., Rowland S. L., Mullen G. P., Rothfield L. I. Nat. Struct. Biol. 2000;7:1013–1017. doi: 10.1038/80917. [DOI] [PubMed] [Google Scholar]
- 44.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 45.Bartels C., Xia T.-H., Billeter M., Güntert P., Wüthrich K. J. Biomol. NMR. 1995;5:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann T., Guntert P., Wuthrich K. J. Mol. Biol. 2002;319:209–227. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 47.Guntert P. Methods Mol. Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 48.Brünger A. T. x-plor. New Haven, CT: Yale Univ. Press; 1992. Version 3.1. [Google Scholar]
- 49.Thompson J. D., Higgins D. G., Gibson T. J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koradi R., Billeter M., Wüthrich K. J. Mol. Graphics. 1996;14:51–55. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 51.DeLano W. L. The pymol Molecular Graphics System. San Francisco: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.