Abstract
Xin was first cloned using differential mRNA display from the developing chicken heart. Chick Xin (cXin) participates in a BMP-Nkx2.5-MEF2C pathway to regulating cardiac morphogenesis. Through subsequent EST database searches and cDNA cloning, two mouse Xin genes, mXinα and mXinβ were identified and cloned. The human homologue of mXinα (named Cmya1) was mapped to chromosome 3p21.2-p21.3 by radiation hybrid analysis and recently to 3p22.2 by DNA sequencing, which is near the loci for a dilated cardiomyopathy with conduction defect-2 and arrhythmogenic right ventricular dysplasia-5. The predicted human homologue of mXinβ (named Cmya3) was mapped to chromosome 2q24.3 by DNA sequencing. Predicted Xin proteins all contain a novel 16-amino acid repeating unit (Xin repeat), a putative DNA binding domain and nuclear localization signal, as well as a proline-rich region. All three Xin genes from chick and mouse have a similar tissue expression profile, which is restricted to striated muscle. The expression of mXinα in Nkx2.5 or MEF2C knockout mouse embryos was drastically reduced, suggesting that mXinα is a downstream target of the Nkx2.5 and MEF2C transcription factors. On the other hand, the expression of mXin was up-regulated when mice were subjected to pressure overload-induced cardiac hypertrophy. Xin protein co-localizes with N-cadherin and β-catenin throughout mouse embryogenesis and into adulthood. Furthermore, mXinα appears to interact directly with β-catenin. The Xin repeats bind to actin filaments and may also organize microfilaments into networks. These results may suggest that Xin acts by integrating adhesion, by organizing actin filament arrangement at the insertion sites, and by regulating Wnt/β-catenin-and N-cadherin-mediated signaling pathways required for cardiac development and cardiac function.
Keywords: Xin repeats, β-catenin-binding protein, intercalated disc, cardiomyopathy, Nkx2.5
Introduction
Cardiac morphogenesis is a dynamic, progressive, and intricate process. Defects in this process lead to congenital heart diseases. Only through understanding normal development can intervention strategies be developed to alleviate developmental defects. Intensive studies on the genetic and molecular mechanisms controlling cardiac development and function have led to the discovery of many novel genes and pathways involved in cardiac development and disease (for a review, see 1-5). One of the master regulatory proteins, cardiac-restricted homeobox transcription factor Nkx2.5, controls many other cardiac muscle genes and is essential for normal heart morphogenesis and function. Human mutations in Nkx2.5 result in atrial septal defects, outflow tract defects, progressive cardiomyopathy and conduction defects 6-10. Although the mechanisms are not completely understood, recent studies with Nkx2.5 ventricular-restricted knockout mice reveals that the formation and maturation of the atrioventricular (AV) nodal and ventricular myocytes 11 depend on functional Nkx2.5 protein. Further bioinformatics and microarray analyses identify many downstream target genes including bone morphogenetic protein-10 (BMP-10), minK, and connexin 40 (Cx40) that are deregulated in hearts lacking Nkx2.5. Therefore, the molecular mechanisms underlying the progressive cardiomyopathy and conduction defects are very complicated. Recently, a novel Xin protein localized to the adherens junctions of intercalated discs has been identified as a downstream target of Nkx2.5, which may play an important role in cardiac morphogenesis and function. In this review, we briefly describe the discovery of Xin and then focus on its domain structure, tissue expression and regulation, as well as potential functions in the heart.
Discovery of Xin
In a search for genes involved in heart development, we previously used the differential mRNA display method in conjunction with whole mount in situ hybridization to clone a novel differentially expressed gene, Xin, from the chick 12. Incubation of chick embryos with Xin antisense oligonucleotides resulted in abnormal cardiac morphogenesis and altered cardiac looping, suggesting that chick Xin (cXin) plays a role in cardiac development 13. Shortly after its discovery, the protein was named Xin, which in Chinese means “heart”. The first mouse Xin homologue (mXinα) was identified by searching the EST public database with the cXin sequence and using the resulting EST to probe a mouse skeletal cDNA library 13. The full length cDNA sequences of cXin and mXinα were submitted to GenBank with accession numbers of AF051944 and AF051945, respectively. The human homologue of mXinα (named cardiomyopathy associated 1, Cmya1) was further identified and mapped to the region of 3p21.2-p21.3 by radiation hybrid analysis 14 and to 3p22.2 by DNA sequencing (http://www.ncbi.nlm.gov/entrez). It is interesting to note that arrhythmogenic right ventricular dysplasia-5 (ARVD5, 3p21.3-p23) and dilated cardiomyopathy with conduction defect 2 (CDCD2, 3p25-p22) have been mapped to this region (OMIM, Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/Omim/getmap). Low stringency Southern blot analysis of BamHI, EcoRI, HindIII or KpnI-digested mouse genomic DNAs with cXin cDNA probe revealed the presence of a second mXinβ gene in the mouse (data not shown). The human homologue of mXinβ (named Cmya3) was mapped to chromosome 2q24.3 by DNA sequencing. Nearby disease-associated loci (OMIM) include dilated cardiomyopathy 1H (CMD1H, 2q14-q22) and 1G (CMD1G, 2q24.3), Edstrom myopathy (2q24-q31), and Arrhythmogenic right ventricular dysplasia-4 (ARVD4, 2q32.1-32.3).
The structure of Xin
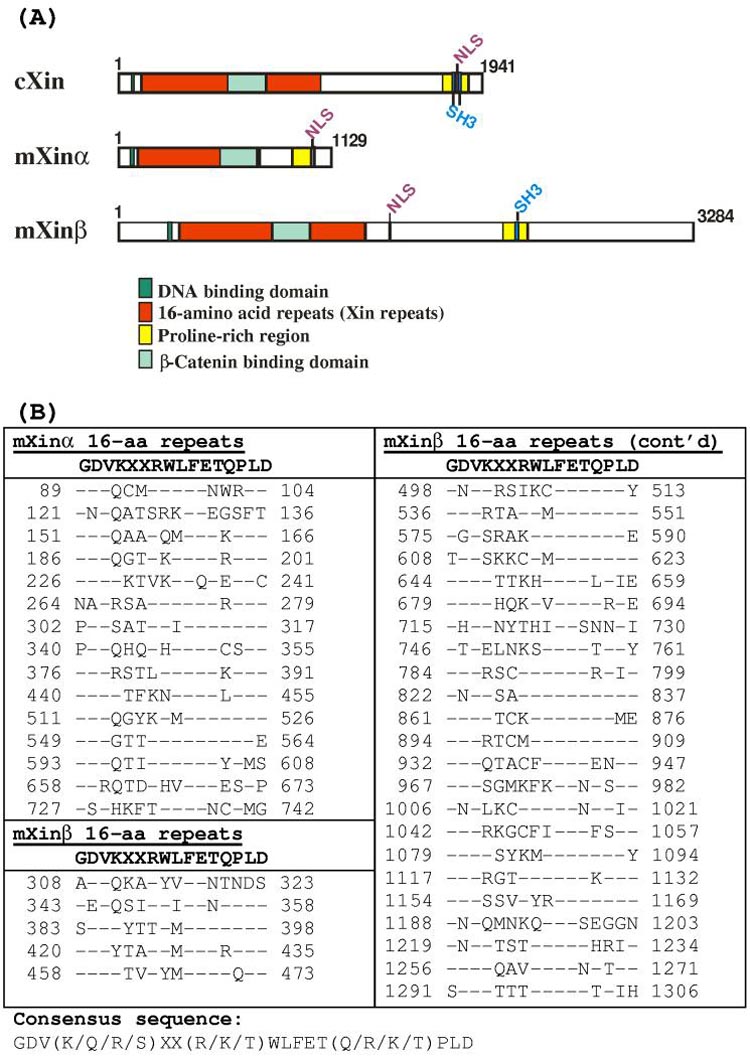
The Xin gene encodes a protein containing a proline-rich region (some with a putative SH3 domain), a putative DNA binding domain, a potential nuclear localization signal, and a region with many 16-amino acid (aa) repeating units (called Xin repeat) (Fig. 1A). The predicted cXin protein has 26 Xin repeats (data not shown), whereas mXinα and mXinβ contain 15 and 28 repeats, respectively (Fig. 1B). The consensus sequence for this Xin repeat is GDV(K/Q/R/S)XX (R/K/T)WLFET(Q/R/K/T)PLD. The same consensus sequence of Xin repeats was recently found in both human Cmya1 and Cmya3 proteins 15. Database searches with this Xin repeat revealed genomic clones, cDNAs and expressed sequence tags in all vertebrates, including mammals, birds, amphibians and fishes but no sequence similarities were found in invertebrates. The Xin repeat appears to bind to actin filaments and define a new class of actin binding domain 15. The putative DNA binding domain is similar to that found in oncogene Myb-A and Myb-B. The amino acid sequence of this domain and its flanking residues, EL(K/R/N)RLY(R/K)H(M/I)HPELRKNL, are highly conserved among chick, mouse and human Xin proteins. Although a DNA binding domain and nuclear localization signal are both present in all Xin proteins, the nuclear localization of Xin has not been detected. These results may suggest a novel yet unidentified function for this conserved DNA binding domain. Using coimmunoprecipitation and yeast 2 hybrid assays, we have recently shown that Xinα interacts directly with β-catenin and the β-catenin binding domain is mapped to aa #533-746 within the last 4 Xin repeats on the mXinα molecule.
Fig. 1.
(A). Schematic diagrams of domain structures in chick Xin (cXin) and mouse Xin (mXinα and mXinβ). All Xin proteins contain a putative DNA binding domain and nuclear localization signal (NLS), a 16-amino acid repeating unit called the Xin repeat, a conserved β-catenin-binding domain, and a proline rich region within which an SH3 domain is found in cXin and mXinβ. (B). The amino acid sequences of the Xin repeats found in mXinα and mXinβ proteins. Dashes within sequences represent amino acid residues identical to that of the consensus sequence (GDVKXXRWLFETQPLD). cXin has 26 Xin repeats (data not shown), whereas mXinα and mXinβ contains 15 and 28 Xin repeats, respectively.
The Expression and Potential Functions of Xin
(1) cXin expression is induced by BMP-2
During embryogenesis, the chick cXin is first detected at HH (Hamburger-Hamilton) stage 8 in a paired lateral plate mesoderm by whole-mount in situ hybridization 13. At stage 9, cXin expression increases substantially in the heart forming fields, which migrate anteriorally and ventrally toward the midline of the embryo. The cXin gene is exclusively expressed in the primitive heart tube of a stage 10 embryo, in which the heart looping process has not yet begun. Cardiac specific expression of cXin continues until stage 15, when somite expression begins to be detected. Both skeletal and cardiac muscle-specific expression of cXin continues throughout development and into adulthood. This tissue expression pattern of the cXin gene is further confirmed by Northern blot analysis 13 at the message level, and by Western blot analysis and immunofluorescence 16,17 at the protein level. The message size of cXin is 8.815 kb. It has been previously reported that Nkx2.5 expression can be induced by BMP-2 in the anterior medial mesoendoderm of stage 6 chick embryos, a tissue that normally does not express cardiac muscle genes 18. Using this explant system, we show that cXin expression is also strongly induced by BMP-2 treatment 13, as seen for the induction of Nkx2.5 and MEF2C. However, the induction of cXin follows the activation of Nkx2.5 and MEF2C, but clearly precedes expression of ventricular myosin heavy chain 13. These results suggest that cXin may participate in a BMP-2-Nkx2.5-MEF2C pathway to regulate cardiac morphogenesis.
(2) mXinα is a downstream target of Nkx2.5 and MEF2C
Using trans-activation experiments, we further show that either the MEF2C or Nkx2.5 transcription factor alone is able to activate the expression of a Luciferase reporter gene driven by mXinα promoter in nonmuscle cells 13. To further confirm this regulation of mXinα expression by MEF2C and Nkx2.5, we have analyzed the expression of mXinα in MEF2C and Nkx2.5 knockout mouse embryos. RT-PCR (reverse transcription-polymerase chain reaction) analysis of embryonic day 9.5 (E9.5) mice reveals that the mXinα message in MEF2C−/− is down-regulated to undetectable levels while the message for a ribosomal L7 gene is not affected (Fig. 2A). Whole-mount in situ hybridization also shows a drastic reduction in mXinα expression in the Nkx2.5 knockout E9.5 mouse as compared to the wild type littermate (Fig. 2B). These results, taken together with explant induction studies, suggest a BMP2-Nkx2.5-MEF2C-Xin pathway in cardiac differentiation.
Fig. 2.
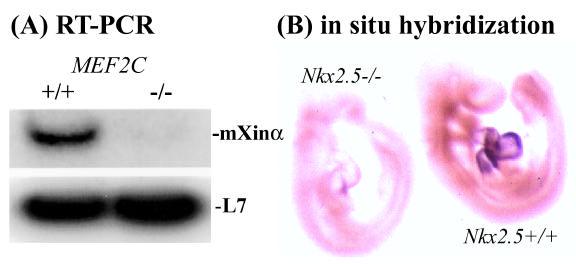
A drastic reduction of mXinα expression is observed in MEF2C or Nkx2.5 knockout mouse embryos. (A). RT-PCR analysis of RNAs prepared from E9.5 mouse wild type (+/+) and MEF2C homozyogous (−/−) embryos shows that the mXinα message is barely detectable in the RNA sample from MEF2C−/− embryos. In contrast, the message for a ribosomal L7 gene is not affected in MEF2C knockout embryos. (B) Whole-mount in situ hybridization analysis of E9.5 wild type (+/+) and Nkx2.5 knockout (−/−) embryos with a mXinα riboprobe shows a significant reduction in the mXinα message in the knockout heart.
(3) Xin is a striated muscle-specific gene, whose gene product is localized to the adherens junctions of intercalated discs in the adult heart
Similar to cXin and mXinα tissue expression patterns 13, mXinβ was detected in the skeletal muscle and heart with additional weak expression in the lung of adult mice (Fig. 3). The low level of expression in the lung, which was also occasionally detected for cXin and mXinα, may be due to the associated cardiomyocytes in the pulmonary vein of lung tissue. The mXinβ probe hybridized to a 12kb mRNA band, much larger than the 5.8 kb mXinα message and also the 8.8 kb cXin message 13. Furthermore, the striated muscle-restricted expression of Xin was also observed in the developing embryos and adult animals by in situ hybridization assays. Therefore, Xin is a striated muscle-specific gene.
Fig. 3.
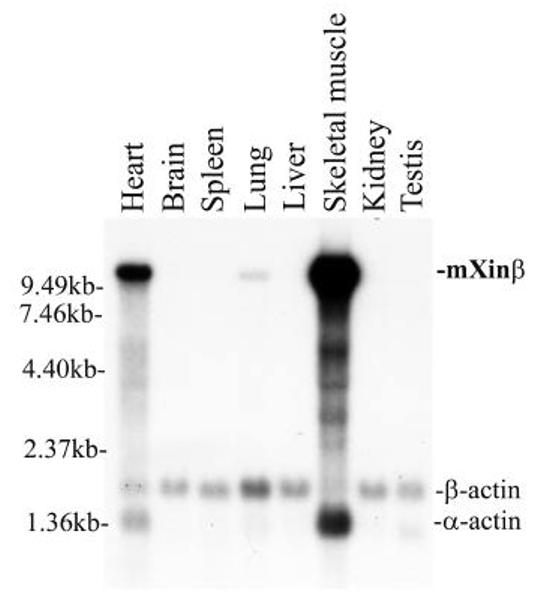
Multiple tissue Northern blot analysis of mXinβ expression. A multiple adult tissue RNA blot was probed with a 2.2 kb mXinβ cDNA. A high molecular weight band, 12 kb, was detected in the skeletal muscle and heart with weak expression in the lung. The same blot was further hybridized with the β-actin probe as a RNA loading control. The β-actin probe cross hybridized with α-actin in skeletal and cardiac muscles.
The mXinα protein is first detected within the heart tube of E8.0 embryos, exhibiting a peripheral localization within the cardiomyocytes 16. Colocalization of mXinα with both β-catenin and N-cadherin is observed throughout embryogenesis and into adulthood 16. At E15.5, a transition occurs with mXinα and the adherens junction proteins, N-cadherin or β-catenin, moving into a more puntate staining pattern around the cardiomyocytes 16. mXinα is detected earlier than vinculin in the developing heart and colocalizes with vinculin only at the intercalated disc but not at the sarcolemma (costamere) within embryonic and post-natal hearts 16. These results strongly suggest that Xin may play a role in the formation and maintenance of the intercalated disc and myofibril integrity.
(4) mXinα directly interacts with β-catenin and actin filaments
To explore the interactions of mXinα with N-cadherin and β-catenin, a yeast 2-hybrid analysis was employed. Using full length mXinα fused with the DNA binding domain of the Gal4 transcription factor as bait, and β-catenin fused with the activation domain of Gal4 as prey, we have demonstrated a direct protein-protein interaction between mXinα and β-catenin. However, using the N-cadherin cytoplasmic domain fused with the Gal4 activation domain as prey, no interaction was detectable. To determine more specifically where mXinα is interacting with β-catenin, several deletion constructs lacking either a portion of the Xin repeats or C-terminal half of mXinα were made and used as bait in yeast 2 hybrid assays. Preliminary data using these constructs revealed that a region of sequence (aa#533-746) including the last 4 Xin repeats is required for this interaction. Furthermore, within this region, there is a sequence homology to that found in a known β-catenin-binding protein, APC (adenomatosis polyposis coli). Whether this region alone is sufficient for the binding to β-catenin remains to be determined.
Using actin-binding assays and transfection experiments with the Xin repeat, Pacholsky et al. 15 demonstrated that the Xin repeat is a new class of actin-binding motif, capable of binding to actin filaments and organizing microfilaments into networks. A minimum of 3 Xin repeats is required for the binding to actin filaments. These results are consistent with our early report that a portion of mXinα can be co-localized to the tropomyosin-containing actin bundles of differentiating C2C12 myotubes 16. By using full length mXinα cDNA fused with the Gal4 DNA binding domain as bait to screen a yeast 2 hybrid library prepared from mouse heart cDNAs, we have obtained several clones encoding thin filament proteins/actin binding proteins, including actin, troponin C, troponin T, and gelsolin. This finding further supports that Xin is an actin binding protein. An interesting question is then why mXinα is not localized to the thin filaments in the cardiomyocytes. The β-catenin-binding domain that we identified within the last 4 Xin repeats may provide a way for the exclusive localization of mXinα at the adherens junctions of the intercalated discs. Moreover, our preliminary results from experiments designed to map the actin-interacting domain on the mXinα molecule reveal an interesting phenomenon. Using full-length mXinα as bait in a 2 hybrid interaction assay, a weak interaction with actin prey was always observed, whereas a much stronger interaction could be obtained with a C-terminal half deleted mXinα. In the same assay, no interaction was detected when using mXinα deletion mutants lacking the Xin repeats as bait. These results further suggest a potential model whereby the C-terminus of mXinα may prevent the full-length molecule from binding to actin, until the β-catenin-binding domain on mXinα is occupied by the β-catenin molecule. The binding of mXinα to β-catenin at the adherens junction would then open the stronger binding sites for actin and its components. Fig. 4A shows this working model for mXinα organization at the adherens junction of the intercalated disc. The interaction of mXinα with β-catenin would provide a way to maintain the integrity of the N-cadherin-mediated adhesion and the thin filament insertion in cardiomyocytes. It is known that β-catenin plays a central role in regulating Wnt signaling and cadherin-mediated adhesion in many cell types and tissues 19. In the absence of the Wnt signal, cytoplasmic β-catenin is complexed with Axin, APC (adenomatosis polyposis coli), and GSK3β (glycogen synthase kinase-3β), subsequently phosphorylated, recognized by β-TrCP (an E3 ubiquitin ligase), and targeted for degradation 19,20(Fig. 4B). Conventional Wnt ligands bind to cell receptors, Frizzled and Arrow/LPR5/6 (lipoprotein receptor-related proteins 5 and 6), thus activating Dishevelled (Dsh), which results in uncoupling β-catenin from degradation. The accumulation of cytoplasmic β-catenin is then available to bind Tcf (HMG-box transcription factor of the T-cell factor family) and enter into the nucleus to induce gene expression (Fig. 4B). Accumulated lines of evidence have suggested that conventional Wnt signaling plays a suppressive effect on cardiogenesis. In Xenopus, the inhibition of Wnt signaling from β-catenin to Tcf by activated GSK3β expression or by Wnt signaling inhibitors such as Cresent and Dickkopf-1 can promote cardiogenesis 21. In mice, conditional deletion of β-catenin in embryonic endoderm leads to the formation of ectopic hearts 22. This evidence certainly supports the role of Wnt signaling in developmental growth of the heart. Interestingly, we have shown that at mouse embryonic day 15.5, β-catenin, the essential component of Wnt signaling, together with mXinα begin to coalesce into discrete bands as intercalated discs 16. Therefore, mXinα may potentially play a linking role between the Wnt signaling pathway and formation of the intercalated disc during development.
Fig. 4.
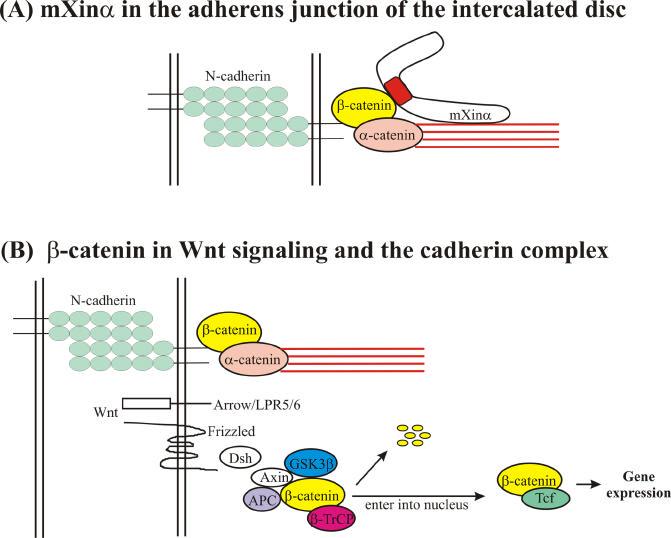
(A). Proposed model for mXinα localization at the adherens junction of the intercalated disc. The extracellular domains of N-cadherin molecules projecting from the plasma membrane of each cardiomyocyte form a homotypic interaction. The cytoplasmic domain of N-cadherin recruits β-catenin and α-catenin, and subsequently allows the assembly of actin thin filaments at the adherens junction. Full-length mXinα may be present at an auto-inhibited state due to the C-terminal proline-rich region, which may prevent the Xin repeats from binding to actin filaments. Once β-catenin binds to the β-catenin-binding domain on mXinα, a conformational change leads to an open state, which has a higher binding affinity of the Xin repeats to actin filaments. (B). β-catenin plays a central role in regulating Wnt signaling and the N-cadherin-mediated complex (adapted from Nelson and Nusse 19). In the absence of a Wnt signal, cytoplasmic β-catenin is complexed with Axin, APC (adenomatosis polyposis coli), and GSK3β, phosphorylated, recognized by β-TrCP (an E3 ubiquitin ligase) and then targeted for degradation. In the presence of a Wnt signal, which binds to Frizzled or Arrow/LPR5/6 receptors, Dsh (Dishevelled) becomes activated and uncouples β-catenin from degradation. Accumulated, cytoplasmic β-catenin is then available to complex with the Tcf transcription factor in the nucleus and thus change target gene expression. β-catenin appears to play a central role in regulating N-cadherin-mediated adhesion and the Wnt/β-catenin signaling pathway. The mXinα protein interacts directly with β-catenin and actin filaments and subsequently, may stabilize the N-cadherin/β-catenin complex. Thus, mXinα not only is a component of the adherens junction but also is a potentially important factor that may affect the level of β-catenin available to control gene expression in cardiomyocytes.
N-cadherin-mediated adhesion and signaling are believed to require β-catenin and α-catenin to link to the actin cytoskeleton and/or thin filaments, although the quaternary complex has not been demonstrated in vitro or in vivo. As mXinα possesses both β-catenin-binding and actin-binding domains potentially strengthens this linkage and plays an important role in this signaling pathway. Transgenic mice overexpressing N-cadherin in the heart develop cardiomyopathy. Furthermore, ectopic expression of E-cadherin in the heart leads to a much more severe cardiomyopathy 23. Thus, the importance of N-cadherin-mediated adhesion and signaling in cardiac development and function becomes obvious. Using a cardiac-specific tamoxifen-inducible Cre transgene to delete N-cadherin in the adult myocardium, Kostetskii et al. have recently shown that loss of N-cadherin results in the disassembly of the intercalated disc structure, including adherens junctions and desmosomes, and in a significant decrease in the gap junction protein, connexin 43 24. As a consequence, the mutant mice exhibit dilated cardiomyopathy, impaired cardiac function, and die abruptly by the onset of spontaneous ventricular tachycardia. In summary, either too much or too little N-cadherin-mediated adhesion in the heart leads to dilated cardiomyopathy. Ectopic expression of E-cadherin in the heart would interfere with the N-cadherin-mediated signal and result in cardiomyopathy. These results suggest that the N-cadherin-mediated adhesion and signaling pathway are essential for the structural integrity and function of the heart. Again, β-catenin is an integral component of this signaling pathway. Therefore, interactions between Wnt/β-catenin- and N-cadherin-mediated signaling pathways are likely present in the adult heart and the regulation of these interactions may be through the β-catenin binding proteins such as mXinα and/or tyrosine kinase. It has been shown in the nervous system that tyrosine phosphorylation of β-catenin in response to extracellular cues is always associated with loss of the adhesive function 25.
(5) Xin expression is significantly up-regulated in pressure-overload animals
Unlike skeletal muscle cells, in which proliferation and differentiation are mutually exclusive, embryonic cardiomyocytes can proliferate, differentiate and assemble functional sarcomeres. Even in the neonate, cardiomyocytes grow through physiological hypertrophy rather than hyperplasia. In response to abnormal stress, such as hypertension, pressure overload, endocrine disorders, myocardial infarction and contractile dysfunction from inherited mutations in sarcomeric or cytoskeletal proteins, adult cardiomyocytes undergo pathological hypertrophy. This hypertrophy can be a compensatory mechanism that helps to preserve pump function in pathological conditions. Frequently, this hypertrophy progresses to dilated cardiomyopathy. Studies using several mouse hypertrophy models including pressure overload-induced by thoracic aortic banding 26-28 have led to a better understanding of the molecular mechanism of hypertrophy and cardiomyopathy. In general, several transformations within the myocardium will accompany hypertrophy, including changes in gene expression and structural changes at the intercalated disc. We have detected an increase in the expression of both mXinα and mXinβ messages in pressure overload hypertrophied hearts, suggesting that mXin may play a role in the compensatory response to an increase in cardiac workload. Upregulation of mXin expression may be involved in organizing additional sarcomere insertion sites. This is in accordance with our hypothesized role for mXinα in intercalated disc formation and myofibrillogenesis 16.
Recently, another adhesion system, nectin/l-afadin/ponsin, has been reported to be co-localized with cadherins in adherens junctions of epithelia and intercalated discs of the heart 29,30. Moreover, it is reported that α-catenin from the N-cadherin system is capable of interacting with l-afadin from the nectin adhesion system 30, suggesting a possible cross-talk between two adhesion systems. Although there is no other evidence besides the close proximity of mXin, it is possible that one function of mXin may be regulating this cross-talk.
(6) mXinα knockout mice exhibit spontaneous hypertrophy in the hearts
To determine the function of mXin, mXinα-null mice were created. Embryonic lethality was expected based on previous antisense oligonucleotide experiments in chick; however, viable and fertile knockout mice were observed. This viability results from functional compensation through upregulation of mXinβ, as both heterozygous and homozygous mouse hearts showed proportionally increased levels of the mXinβ message. Nevertheless, mXinα knockout mice exhibited spontaneous cardiac hypertrophy. Ultrastructural studies of hearts from mXinα-null mice revealed a significant disruption of the intercalated disc ultrastructure and disarray of myofilaments at this region. These results suggest that mXinα is involved in the regulation of the hypertrophy response and maintenance of the intercalated disc membrane associations in normal mice. Lack of mXinα may contribute to the development of hypertrophic cardiomyopathy and conduction defects as a result of these intercalated disc abnormalities.
Conclusions
The striated muscle-specific Xin protein contains a β-catenin-binding domain and many Xin repeats that bind to actin filaments. Xin is a downstream target of Nkx2.5 and MEF2C transcription factors and may play a role in a BMP2-Nkx2.5-MEF2C-Xin pathway to regulate cardiac morphogenesis and differentiation. The Xin protein may act by integrating the N-cadherin-mediated adhesion and by regulating actin assembly at the adherens junctions of the intercalated discs. Through the interaction with β-catenin, Xin can potentially stabilize β-catenin at the adherens junctions and subsequently influence the classical Wnt/β-catenin signaling pathway for cardiac morphogenesis. In the chick, cXin antisense oligo treated embryos exhibit abnormal cardiac development and cardiac looping, supporting a role for Xin in cardiac morphogenesis and differentiation. mXinα knockout mice have been generated and appear to develop normally. However, mXinβ is upregulated in the mXinα knockout hearts, suggesting that mXinβ may partially compensate for the loss of mXinα during development. Further analysis of mXinα knockout mice reveals that the adult hearts show disruption of intercalated disc ultrastructure and exhibit spontaneous hypertrophy. The molecular mechanisms underlying the cardiac hypertrophy in the mXinα knockout mice remain to be determined. Future studies include characterization of mXinα and mXinβ single knockouts, as well as mXinα/mXinβ double knockout mice, to define both distinct and overlapping functions of the two Xin proteins in the mouse. Continued studies on the mXin-interacting proteins will advance our understanding of how the Xin protein works. For example, evaluation of the binding affinity of mXinα to β-catenin and to actin filaments should prove our current working hypothesis that full-length mXinα requires β-catenin binding to initiate and enhance its actin binding affinity.
Acknowledgements
We would like to thank Dr. Joseph Hill for providing pressure overload mouse hearts for mXinα and mXinβ expression studies, Dr. Eric Olson for providing MEF2C knockout embryos for RT-PCR analysis of mXinα expression, and Dr. Val Sheffield's lab for performing radiation hybridization analysis for hXinα. This work was supported by US National Institutes of Health grant RO1 HL075015.
References
- 1.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 2.Chien KR, Olson EN. Converging pathways and principles in heart development and disease: CV@CSH. Cell. 2002;110:153–162. doi: 10.1016/s0092-8674(02)00834-6. [DOI] [PubMed] [Google Scholar]
- 3.Olson EN, Schneider MD. Sizing up the heart: developmental redux in disease. Genes & Dev. 2003;17:1937–1956. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 4.Bruneau BG. The developing heart and congenital heart defects: a make or break situation. Clin. Genet. 2003;63:252–261. doi: 10.1034/j.1399-0004.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- 5.Olson EN. A decade of discoveries in cardiac biology. Nat. Med. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 6.Schott J-J, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor Nkx2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- 7.Benson DW, Silberbach M, Kavanaugh-Mchugh A, Cottrill C, Zhang Y, Riggs S, Smalls O, Johnson M, Watson MS, Seidman JG, Seiderman CE, Plowden J, Kugler JD. Mutations in the cardiac transcription factor Nkx2.5 affect diverse cardiac developmental pathways. J. Clin. Invest. 1999;104:1567–1573. doi: 10.1172/JCI8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldmuntz E, Geiger E, Benson DW. Nkx2.5 mutations in patients with tetralogy of Fallot. Circulation. 2001;104:2565–2568. doi: 10.1161/hc4601.098427. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Roelens I, Sluysmans T, Gewillig M, Devriendt K, Vikkula M. Progressive AV-block and anomalous venous return among cardiac anomalies associated with two novel missense mutations in the Csx/Nkx2-5 gene. Hum. Mutat. 2002;20:75–76. doi: 10.1002/humu.9041. [DOI] [PubMed] [Google Scholar]
- 10.Elliott DA, Kirk EP, Yeoh T, Chandar S, McKenzie F, Taylor P, Grossfeld P, Fatkin D, Jones O, Hayes P, Feneley M, Harvey RP. Cardiac homeobox gene Nkx2-5 mutations and congenital heart disease. J. Am. Coll. Cardiol. 2003;41:2072–2076. doi: 10.1016/s0735-1097(03)00420-0. [DOI] [PubMed] [Google Scholar]
- 11.Pashmforoush M, Lu JT, Chen H, St. Amand T, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles WR, Ho SY, Benson DW, Silberbach M, Shou W, Chein KR. Nkx2-5 pathways and congenital heart disease: Loss of ventricular myocytes lineage specification leads to progressive cardiomyopathy and complete heart block. Cell. 2004;117:373–386. doi: 10.1016/s0092-8674(04)00405-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang D-Z, Hu X, Lin JL-C, Kitten GT, Solursh M, Lin JJ-C. Differential display of mRNAs from the atrioventricular region of developing chicken hearts at stages 15 and 21. Frontier in Biosciences. 1996;1:1–15. doi: 10.2741/a100. [DOI] [PubMed] [Google Scholar]
- 13.Wang D-Z, Reiter RS, Lin JL-C, Wang Q, Williams HS, Krob SL, Schultheiss TM, Evans S, Lin JJ-C. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development. 1999;126:1281–1294. doi: 10.1242/dev.126.6.1281. [DOI] [PubMed] [Google Scholar]
- 14.Wang D-Z, Sheffield VC, Lin JJ-C.Mapping of the human homologue of mouse Xinα by radiation hybrid ananlysis unpublished data 1998 [Google Scholar]
- 15.Pacholsky D, Vakeel P, Himmel M, Lowe T, Stradal T, Rottner K, Furst DO, van der Ven PFM. Xin repeats define a novel actin-binding motif. J. Cell Sci. 2004;117:5257–5268. doi: 10.1242/jcs.01406. [DOI] [PubMed] [Google Scholar]
- 16.Sinn HW, Balsamo J, Lillien J, Lin JJ-C. Localization of the novel Xin protein to the adherens junction complex in cardiac and skeletal muscle during development. Dev. Dyn. 2002;225:1–13. doi: 10.1002/dvdy.10131. [DOI] [PubMed] [Google Scholar]
- 17.Lin JJ-C, Wang D-Z, Reiter RS, Wang Q, Lin JL-C, Williams HS. Differentially expressed genes and cardiac morphogenesis. In: Tomanek RJ, Runyan R, editors. Formation of the Heart and Its Regulation. Birkhauser; Boston, MA: 2001. pp. 75–96. [Google Scholar]
- 18.Schultheiss TM, Burch JBE, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes & Dev. 1997;11(4):451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 19.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polakis P. Casein kinase 1: A Wnt'er of disconnect. Curr. Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- 21.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes & Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM. Formation of multiple hearts in mice following deletion of β-catenin in the embryonic endoderm. Dev. Cell. 2002;3:171–181. doi: 10.1016/s1534-5807(02)00206-x. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira-Cornwell MC, Luo Y, Narula N, Lenox JM, Lieberman M, Radice GL. Remodeling the intercalated disc leads to cardiomyopathy in mice misexpressing cadherins in the heart. J. Cell Sci. 2002;115:1623–1634. doi: 10.1242/jcs.115.8.1623. [DOI] [PubMed] [Google Scholar]
- 24.Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, Molkentin JD, Radice GI. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ. Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 25.Lilien J, Balsamo J, Arregui C, Xu G. Turn-off, drop-out: functional state switching of cadherins. Dev. Dyn. 2002;224:18–29. doi: 10.1002/dvdy.10087. [DOI] [PubMed] [Google Scholar]
- 26.Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–2869. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 27.Rockman HA, Knowlton KU, Ross J J, Chien KR. In vivo murine cardiac hypertrophy. Circulation. 1993;87(suppl VII):VII14–VII21. [Google Scholar]
- 28.Friddle CJ, Koga T, Rubin EM, Bristow J. Expression profiling reveals distinct sets of genes altered during induction and regression of cardiac hypertrophy. Proc. Nat. Acad. Sci. 2000;97:6745–6750. doi: 10.1073/pnas.100127897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplsmic domain-associated proteins. J. Cell Biol. 2000;150:1161–1175. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of α-catenin. J. Biol. Chem. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]