Abstract
We have studied the effects of cAMP on synaptic transmission at the calyx of Held and found that forskolin (an activator of adenylate cyclase) and 8-Br-cAMP (a membrane-permeable analog of cAMP) potentiated excitatory postsynaptic currents (EPSCs). Direct sampling of miniature EPSCs (mEPSCs) and nonstationary fluctuation analysis showed that mEPSCs were not modulated by cAMP, suggesting that the locus of modulation is presynaptic. Deconvolution was used to examine effects of cAMP on quantal-release rates. By using this method, it was shown recently that release probabilities of readily releasable vesicles are heterogeneous. Here, we show that cAMP selectively increases the number of vesicles with higher release probabilities, whereas a slow component of the EPSC, representing vesicles that fuse more slowly, is unchanged. cAMP increases the apparent Ca2+ sensitivity for secretion, but this increase does not reflect an increase in release probability necessarily but rather an increase in the number of highly sensitive vesicles.
The synaptic strength is modified in an activity-dependent manner as well as by neuromodulators and second messengers (1–3). For elucidating the underlying mechanisms of these modulations, it is important to understand these various forms of synaptic plasticity in terms of changes in specific parameters of quantal release.
The calyx of Held allows simultaneous voltage-clamp recording from presynaptic and postsynaptic compartments (4, 5). By taking advantage of this technique and using a recently developed deconvolution method (6), we have shown that release probability (Pr) of readily releasable quanta is heterogeneous (7). Approximately one-half of the total number of quanta is released with a Pr that is about 2- to 5-fold higher than that of the other half. Similar findings have been obtained at other synapses (8, 9). In this work, we investigated whether different populations of synaptic vesicles with distinct Pr are modulated differentially. Such different modulation would also provide that the quanta with different Pr are biochemically, not just electrophysiologically, distinct.
Based on this idea, we tried several pharmacological manipulations and found that drugs that elevate cAMP concentration lead to potentiation of excitatory postsynaptic current (EPSC) amplitude (see also ref. 10). In most preparations, cAMP potentiates quantal release presynaptically by activating protein kinase A (PKA; refs. 1 and 11–19). cAMP did not increase the amplitude of miniature EPSCs (mEPSCs) at the calyx of Held, suggesting a presynaptic origin of cAMP action. Analysis of quantal-release rates performed by using the deconvolution method (6) revealed that cAMP increased the apparent sensitivity of quanta to Ca2+ influx, and it did so by selectively increasing the number of a subpopulation of readily releasable quanta with high Pr.
Methods
Slice Preparations and Recordings.
Transverse slices of brainstem (150 to 200 μm in thickness) were cut from 8- to 10-day-old Wistar rats (4, 20). The recording chamber was perfused continuously with normal extracellular solution at a rate of 1 ml/s. Normal extracellular solution contained (in mM) 125 NaCl , 2.5 KCl, 2 CaCl2, 1 MgCl2, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 0.4 ascorbic acid, 3 myoinositol, and 2 Na-pyruvate, bubbled with 95% O2 and 5% CO2 (pH 7.4, 320 milliosmolarity). A calyx terminal and the postsynaptic principal neuron were simultaneously whole-cell clamped at −80 mV with an EPC-9/2 amplifier (HEKA-Electronics, Lambrecht/Pfalz, Germany). The presynaptic patch pipette (4–6 MΩ) was filled with a solution containing (in mM) 130–135 Cs-gluconate, 20 tetraethylammonium-Cl, 10 Hepes, 5 Na2-phosphocreatine, 4 MgATP, 0.3 GTP, and 0.5 EGTA (pH 7.2 with CsOH; 310 milliosmolarity). EGTA (0.5 mM) was used to block facilitation, which masks the heterogeneity of Pr. In some experiments (Fig. 5), 0.05 mM BAPTA [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate] was used instead of EGTA. Then, 30–90% of the presynaptic series resistance (8–30 MΩ) was compensated. For the postsynaptic pipette (2–3.5 MΩ) solution, the concentration of EGTA was increased to 5 mM. The postsynaptic series resistance (3–10 MΩ) was compensated so that the uncompensated resistance was 2–3 M Ω. EPSC amplitudes were corrected off-line for the remaining deviation from the holding potential.
During recordings, 0.5 μM tetrodotoxin, 10 mM tetraethylammonium, 50 μM D-AP5, 100 μM cyclothiazide, and 1 mM kynurenic acid (Kyn) were added to the extracellular solution. Forskolin (50 μM; Calbiochem), 8-Br-cAMP (1 mM; sigma), and 3-isobutyl-1-methylxanthine (IBMX) (100 μM; Calbiochem) were added to the extracellular solution as indicated. When mEPSCs were sampled, bicuculline (10 μM) and strychnine (2 μM) were added to the extracellular solution, and Kyn was omitted from the extracellular solution. PKA inhibitors Rp-cAMP and KT-5720 were obtained from Calbiochem, and H-89 was from Sigma.
Deconvolution and Fluctuation Analysis.
Release rates were estimated by using the deconvolution method adapted for the case of glutamatergic synapses (6). This method assumes that the total EPSC can be separated into a residual current because of residual glutamate in the synaptic cleft and a current component directly induced by quantal release. It determines residual glutamate by means of a simple diffusion model incorporated into the deconvolution algorithm. Because we had shown before that deconvolution is valid in the presence of cyclothiazide and Kyn (6), which block desensitization and saturation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, all experiments were carried out in their presence.
To estimate quantal sizes underlying evoked EPSCs, nonstationary fluctuation analysis was applied (6). A given protocol was repeated three to five times, and nonstationarities were eliminated by subtracting consecutive traces from one another and by bandpass filtering. Variance was calculated and smoothed by a gliding window. The resulting estimate of variance was corrected for AMPA channel noise. Finally, we divided variance by the release rate, multiplied the result by a correction factor, which takes into account bandpass filtering of variance, and used this value as an index of mEPSC amplitudes.
Results
Potentiation of Evoked EPSCs by cAMP.
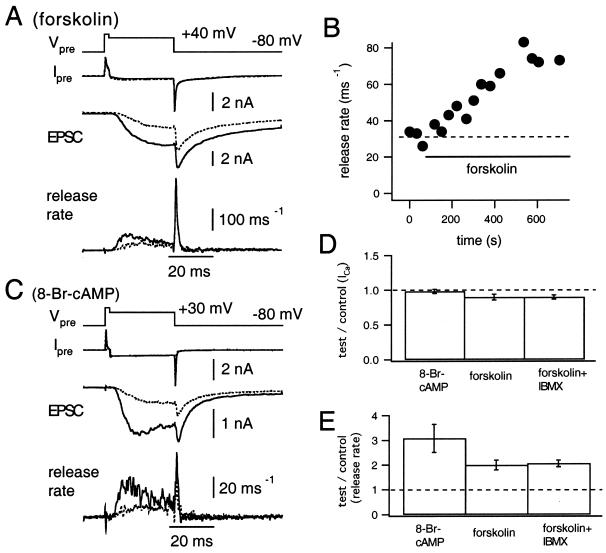
The presynaptic Ca2+ current (Fig. 1A, Ipre, dotted line) was elicited by depolarization to +70 mV for 2 ms then repolarized to +40 mV for 30 ms (Fig. 1A, Vpre). This voltage protocol evoked a slow AMPA-receptor mediated EPSC (Fig. 1A, EPSC, dotted trace). We calculated the release rate (Fig. 1A, release rate, dotted trace), the peak of which was around 30 quanta ms−1 (Fig. 1B). Extracellular application of forskolin (50 μM), an activator of adenylate cyclase, induced a marked potentiation of the EPSC (Fig. 1A, EPSC, solid trace) and of the release rate (Fig. 1A, release rate, solid trace) without changing the presynaptic Ca2+ current significantly. Here, the release rate was calculated assuming that forskolin did not modulate mEPSCs (see below). Potentiation of the release rate was more pronounced during the early period of the depolarizing pulse and less significant during the late period. The peak release rate was potentiated usually within 5–10 min after application of forskolin (Fig. 1B). Unfortunately, potentiation could not be documented for periods longer than 15 min because of run-down of transmitter release.
Figure 1.
Potentiation of EPSC induced by the application of forskolin. (A) The presynaptic terminal was depolarized from −80 to +70 mV for 2 ms and then repolarized to +40 mV (Vpre) for 30 ms. Presynaptic Ca2+ influx (IPre), the evoked EPSC, and the release rates are shown. Dotted and solid traces represent the data before and after application of forskolin (50 μM), respectively. (B) Peak release rates obtained during the experiment shown in A are plotted against time. The dotted line indicates the mean peak release rate during the control period. (C) The same experiment as in A, except that 8-Br-cAMP (1 mM) was applied. (D and E) Summary of the effect of 8-Br-cAMP (1 mM; n = 6), forskolin (50 μM; n = 4), and forskolin (50 μM) + IBMX (100 μM; n = 6) on the Ca2+ current amplitude (D) and the peak release rate (E). Amplitudes of the Ca2+ current (D) and peak release rate (E) were normalized to control values before application of drugs.
When the terminal was depolarized to values between +30 and +40 mV, the peak release rate at 5–10 min after forskolin was 200 ± 20% (mean ± SEM) of the control, whereas the Ca2+ current amplitude was 90 ± 4% of the control value (677 ± 74 pA; n = 4, Fig. 1 D and E). In an attempt to get larger potentiation, we coapplied forskolin and IBMX (100 μM), an inhibitor of phosphodiesterase (which hydrolyzes cAMP or cGMP). Although the release rate was potentiated slightly faster after application, we observed almost identical potentiation of the peak rate (206 ± 14%, n = 6; Fig. 1E) without changing the presynaptic Ca2+ current (90 ± 3% of the control). 8-Br-cGMP (1 mM), a membrane-permeable analog of cGMP, potentiated neither the peak release rate (110 ± 9%, n = 4) nor the Ca2+ current (98 ± 5%). Therefore, it seems unlikely that cGMP potentiates EPSCs at the calyx of Held.
To confirm that potentiation was caused by cAMP, 1 mM 8-Br-cAMP was applied extracellularly. 8-Br-cAMP, a membrane-permeable analog of cAMP, potentiated the EPSC and the release rate (Fig. 1C, dotted traces; control, solid traces; in the presence of 8-Br-cAMP). Similar to forskolin, 8-Br-cAMP potentiated the release rate in the early period but much less in the later period of the depolarizing pulses (Fig. 1C). On average, the peak release rate and the Ca2+ current amplitude were 308 ± 57% and 98 ± 3% of control, respectively (n = 5; Fig. 1 D and E).
Effect of cAMP-Related Pharmacological Manipulations on mEPSCs.
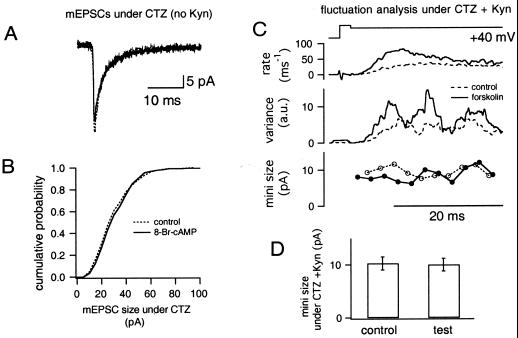
To examine postsynaptic factors contributing to the potentiation, we examined whether mEPSCs were modulated by varying cAMP levels. Kyn was omitted from the extracellular solution to resolve mEPSCs reliably. Fig. 2A shows averaged mEPSCs before (dotted trace) and after (solid trace) application of 1 mM 8-Br-cAMP, and amplitudes and the time course of mEPSCs were similar. In addition, the cumulative mEPSC-amplitude distribution was identical (Fig. 2B). Mean mEPSC amplitudes were similar in control measurements (34.8 ± 2.9 pA, n = 12) and after the application of drugs (ratios to the control; forskolin, 1.08 ± 0.06; 8-Br-cAMP, 1.06 ± 0.03; forskolin + IBMX, 1.07 ± 0.03; n = 4 in each condition). The mEPSC decay, which was fitted with a double exponential [time constants of τ1 = 1.59 ± 0.14 ms (53%) and τ2 = 8.89 ± 0.92 ms in the control], did not change significantly (ratios to control, τ1 = 0.98 ± 0.07; proportion of fast component: 0.95 ± 0.08; τ2 = 0.95 ± 0.08; n = 12). An increase in mEPSC frequency was observed only sometimes after the application, and we did not study it further.
Figure 2.
Effects of cAMP-related drugs on quantal parameters. (A) Averaged mEPSCs obtained before (dotted trace) and after (solid trace) application of 8-Br-cAMP. Kyn was omitted from the external solution to be able to resolve mEPSCs. CTZ, cyclothiazide. (B) The cumulative amplitude distribution of mEPSCs before (dotted trace) and after (solid trace) application of 8-Br-cAMP. Data were obtained from the same cell as in A. (C) Nonstationary fluctuation analysis under CTZ and Kyn. The presynaptic terminal was depolarized to +40 mV for 30 ms, and release rates as well as variances associated with the EPSCs were calculated and averaged among several traces during the control period (dotted traces) and after application of forskolin (solid traces). Variances are shown with arbitrary units (a.u.). The quantal size before (○) and after (●) application of forskolin was estimated by dividing variance over release rate and multiplying by a correction factor that takes into account bandpass filtering (Bottom). (D) The quantal size estimated before (left) and after (right) the application of drugs. The data shown are averaged values from the application of forskolin (n = 2), 8-Br-cAMP (n = 5), and forskolin + IBMX (n = 5).
Although the properties of spontaneous mEPSCs are not changed by cAMP-related drugs, it is possible that quantal events during massive exocytosis may be modulated by cAMP. Thus, we used nonstationary fluctuation analysis of evoked EPSCs to determine the properties of evoked mEPSCs. In Fig. 2C, we compared mEPSC amplitudes (under Kyn; ref. 6) measured before (○ with dotted line) and after (● with solid line) the forskolin application. In both cases, mEPSC amplitudes were the same (≈11 pA). Fig. 2D shows the mean quantal amplitude obtained from fluctuation analysis before (left, 10.3 ± 1.2 pA) and after (right, 10.0 ± 1.2 pA; n = 2 from forskolin, n = 5 from forskolin + IBMX, n = 3 from 8-Br-cAMP) application of cAMP-related drugs. Estimated amplitudes are slightly smaller than previous estimates under similar conditions (≈15 pA under cyclothiazide + Kyn; ref. 6). mEPSC amplitudes in the presence of drugs were 98 ± 3% of controls, and there were no differences among different types of drugs (forskolin, 98%; forskolin + IBMX, 101%; 8-Br-cAMP; 95%).
Effect of PKA Inhibitors on EPSCs.
In many preparations, quantal release is modulated by cAMP by the activation of PKA (14–18). To examine the role of PKA in cAMP-induced potentiation, we included 1 mM Rp-cAMP, a blocker of PKA, in the presynaptic patch pipette and applied the same protocol as shown in Fig. 1. Nevertheless, forskolin similarly potentiated the EPSC and the peak release rate (198 ± 9% of the control, n = 5) in a manner similar to control. Likewise, the intracellular application of 9 μM KT-5720, another blocker of PKA, failed to block potentiation (224 ± 7% relative to control before forskolin, n = 3). Furthermore, we preincubated the slice with 10 μM H-89, another PKA blocker, for 30–60 min and also applied 10 μM H-89 intracellularly, but forskolin still potentiated release (216 ± 19% relative to control before forskolin, n = 3). These results indicate that cAMP-induced potentiation described here is not mediated by PKA, which may also be the case in other preparations (21, 22).
cAMP-induced enhancement of synaptic transmission at the crayfish neuromuscular junction is mediated by modulation of presynaptic Ih (22). However, the extracellular application of 1 mM Cs+, which blocks Ih, had no effect on forskolin-induced potentiation (n = 4).
cAMP Potentiates the Number of Fast-Releasing Synaptic Vesicles.
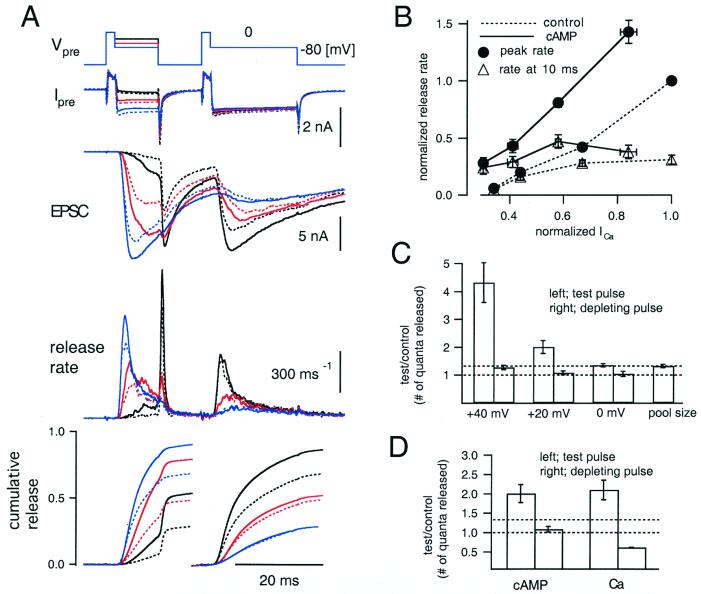
To examine the Ca2+ dependence of cAMP-induced potentiation, the presynaptic terminal was depolarized to three different potentials (Fig. 3A; black, +40 mV; red, +20 mV; blue, 0 mV) for 10 ms (test pulse). After an interval of 10 ms, the terminal was held at 0 mV for 20 ms to deplete the RRP of quanta (depleting pulse). Note the increase in both Ca2+ influx and release response during the test pulse when comparing the three conditions. An opposite relationship is observed during the depleting pulses, suggesting a limited number of quanta available for release. These three protocols were applied before (dotted traces) and after (solid traces) application of drugs (three cells each from forskolin, forskolin + IBMX, and 8-Br-cAMP). When the currents elicited by three different test potentials are compared, it can be seen that the release rates are potentiated more when presynaptic Ca2+ influx is smaller. As a result, cAMP shifts the Ca2+-release relationship to the left (Fig. 3B; cAMP, ● with solid line; control, ● with dotted line). However, when the release rate at the end of the test pulse was plotted against the Ca2+ current amplitude, potentiation turned out to be small, particularly at large Ca2+ influx (Fig. 3B; cAMP, ▵ with solid line; control, ▵ with dotted line). Remarkably, we observed only small (Fig. 3, when the test pulse was to +40 mV) or almost negligible potentiation (Fig. 3A, when the test pulse was to +20 and 0 mV) during depleting pulses. These findings suggest that cAMP does not simply increase the size of the RRP uniformly. In addition, cAMP does not simply increase Pr because in that case quanta should have been depleted faster.
Figure 3.
Calcium dependence of cAMP-induced potentiation of quantal release. (A) The presynaptic terminal was depolarized from −80 to +70 mV for 2 ms, followed by 10-ms periods of +40 mV (black), +20 mV (red), and 0 mV (blue) to elicit different amounts of Ca2+ influx (test pulses). After an interval of 10 ms, the presynaptic terminal was depolarized to 0 mV for 20 ms to deplete the readily releasable pool (RRP, depleting pulse). The curves shown in each panel are, from Top to Bottom: holding potential of the terminal (Vpre); the presynaptic current (Ipre); EPSC; release rate; and cumulative release evoked during the test and subsequent depleting pulses. Traces for cumulative release were normalized to the total pool size in the control (1,926 ± 314 quanta) and were averaged over nine cell pairs (three cells each from forskolin, 8-Br-cAMP, and forskolin + IBMX). Broken and solid traces represent control and after treatment with 8-Br-cAMP or after the application of other drugs (cumulative release), respectively. (B) The peak release rates (●) and release rates at the end of test pulses immediately before repolarization (▵) were plotted against the amplitude of the Ca2+ current. Solid lines and corresponding data points represent data in the presence of the drugs, and the dotted lines are from controls before application of drugs. The data were normalized first to the peak release rate during the test pulse to 0 mV in the control period in each cell pair and then averaged over nine cell pairs. (C) From left, the data corresponding to the test pulse to +40 mV, +20 mV, and 0 mV are shown (protocols of A). In each condition, the number of quanta released during the test pulse (left column) and the depleting pulse (right column) was calculated, and the values in the presence of drugs are shown relative to those in control. At the very right, the relative increment of the total RRP size after the drug application is shown. (D) On the left side, the numbers of quanta released during the test pulse (+20 mV; left bar) and the depleting pulse (right bar) were calculated. Values after the drug application relative to those in control are shown. On the right, the number of quanta released during different test pulses (+20 and 0 mV; left bar) and subsequent depleting pulses (right bar) in control conditions were compared by taking ratios (0 mV over 20 mV).
To quantify the Ca2+ dependence of cAMP-induced potentiation, cumulative release during the test and the depleting pulses was calculated (Fig. 3A Bottom). Cumulative release during the test pulse was potentiated more when the Ca2+ influx was smaller. When the test pulse was +40 mV, cAMP potentiated the amount of release to 432 ± 71% of the control (Fig. 3C, n = 9). Potentiation was less when the Ca2+ influx became larger (Fig. 3C, +20 mV; 201 ± 23% of the control, 0 mV; 136 ± 6%).
During the depleting pulse, cumulative release was increased only when the test pulse was to +40 mV (127 ± 8%; Fig. 3C). For the two other test potentials, cumulative release during the depleting pulse was found to be the same between before and after application of drugs (Fig. 3C), and time courses were almost identical (Fig. 3A). It has been shown that prolonged presynaptic depolarization to 0 mV evokes two components of quantal release with time constants of 2–3 ms and ≈10 ms, respectively, when the presynaptic pipette contains 0.5 mM EGTA to block overlapping facilitation (7). Then, in the protocols of Fig. 3A, the fast component should be almost depleted during the test pulse to +20 or 0 mV, and release during the depleting pulse should reflect the slow component. Thus, the slow component of quantal release was not modulated by cAMP (see also Fig. 1 A and C). cAMP potentiated the total RRP size to 133 ± 6% of the control (1,926 ± 314 quanta; Fig. 3C).
For comparison, we increased Pr simply by eliciting more Ca2+ influx during the test pulse. We compared the amount of release in response to test pulses between +20 and 0 mV under control conditions. The amount of release during test pulses at 0 mV was 210 ± 25% of those at +20 mV, whereas the amount of release during depleting pulses decreased (61 ± 1%, Fig. 3D). The total pool size was almost the same (107 ± 2%) for both depolarization levels. Thus, cAMP-induced potentiation is different from simple up-regulation of Pr (Fig. 3D).
Comparison Between cAMP-Induced Potentiation of Quantal Release and Facilitation Induced by Residual Ca2+.
It has been shown that facilitation (induced by presynaptic residual Ca2+) increases Pr (23). To obtain more insights into cAMP-induced potentiation, we compared these two forms of synaptic modification.
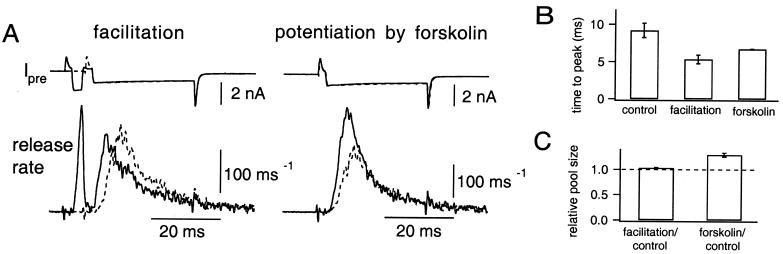
To allow facilitation to occur, the presynaptic patch pipette was filled with a low Ca2+ buffering solution (0.05 mM BAPTA). The terminal was depolarized to +80 mV for 2 ms and was repolarized to +20 mV for 30 ms (Fig. 4A Left), which was long enough to deplete most of the RRP. Facilitation was induced by predepolarization of the terminal to 0 mV for 2–3 ms, which evoked the release of 287 ± 53 quanta, 17. 9 ± 1.6% of the total RRP size (n = 5). Prepulses shortened the time to peak release rate (Fig. 4B, when facilitated, 5.33 ± 0.58 ms; control, 9.20 ± 0.98 ms). Consistent with the idea that facilitation is caused by an increase in Pr only (23, 24), the release rate declined earlier, and the RRP size was unchanged (Fig. 4C, 103 ± 1%).
Figure 4.
Comparison between Ca2+-dependent facilitation and cAMP-induced potentiation. (A) The presynaptic terminal was depolarized to +80 mV for 2 ms and subsequently repolarized to +20 mV for 30 ms (test pulse). (Left) A prepulse (depolarization to 0 mV for 3 ms before the test pulse) was applied to elicit facilitation (solid trace). The trace without the prepulse (control) is superimposed (dotted trace). (Right) The data obtained after the application of forskolin (solid trace) are superimposed on a control trace (dotted). The data were from the same cell pair. (B) Time to the peak release rate (n = 5) observed during the test pulse obtained during control condition (left), facilitation by a prepulse (center), and forskolin application (right). (C) Relative sizes of the total RRP to the size of control are shown. Pool sizes are compared during the presence of either a prepulse (left) or forskolin (right) relative to the control.
Forskolin also shortened the time to the peak release rate (Fig. 4A Right), and the time to the peak release rate was 6.72 ± 0.05 ms (Fig. 4B). In contrast to facilitation, forskolin increased the amount of release in the early depolarization period without changing the late phase (Fig. 4A). As a result, the cumulative amount of release during the pulse increased to 129 ± 4% (Fig. 4C) of control.
Discussion
We have shown that forskolin and 8-Br-cAMP potentiate EPSCs at the calyx of Held synapse (Fig. 1). Individual quantal events were not modulated by cAMP (Fig. 2), suggesting a presynaptic locus of action. cAMP increased the apparent Ca2+ sensitivity of quantal release (Fig. 3), but potentiation was not simply caused by an increase in Pr, which is the case in facilitation induced by residual Ca2+ (Fig. 4). Whereas facilitation did not change the total size of the RRP, cAMP selectively increased the number of a subset of quanta with high Pr (Figs. 3 and 4). It is unlikely that cAMP-induced potentiation is caused by an increase in presynaptic Ca2+ concentration, because presynaptic Ca2+ currents were not augmented (Fig. 1) and potentiation was observed in the presence of 0.5 mM EGTA, which is sufficient to chelate residual Ca2+ (25). In addition, depletion of releasable quanta should be faster if presynaptic Ca2+ concentration is elevated (Fig. 4), which was not observed in cAMP-induced potentiation.
Although specific inhibitors of PKA (Rp-cAMP, KT-5720, and H-89) effectively block the potentiation of transmitter release in other preparations (17, 21), they were ineffective at the calyx synapse. We do not know the exact reason why the inhibitors are ineffective. However, a recent finding by Beaumont and Zucker (22) indicates that at the neuromuscular junction, cAMP enhances quantal release through the up-regulation of Ih channels. This mechanism is unlikely to apply to the calyx of Held, because application of Cs+ had no effect on forskolin-induced potentiation. Interestingly, Beaumont and Zucker (22) observed a remaining PKA-independent potentiation of release that is not mediated by Ih channels. Thus, potentiation may partially share a common mechanism in both preparations. It will be interesting to determine whether some proteins involved in exocytosis possess cAMP-binding sites, such as recently identified cAMP sensitive guanine nucleotide exchange factors (26, 27).
In many preparations, cAMP-induced potentiation of quantal release is caused by an increase in Pr (14, 19). The present results are partially consistent with these findings, because the relationship between Ca2+ influx and release rate was shifted toward lower Ca2+ influxes (Fig. 3). However, potentiation is not caused simply by modulation of Pr, which becomes evident from a comparison between cAMP-mediated potentiation and facilitation by residual Ca2+ (Fig. 4), which is generally attributed to an increase in Pr only (23).
The simplest explanation for the mechanism of potentiation is that potentiation is caused by a selective increase in the number of those synaptic vesicles (see also refs. 28 and 29) which have high Pr. Large potentiation can be observed during smaller presynaptic Ca2+ influxes and especially at the very beginning of a stimulation episode when quanta, which have higher sensitivity for Ca2+, will be the first ones to be released. Less significant potentiation is observed during larger Ca2+ influxes and during the late phase of a prolonged depolarization, because only low Pr quanta, which are not modulated by cAMP, will remain to be released then. Simple simulations suggest that the cAMP-induced shortening of the time to peak release (Fig. 4), which might be interpreted as an increase in Ca2+ sensitivity, can also be explained by a selective increase in the number of high Pr vesicles without changing the Ca2+ sensitivity of such a population (data not shown).
On the other hand, it is possible that an increase in Ca2+ sensitivity and an increase in the number of synaptic vesicles with high Pr occur simultaneously (30). Alternatively, cAMP may modulate the adaptation of exocytotic machineries (31), although we have no evidence for such phenomena at the calyx of Held (7). Furthermore, cAMP may change the degree of colocalization of Ca2+ channels and synaptic vesicles.
Selective modulation of different vesicle populations might also be the mechanism of other forms of synaptic modulation. Augmentation induced by protein kinase C is suggested to result from an increase in the size of the RRP (32), but Yawo (33) suggested that the potentiation at the ciliary ganglion is caused instead by an increase in Pr (see also ref. 34). Perhaps both results may be reconciled if one assumes that the number of high Pr quanta is increased.
Under physiological conditions, the calyx of Held shows pronounced synaptic depression (20). cAMP is expected to potentiate the first few excitatory postsynaptic potentials (EPSPs) during the train, but keeps subsequent EPSPs almost intact (35). The impact of this form of synaptic plasticity on computational aspects of auditory signal processing requires more detailed studies on neural coding (36, 37).
Acknowledgments
We thank Ralf Schneggenburger for very helpful advice during the course of this study. We also thank Jens Retting and Sonja Pyott for critical comments on the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 406 to E.N. and by an Alexander von Humboldt fellowship (to T.S.).
Abbreviations
- EPSC
excitatory postsynaptic current
- PKA
protein kinase A
- mEPSC
miniature excitatory postsynaptic current
- Kyn
kynurenic acid
- IBMX
3-isobutyl-1-methylxanthine
- RRP
readily releasable pool
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021541098.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021541098
References
- 1.Byrne J H, Kandel E R. J Neurosci. 1996;16:425–443. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L G, Saggau P. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 3.Zucker R S. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
- 4.Borst J G, Helmchen F, Sakmann B. J Physiol (London) 1995;489:825–840. doi: 10.1113/jphysiol.1995.sp021095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T, Forsythe I D, Tsujimoto T, Barnes-Davies M, Onodera K. Science. 1995;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 6.Neher, E. & Sakaba, T. (2001) J. Neurosci., in press.
- 7.Sakaba, T. & Neher, E. (2001) J. Neurosci., in press.
- 8.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 9.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Hori T, Kajikawa T, Tsujimoto T. Science. 2000;289:460–463. doi: 10.1126/science.289.5478.460. [DOI] [PubMed] [Google Scholar]
- 11.Goy M F, Kravitz E A. J Neurosci. 1989;9:369–379. doi: 10.1523/JNEUROSCI.09-01-00369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Llano I, Gerschenfeld H M. J Physiol (London) 1993;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavez-Noriega L E, Stevens C F. J Neurosci. 1994;14:310–317. doi: 10.1523/JNEUROSCI.14-01-00310.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weisskopf M G, Castillo P E, Zalutsky R A, Nicoll R A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y Y, Li X C, Kandel E R. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 16.Capogna M, Gähwiler B H, Thompson S M. J Neurosci. 1995;15:1249–1260. doi: 10.1523/JNEUROSCI.15-02-01249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trudeau L E, Emery D G, Haydon P G. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- 18.Kondo S, Marty A. J Physiol (London) 1997;498:165–176. doi: 10.1113/jphysiol.1997.sp021849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen C, Regehr W G. J Neurosci. 1997;17:8687–8694. doi: 10.1523/JNEUROSCI.17-22-08687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Gersdorff H, Schneggenburger R, Weis S, Neher E. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renstrom E, Eliasson L, Rorsman P. J Physiol (London) 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beaumont V, Zucker R S. Nat Neurosci. 1999;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 23.Zucker R S. J Physiol (London) 1973;229:787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vyshedskiy A, Lin J-W. J Neurophysiol. 1997;78:1791–1977. doi: 10.1152/jn.1997.78.4.1791. [DOI] [PubMed] [Google Scholar]
- 25.Helmchen F, Borst J G, Sakmann B. Biophys J. 1997;72:1458–1471. doi: 10.1016/S0006-3495(97)78792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Nature (London) 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki H, Springett G M, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman D E, Graybiel A M. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 28.Chavis P, Mollard P, Bockaert J, Manzoni O. Neuron. 1998;20:773–781. doi: 10.1016/s0896-6273(00)81015-6. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Zablow L, Kandel E R, Siegelbaum S A. Nat Neurosci. 1999;2:24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- 30.Vyshedskiy A, Delaney K R, Lin J-W. J Neurosci. 1998;18:5160–5169. doi: 10.1523/JNEUROSCI.18-14-05160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu S F, Augustine G J, Jackson M B. Neuron. 1996;17:501–512. doi: 10.1016/s0896-6273(00)80182-8. [DOI] [PubMed] [Google Scholar]
- 32.Stevens C F, Sullivan J M. Neuron. 1998;21:885–893. doi: 10.1016/s0896-6273(00)80603-0. [DOI] [PubMed] [Google Scholar]
- 33.Yawo H. J Physiol (London) 1999;515:169–180. doi: 10.1111/j.1469-7793.1999.169ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olekevich S, Walmsley B. J Physiol (London) 2000;526:349–357. doi: 10.1111/j.1469-7793.2000.t01-1-00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markram H, Tsodyks M. Nature (London) 1996;382:807–810. doi: 10.1038/382807a0. [DOI] [PubMed] [Google Scholar]
- 36.Abott L F, Sen K, Varela J A, Nelson S B. Science. 1997;275:220–222. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- 37.Tsodyks M V, Markram H. Proc Natl Acad Sci USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]