Abstract
Sleep promotes the integration of recently acquired spatial memories into cerebral networks for the long term. In this study, we examined how sleep deprivation hinders this consolidation process. Using functional MRI, we mapped regional cerebral activity during place-finding navigation in a virtual town, immediately after learning and 3 days later, in subjects either allowed regular sleep (RS) or totally sleep-deprived (TSD) on the first posttraining night. At immediate and delayed retrieval, place-finding navigation elicited increased brain activity in an extended hippocampo-neocortical network in both RS and TSD subjects. Behavioral performance was equivalent between groups. However, striatal navigation-related activity increased more at delayed retrieval in RS than in TSD subjects. Furthermore, correlations between striatal response and behavioral performance, as well as functional connectivity between the striatum and the hippocampus, were modulated by posttraining sleep. These data suggest that brain activity is restructured during sleep in such a way that navigation in the virtual environment, initially related to a hippocampus-dependent spatial strategy, becomes progressively contingent in part on a response-based strategy mediated by the striatum. Both neural strategies eventually relate to equivalent performance levels, indicating that covert reorganization of brain patterns underlying navigation after sleep is not necessarily accompanied by overt changes in behavior.
Keywords: functional MRI, hippocampus, sleep deprivation, memory consolidation, striatum
The hypothesis that sleep represents a crucial, albeit not always indispensable, neurophysiological state that actively promotes learning-dependent brain plasticity (1–5) has become a main topic of interest in neuroscience. In this view, an active processing of freshly acquired memories may occur in the sleeping brain, supporting the gradual consolidation process by which labile recent memories are restructured and incorporated into stable memories for the long term (6, 7).
Memories that can be consciously and symbolically expressed belong to the declarative memory system. At the neuroanatomical level, their encoding and initial maintenance rely upon the integrity of the medial temporal lobe, with the hippocampus at its core (8, 9). Episodic memory for personally experienced events set in a spatiotemporal context is defined as a subclass of declarative memory (10). Animal (11) and human (12) studies suggest that the hippocampus is involved in spatial learning, because encoding is based on a flexible knowledge of relationships between environmental cues. Hippocampus-dependent spatial memory in animals is thus phylogenetically viewed as a homologue of human episodic/declarative memory (13, 14). However, spatial navigation in a well learned environment may also be mediated by the striatum through the expression of stimulus–response associations (15, 16). Current research further suggests that rodents (17, 18) and humans (19) tend to initially use a hippocampus-dependent scheme early in training and then a striatum-dependent response strategy after repeated practice.
Several studies showed a favorable effect of sleep on the consolidation of hippocampus-dependent memories in human, especially non-rapid-eye-movement sleep that includes slow-wave sleep (SWS) and stage II sleep. SWS deprivation (20, 21) and administration of the cholinergic agonist physostigmine during SWS (21) impair the sleep-dependent retention of lists of word pairs, whereas transcranial direct current stimulation during SWS, but not wakefulness, positively affects their retention (22). In addition, performance at morning or evening recall of word pairs (23) and overnight retention of face–name associations (24) correlate with spindle density during intervening stage II sleep. Likewise, recognition memory performance for landscapes correlates with amounts of SWS gained during a postlearning nap (25). SWS deprivation impairs the retention of spatial rotation abilities (26), and memory for spatial locations is impaired in proportion to lower amounts of SWS in schizophrenic patients (27). Besides, extensive navigation in a virtual maze increases stage II sleep duration on the first posttraining night (28). Finally, our group reported a positron emission tomography study showing that hippocampal activity associated with place learning in a large-scale virtual town during wakefulness increases during SWS (and stage II sleep) on the first posttraining night and more so than after procedural learning (29). Importantly, overnight improvement in navigation accuracy correlated with hippocampal activity during posttraining SWS. These functional brain imaging data contributed to complement the results of electrophysiological studies in rodents, which demonstrated at the cellular level the reexpression of hippocampal and neocortical patterns of spatial-related activity mainly during non-rapid-eye-movement sleep (30, 31).
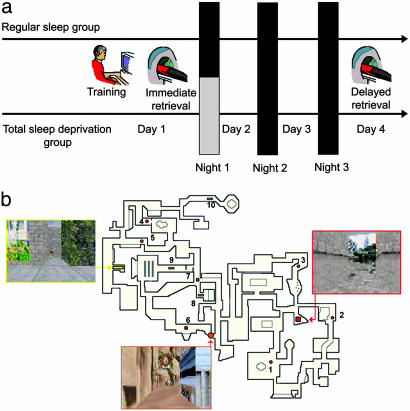
In the present study, we tested the hypothesis that sleep deprivation after spatial learning should hinder the plastic changes in cerebral activity that underpin the long-term consolidation of recent memory traces. To do so, we used functional MRI (fMRI) to map the brain activity of subjects engaged in place-oriented navigation within a complex virtual town, both immediately and 3 days after learning (Fig. 1a and b). On the first posttraining night, half of the subjects were totally sleep-deprived (TSD), whereas the others benefited from regular sleep (RS). All were then allowed two nights of RS before the delayed retrieval session, to preclude in TSD subjects the confounding aftereffect of extended wakefulness on regional cerebral activity, alertness, and behavioral performance on the next day (32, 33).
Fig. 1.
Experimental protocol and memory task. (a) Experimental design. After training on a desktop computer and subsequent testing in the scanner on day 1 (immediate retrieval), subjects were either TSD or allowed RS on the first posttraining night. They were all retested under identical conditions during a second fMRI session on day 4 (delayed retrieval). (b) The map depicts an aerial view of the color 3D virtual town in which subjects navigated at the ground level. Snapshots show the three locations used as targets for testing during the fMRI sessions. The 10 starting points are represented by numbers, with associated symbols indicating the target location to reach.
Results
Navigational Performance.
For each of the 10 tests of place finding administered within each fMRI session, subjects were required to reach, as fast as possible, a given target from one designated starting point. Distance traveled toward destination was the main measure of navigation performance (see Methods). Mean distance scores per session for the TSD group were 6.8 (arbitrary units, SD = 7.2) and 6.2 (SD = 4.2) at immediate (day 1) and delayed retrieval (day 4), respectively. Distances scores were 8.1 (SD = 7.8) at immediate and 8.2 (SD = 5.7) at delayed retrieval for the RS group (Fig. 2a). A two-way ANOVA computed on average within-session individual scores with group (TSD vs. RS) and retrieval session (immediate vs. delayed) factors did not show any significant main effects of group or session or a group-by-session interaction effect [all F(1,20) < 0.45, P > 0.5]. These results suggest that both groups gained a moderate knowledge of the virtual town that persisted 3 days after learning. Most importantly, they revealed that sleep deprivation on the first posttraining night did not alter subjects' ability to find their way in the virtual town at delayed retrieval. Other behavioral measures did not differ between groups at delayed retrieval: number of stops [TSD vs. RS; 1.3 ± 0.6 vs. 1.6 ± 0.7; t(20) = −0.8, P > 0.4] and effective navigation speed [TSD vs. RS; 26.3 ± 3.8 vs. 27.1 ± 3; t(20) = −0.52, P > 0.6].
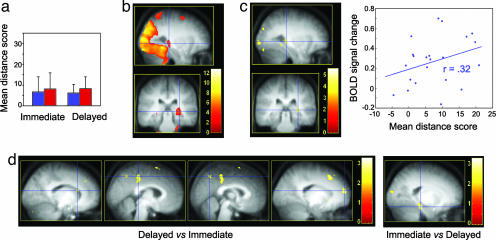
Fig. 2.
Navigation accuracy and place-finding-related brain network. Contrasts are displayed at P < 0.001 (uncorrected) superimposed on the average T1-weighted MRI scan. Color bars code the value of the t statistic associated with each voxel. (a) Mean distance scores at the immediate (on the left) and delayed (on the right) retrieval sessions for the RS (blue) and TSD (red) groups. Error bars are standard deviations. (b) Hippocampo-neocortical network activated in both populations during navigation in the virtual town at immediate retrieval (sagittal and coronal sections). Blue crosshair, right hippocampus (30, −30, 0 mm; Z = 4.33). (c) Brain areas whose activity positively correlated, at the between-subjects level, with accuracy of place finding at immediate retrieval (sagittal and coronal sections). Blue crosshair, right hippocampus (30, −32, 2 mm; Z = 3.65). The scatter plot shows the positive correlation (r = 0.32) between each subject's overall performance and level of activity in the right hippocampus, at the same coordinates. (d) Changes in place-finding-related brain activity between immediate and delayed retrieval sessions (coronal sections). (Left) Brain areas more activated at delayed than immediate retrieval. Blue crosshair, left caudate nucleus (−14, 20, 18 mm; Z = 3.57), right middle cingulate cortex (4, −34, 50 mm; Z = 3.81), right precuneus (2, −62, 50 mm; Z = 3.34), and right dorsolateral prefrontal cortex (18, 58, 18 mm; Z = 4.31). (Right) Decreased activity in the hippocampal complex at delayed as compared with immediate retrieval. Blue crosshair, left hippocampal area (−20, −28, −18 mm; Z = 4.40).
Navigation-Related Brain Activity.
Brain imaging results are reported descriptively at P < 0.001 (uncorrected) in Tables 1–3, which are published as supporting information on the PNAS web site. Only activations found significant after correction for multiple comparisons in a small spherical volume (radius 10 mm; psvc(10 mm) < 0.05; ref. 34) based on a priori coordinates (Table 4, which is published as supporting information on the PNAS web site) will be discussed hereafter.
A random effects analysis (35) revealed increased blood–oxygen level-dependent (BOLD) response in an extended hippocampo-neocortical network during place finding in both the TSD and RS groups (Fig. 2b). At immediate retrieval, activations were observed bilaterally in the hippocampus [30, −30, 0 mm in standard stereotactic space; Z = 4.33; −26, −30, −4; Z = 3.22; psvc(10 mm) <0.05] and surrounding cortex as well as in occipital, parietal, and localized frontal and cerebellar areas (Table 1). Place finding at delayed retrieval elicited increased BOLD activity in the same set of brain structures, including the hippocampus (30, −33, 2; Z = 4.52; psvc(10 mm) <0.05). Correlation analyses indicated a positive relationship between an individual's performance score and navigation-related response in the right hippocampus (Fig. 2c), both at immediate (30, −32, 2; Z = 3.65; Pearson correlation coefficient r = 0.32) and delayed (24, −32, 6; Z = 4.25; r = 0.42) retrieval (psvc(10 mm) <0.05).
Besides these commonalities, variations in brain activity were found between immediate and delayed retrieval sessions, both in TSD and RS subjects. A conjunction analysis using individual between-sessions contrasts from the two groups showed higher activity during place finding at delayed than immediate retrieval in the left (−14, 20, 18; Z = 3.57; psvc(10 mm) <0.05) and right (18, 20, 14; trend Z = 2.74; P < 0.005, uncorrected) caudate nucleus and in a set of neocortical areas located in the middle cingulate cortex, the precuneus, the superior parietal lobule, and the temporal and frontal cortices. Conversely, decreased activity was found in the right (16, −18, −26; Z = 4.34) and left (−20, −28, −18; Z = 3.57) hippocampal regions (psvc(10 mm) <0.05) at delayed (vs. immediate) retrieval (Fig. 2d; see Table 2).
Posttraining Sleep-Dependent Reorganization of Brain Activity.
At delayed retrieval, brain activity during place finding was higher in RS than TSD participants in the right (14, 8, 18; Z = 3.73) and left (−16, 4, 20; Z = 3.24) caudate nucleus (psvc(10 mm) <0.05; Fig. 3a) and in several neocortical areas (Table 3). The opposite contrast (i.e., testing for higher brain response in TSD than RS subjects) did not yield any significant results. Posterior probability maps (36) indicated a very low probability of hippocampal activation in between-groups differences at locations in which variations in activity were observed between sessions (all P values < 13%; see Table 3). Hence, it is unlikely that a lack of significant difference between patterns of hippocampal activation found at delayed retrieval in the TSD and RS groups could be attributed to a failure to detect between-group differences using classical inference statistics.
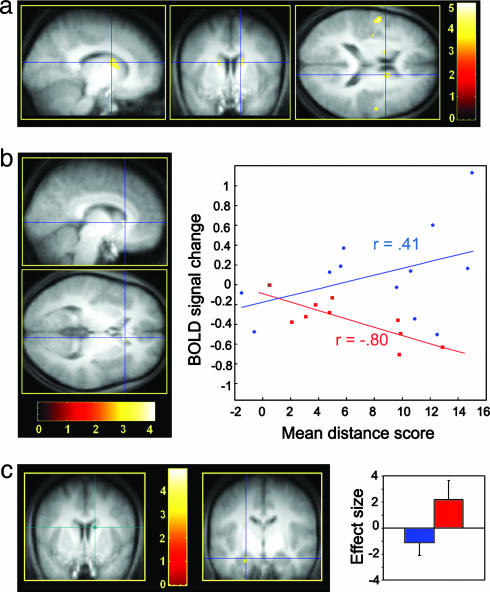
Fig. 3.
Sleep-dependent modulation of brain activity. Contrasts are displayed at P < 0.001 (uncorrected) superimposed on the average T1-weighted MRI scan. Color bars code the value of the t statistic associated with each voxel. (a) Higher activity elicited by place finding for the RS compared with the TSD group at delayed retrieval bilaterally in the caudate nucleus (sagittal, coronal, and axial sections). Blue crosshair, right caudate nucleus (14, 8, 18 mm; Z = 3.73). (b) Between-group regression analyses of the average session performance on cerebral activity at delayed retrieval (sagittal and coronal sections). Blue crosshair, right caudate nucleus (8, 22, 4 mm; Z = 3.45). The scatter plot shows that brain response at this coordinate was correlated positively with performance in the RS group (blue; r = 0.41) but negatively in the TSD group (red; r = −0.80). (c) Psychophysiological interaction analysis using the right caudate nucleus (14, 8, 18 mm; green crosshair) as seed area. The coupling of activity between the caudate nucleus and the left hippocampus (coronal section) was negative in the RS group (blue) but positive in the TSD group (red). Blue crosshair, left hippocampus (22, 12, 22 mm; Z = 3.15). Blue and red plots show the size of effect for each group. Error bars are standard deviations.
A further analysis looked for the brain areas in which activity levels during place finding at delayed retrieval regressed with session mean performance, more (or less) in RS than TSD participants. A significant group by performance interaction was observed in the right caudate nucleus (8, 22, 4; Z = 3.45; psvc(10 mm) <0.05), in which blood–oxygen-level-dependent response was positively coupled with retrieval performance in RS (r = 0.41) but negatively coupled in TSD (r = −0.80) participants (Fig. 3b). These results indicate that higher involvement of the caudate nucleus during place finding is associated with higher navigation accuracy in the RS group but lower accuracy in the TSD group. Because correlations were computed within each group separately, these correlations are independent from the fact that average activity in the caudate nucleus was higher in RS than TSD volunteers at delayed retrieval.
Finally, a psychophysiological interaction analysis (37) tested the hypothesis that the caudate nucleus would additionally establish distinctive functional connections with other brain regions at delayed retrieval in a posttraining sleep-dependent manner. In RS subjects, coupling between the right caudate nucleus (coordinates, 14, 8, 18 mm) and the left hippocampus (−22, −12, −22) activity was negative, whereas it was positive in the TSD group (Z = 3.15, psvc(10 mm) <0.05; Fig. 3c). Similar interaction effects were found in a set of navigation-related neocortical areas (Fig. 4, which is published as supporting information on the PNAS web site). These findings indicate that functional connectivity between the caudate nucleus and the hippocampus was modulated at delayed retrieval by the sleep status on the first posttraining night.
Discussion
The present study shows that sleep after spatial learning promotes the covert reorganization of functional brain activity. In subjects allowed to sleep on the first posttraining night as compared with sleep-deprived subjects, striatal activity was significantly increased during delayed navigation in the learned environment. In addition, the linear relationship between striatal response and navigation accuracy, as well as the functional connectivity between the hippocampus and the striatum, was modulated by the sleep status on the first posttraining night. Importantly, sleep-associated changes in brain activity accompanied equivalent levels of behavioral performance.
It is known from rodent studies that efficient place finding, i.e., the ability to locate a goal within an environment, may rely on either spatial- or response-based (habit) memory systems (11, 13, 15, 38). Spatial memory-related navigation mostly relies upon the hippocampus, which supports an allocentric representation of the environment on the basis of stored relationships between multiple environmental cues. Response memory-related navigation depends on egocentric information processed in the caudate nucleus (striatum) under the form of incrementally acquired stimulus–response associations. Neuropharmacological studies also suggest that rats preferentially perform navigation tasks based on a spatial strategy early in training and on a response scheme later on, after a sufficient amount of practice (17, 18). Likewise, in humans, neuroimaging experiments demonstrated that efficient navigation in virtual environments could be mediated by dissimilar spatial and response cognitive strategies respectively relying on hippocampal and caudate nucleus memory systems (16). fMRI studies in healthy subjects have shown that, as learning of a virtual environment progresses, a shift from a spatial to a response cognitive strategy of navigation may occur, accompanied by increased brain response in the caudate nucleus (19, 39). Moreover, studies conducted in patients suffering from either unilateral hippocampal lesion (39) or caudate nucleus damage arising from Huntington's disease (40) have demonstrated that each system may compensate to some extent for the other one, eventually giving rise to similar levels of behavioral performance.
Changes in memory-related brain activity were observed between retrieval sessions, irrespective of sleep status on the first posttraining night. Whereas hippocampal activity decreased from immediate to delayed retrieval, brain response increased in the caudate nucleus and specific neocortical areas distributed within the temporal, parietal, cingulate, frontal, and prefrontal cortices. These effects cannot be characterized as purely time-dependent, because they might also reflect continued sleep-dependent influences taking place during the two recovery nights. Also, it is unclear whether these activation patterns may reflect the hippocampo-neocortical transfer of information suggested by standard memory consolidation models, which posit that the hippocampus has a time-limited role in the storage of declarative memories, so that their final repository lies in the neocortex (refs. 41 and 42, but see ref. 43). A 3-day interval between sessions might be too short to demonstrate such a transfer effect. Indeed, several previous studies failed to show a diminution of hippocampal involvement in a recognition memory task performed immediately, 1 day or 1 week after learning (44), or even found enhanced retrieval-related hippocampal activity after a consolidation period ranging from 10 min to 1 month (45, 46). However, a recent study revealed a progressive disengagement of the hippocampus during declarative memory retrieval over a time course ranging from a few hours to 3 months (25). Besides, it is unlikely that decreased hippocampal activity in our study resulted from a repetition-suppression effect (47). Stimuli encountered during navigation were already highly familiar at the start of the first fMRI session, and the delay between sessions was probably too long (48). Hence, irrespective of sleep deprivation on the first posttraining night, a relative hippocampal disengagement at delayed retrieval might be accounted for by two forms of systems consolidation processes supporting a gradual transfer of mnemonic representations toward either the neocortex or the striatum.
In the framework of our task, results indicate that sleep on the first posttraining night promotes hippocampo-striatal memory reorganization. The observed patterns of correlation between striatal activity and behavioral performance crucially support the view that the effect of sleep is related to navigation and not to other confounding factors. In addition, the functional connectivity between the hippocampus and the caudate nucleus was modified in a posttraining sleep-dependent manner at delayed retrieval, although the overall level of hippocampal activity was not. As stated above, hippocampus- and caudate nucleus-centered memory systems subtend different cognitive strategies at different time steps of practice, allowing equivalent performance (19, 39). Differential involvement of the hippocampus and striatum at different steps of practice was similarly evidenced by using a classification learning task (49, 50). Additionally, the development of a negative coupling between hippocampal and caudate nucleus responses during learning was reported by using the same task (49, 50). We similarly observed a negative relationship between striatal and hippocampal navigation-related regions at delayed retrieval in the RS group, whereas activity in these regions was positively coupled in TSD subjects. A sensible explanation could be that posttraining sleep-dependent promotion of a response strategy of navigation mediated by the caudate nucleus has competitively prevented the expression of possibly reinforced spatial memories in RS subjects. In the rat hippocampus and striatum, however, acetylcholine release was found to occur in parallel after extended practice within a single session, without intervening sleep (18). The significance of this dissociation in coupling patterns remains to be specifically investigated. In the rat hippocampus, posttraining pharmacological inactivation actually strengthens later caudate nucleus-dependent place finding (51), further showing that interactions between striatal and hippocampal memory systems develop during offline consolidation periods. Our results extend these animal findings by demonstrating that memory systems may interact well beyond the learning episode in humans, a process in which sleep is assigned an important role.
Finally, our behavioral data speak against the confounding possibility that different levels of performance could have explained between-group differences in brain activity. In an unchanged environment, efficiency in place finding could have been equally supported by either a spatial- or response-based strategy, or a combination of both, eventually yielding similar levels of performance. The hippocampo-neocortical network alone is believed to subtend the processing of genuine spatial/declarative memories, thus leading to a map-like cognitive representation of the environment (14, 52), whereas the striatum is thought to support a more automated, less flexible form of navigation (16). The use of navigational tests in which familiar pathways would be blocked, thus forcing subjects to find novel pathways using exclusively a spatial strategy, could possibly lead to the detection of sleep-dependent effects and between-sessions differences in behavioral performance occurring in parallel with changes in brain activity. Note that efficient navigation based on a spatial strategy in TSD subjects may well be explained if hippocampus-dependent memories have been actively maintained during the extended awake period on the first posttraining night in these subjects, and then sleep-dependent consolidation occurred on the following nights. Accordingly, a detrimental influence of sleep deprivation on declarative memory retention was found in studies in which retrieval was assessed immediately after the sleep deprivation episode (3, 20, 26) but not after 1 week (53, 54). Conversely, it could be tested whether navigation is less impaired in the framework of a dual-task paradigm in RS than TSD subjects, under the assumption that the former would be more able to rely on a routine strategy to perform the task in a divided attention condition. Also, a caudate-dependent routine navigation could be characterized by fewer interruptions, that is, stops that would allow explicit retrieval of map-like spatial information needed to plan further moves, provided that the experimental design does not specifically require subjects to be as fast as possible, as in the present study, in which case this behavioral measure could be irrelevant. Future work is needed to assess these hypotheses in the framework of brain imaging studies.
Conclusion
Evidence for a role of sleep in declarative memory processes is mostly based on sleep-dependent changes in behavioral performance. Here, we show that posttraining sleep promotes the long-term reorganization of memory-related brain networks even when overt behavioral performance is unaffected by sleep deprivation. Functional neuroimaging data suggest that sleep favors a shift of brain activity on the long term, so that navigation, which is initially mostly based on a hippocampus-dependent spatial strategy, progressively relies in part on a response-based strategy mediated by the striatum. These findings help to characterize the sleep-for-memory hypothesis by showing that a lack of effect of sleep on overt behavioral performance does not automatically imply that sleep has no covert effect on the brain networks that subtend memory consolidation.
Methods
Subjects.
Twenty-four healthy right-handed volunteers (12 males, 12 females; mean age, 23.5 years; range, 19–29 years) gave their informed written consent to participate in this experiment approved by the Ethics Committee of the University of Liège. They were paid for their participation, were free of neurological or psychiatric disease, and had a normal structural MRI brain scan. In the sleep group (RS; six males, six females), subjects were allowed to sleep as usual at home for the three posttraining nights. In the TSD group (six males, six females), subjects stayed awake in the laboratory the first posttraining night until 7:00 a.m. (Fig. 1a). During this night, participants' physical activity was maintained as low as possible under constant supervision by the experimenters. They had to pursue their usual diurnal activities and slept normally at home on the two following posttraining nights. Data from two TSD subjects were excluded from the analyses because of technical problems during fMRI acquisition. Circadian types were similarly sampled across the two groups. As indicated by self reports, sleep duration and quality were similar between groups during the experimental period except on the first recovery night, when sleep duration was longer for the TSD group than for the RS group, as expected by a stronger drive for sleep after sleep deprivation (55). Further details on sleep parameters are reported in Supporting Text, which is published as supporting information on the PNAS web site.
Navigation Task.
Subjects were trained in a virtual environment adapted from a commercially available computer game (Duke Nukem 3D; 3D Realms Entertainment, Apogee Software, Garland, TX) using the editor provided (Build, Ken Silverman, Realms Entertainment) (29). The environment was a complex town composed of three districts that were made distinct from each other by different visual backgrounds and objects along the streets. Each of these districts contained one target location identified by a rotating medallion. Unknown to the participants during training, the virtual town contained 10 starting points that were each 35 virtual units apart (optimal path) from their associated target location (Fig. 1b). Subjects navigated within the environment at the ground level using a four-direction keypad with their right hand at a constant speed of 1.25 virtual unit per second. During training, the virtual environment was presented on a desktop 800-MHz Pentium III (Siemens, Munich) personal computer (screen size, 17 inches). For testing in the scanner, a mirror allowed the participants to see the display of the virtual town projected on a screen.
On day 1, participants were trained outside of the scanner during a 35-min exploration period. They were explicitly instructed to learn the spatial layout of streets, districts, and target locations by moving freely within the environment. During the entire training session, pictures of the three target locations and their associated names were continuously available to the subject. Immediately after the end of learning on day 1 (immediate retrieval) and on day 4 (delayed retrieval), volunteers were scanned by using fMRI while performing a series of navigation tests in the virtual town. To control for potential circadian interferences, sessions took place at the same time of day (between 10:00 a.m. and 5:00 p.m.) at immediate and delayed retrieval. Each session consisted of 10 blocks of tests, each lasting for 28 s, that alternated with 10 blocks of rest, during which a black screen was displayed for 10–17 s. For each test, participants were assigned a starting point and instructed to reach, as fast as possible, a given target location. Because the target was separated from the starting point by 28 s at constant speed, reaching the destination could be achieved only if they selected the ideal path and, in the meantime, did not stop at all during navigation. After time elapsed, a quantitative measure of behavioral performance was computed by subtracting the shortest distance remaining between the subject's actual location and the final destination from the length of the optimal path. Additional measures of behavior consisted of the number of stops (defined as stops at landmarks or crossroads and U turns) and navigation speed (expressed as the distance effectively traveled during test duration). Within the last 2 s of the rest period preceding each test, the target location for the test was indicated orally through magnetic resonance-compatible headphones. The same 10 tests were administered in a counterbalanced order, both at the between- and within-subjects levels.
Imaging Parameters.
Brain imaging data were obtained by using a 3-T head-only MRI system (Magnetom VISION; Siemens Medical Systems, Erlangen, Germany) equipped with an active shielded gradient coil (30 mT/m). A structural MRI scan using a standard 3D (0.9 × 0.9 × 0.9 mm) T1-weighted sequence was acquired for each subject [repetition time (TR) = 1,960 ms; echo time (TE) = 4.43 ms; flip angle (FA) = 8°; 176 slices; field of view (FoV) = 230 × 173 mm2; matrix size = 256 × 192 × 176; voxel size = 0.9 × 0.9 × 0.9 mm]. For each testing session, 206 functional multislice T2-weighted images were obtained by using a blood–oxygen level-dependent-sensitive single-shot echo planar imaging (EPI) sequence (TR = 2130 ms; TE = 40 ms; FA = 90°; FoV = 220 × 220 mm2; matrix size = 64 × 64 × 32) covering the whole brain (126 mm high). Each functional volume consisted of 32 slices, with a thickness of 3 mm (interslice gap = 1 mm) and a pixel size of 3.4 × 3.4 × 3 mm. The four initial scans of each session were discarded to control for magnetic saturation effects.
fMRI Data Analysis.
A detailed description of fMRI data analysis methods is provided as Supporting Text. Only essential information is reported here. Preprocessing and analysis of functional volumes were performed by using the Statistical Parametric Mapping software spm2 (Wellcome Department of Cognitive Neurology, London) implemented in matlab 6.1 (Mathworks, Sherbom, MA). Preprocessing included realignment and adjustment for movement-related effects, coregistration of functional and anatomical data, spatial normalization into standard stereotactic Montreal Neurological Institute (MNI) space, and spatial smoothing using a Gaussian kernel of 6 mm full width at half maximum (FWHM). Functional data were analyzed by using a mixed-effects model that aimed at showing stereotypical effect in the population from which the subjects are drawn (35). For each subject, a first-level intraindividual analysis tested effects of interest by linear contrasts convolved with a canonical hemodynamic response function, generating statistical parametric maps [SPM(T)]. Movement parameters were included as confounding factors. Cutoff period for high-pass filtering was 128 s. Linear contrasts estimated the main effect of navigation at immediate and delayed retrieval separately, and the difference of navigation-related activity from immediate to delayed retrieval (and conversely). Summary statistic images were thresholded at P < 0.95 (uncorrected) and spatially smoothed (6-mm FWHM Gaussian kernel), then entered into a second-level (random-effects) analysis to evaluate differences and commonalities between groups. To test whether modifications of neuronal activity in navigation-related areas were related to performance in place finding, correlation analyses estimated at the random-effects level the coupling between the mean behavioral performance per session and the effect size at every voxel in individual statistical maps derived from relevant navigation-related contrasts. Additionally, psychophysiological interaction analyses (37) were computed to test the hypothesis that the availability of posttraining sleep would modulate functional connectivity between navigation-related areas. Coordinates of voxels of interest were determined based on results from the random-effects analysis described above. In all analyses, the resulting set of voxel values for each contrast constituted a map of the t statistic [SPM(T)], thresholded at P < 0.001 (uncorrected for multiple comparisons). Statistical inferences were then obtained after corrections at the voxel level by using Gaussian random field theory at psvc(10 mm) < 0.05 corrected in a small spherical volume (34) around a priori coordinates previously reported in neuroimaging experiments that investigated human navigation using virtual reality devices (Table 4). Finally, posterior probability maps enabled conditional or Bayesian inferences about regionally specific effects, allowing us to ensure that a lack of significant statistical effect in a given contrast was not merely due to a failure to detect this effect using classical inferences (36).
Acknowledgments
We thank Dr. V. Bohbot for discussion on this research and E. Van der Loo for help with drawing Fig. 1. This study was funded by the Belgian Fonds National de la Recherche Scientifique (FNRS), Fondation Médicale Reine Elisabeth, Research Fund of the University of Liège, Pole d' Attraction Interuniversitaire P5/04, and Fondation Fyssen. E.B., P.M., and P.O. are supported by FNRS.
Abbreviations
- fMRI
functional MRI
- RS
regular sleep
- SWS
slow-wave sleep
- TSD
totally sleep-deprived.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The neuroimaging data reported in this paper have been deposited with the fMRI Data Center, www.fmridc.org (accession no. 2-2006-121CE).
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Maquet P., Smith C., Stickgold R. Sleep and Brain Plasticity. Oxford: Oxford Univ. Press; 2003. [Google Scholar]
- 2.Peigneux P., Laureys S., Delbeuck X., Maquet P. NeuroReport. 2001;12:A111–A124. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- 3.Gais S., Born J. Learn. Mem. 2004;11:679–685. doi: 10.1101/lm.80504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauchs G., Desgranges B., Foret J., Eustache F. J. Sleep Res. 2005;14:123–140. doi: 10.1111/j.1365-2869.2005.00450.x. [DOI] [PubMed] [Google Scholar]
- 5.Walker M. P., Stickgold R. Annu. Rev. Psychol. 2006;57:1–28. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh J. L. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 7.Dudai Y. Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 8.Scoville W. B., Milner B. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squire L. R., Stark C. E., Clark R. E. Annu. Rev. Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 10.Tulving E. Elements of Episodic Memory. Cambridge, U.K.: Oxford Univ. Press; 1983. [Google Scholar]
- 11.Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrom A. D., Kahana M. J., Caplan J. B., Fields T. A., Isham E. A., Newman E. L., Fried I. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 13.O'Keefe J., Nadel L. The Hippocampus as a Cognitive Map. Cambridge, U.K.: Oxford Univ. Press; 1978. [Google Scholar]
- 14.Burgess N., Maguire E. A., O'Keefe J. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 15.Packard M. G., Knowlton B. J. Annu. Rev. Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 16.Hartley T., Maguire E. A., Spiers H. J., Burgess N. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- 17.Packard M. G. Proc. Natl. Acad. Sci. USA. 1999;96:12881–12886. doi: 10.1073/pnas.96.22.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q., Gold P. E. J. Neurosci. 2003;23:3001–3005. doi: 10.1523/JNEUROSCI.23-07-03001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iaria G., Petrides M., Dagher A., Pike B., Bohbot V. D. J. Neurosci. 2003;23:5945–5952. doi: 10.1523/JNEUROSCI.23-13-05945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plihal W., Born J. J. Cognit. Neurosci. 1997;9:534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 21.Gais S., Born J. Proc. Natl. Acad. Sci. USA. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall L., Molle M., Hallschmid M., Born J. J. Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gais S., Molle M., Helms K., Born J. J. Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemens Z., Fabó D., Halász P. Neuroscience. 2005;132:529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Takashima A., Petersson K. M., Rutters F., Tendolkar I., Jensen O., Zwarts M. J., McNaughton B. L., Fernandez G. Proc. Natl. Acad. Sci. USA. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plihal W., Born J. Psychophysiology. 1999;36:571–582. [PubMed] [Google Scholar]
- 27.Göder R., Boigs M., Braun S., Friege L., Fritzer G., Aldenhoff J. B., Hinze-Selch D. J. Psychiatr. Res. 2004;38:591–599. doi: 10.1016/j.jpsychires.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Meier-Koll A., Bussmann B., Schmidt C., Neuschwander D. Percept. Mot. Skills. 1999;88:1141–1159. doi: 10.2466/pms.1999.88.3c.1141. [DOI] [PubMed] [Google Scholar]
- 29.Peigneux P., Laureys S., Fuchs S., Collette F., Perrin F., Reggers J., Phillips C., Degueldre C., Del Fiore G., Aerts J., et al. Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Wilson M. A., McNaughton B. L. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 31.Skaggs W. E., McNaughton B. L. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 32.Thomas M., Sing H., Belenky G., Holcomb H., Mayberg H., Dannals R., Wagner H., Thorne D., Popp K., Rowland L., et al. J. Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 33.Rogers N. L., Dorrian J., Dinges D. F. Front. Biosci. 2003;8:s1056–s1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 34.Worsley K. J. Hum. Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Penny W., Holmes A. In: Human Brain Function. Frackowiak R., Friston K., Frith C., Dolan R., Price C., Zeki S. J. A., Penny W., editors. London: Academic; 2003. pp. 843–850. [Google Scholar]
- 36.Friston K. J., Penny W. NeuroImage. 2003;19:1240–1249. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 37.Friston K. J., Buechel C., Fink G. R., Morris J., Rolls E., Dolan R. J. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 38.Packard M. G., Hirsh R., White N. M. J. Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohbot V. D., Iaria G., Petrides M. Neuropsychology. 2004;18:418–425. doi: 10.1037/0894-4105.18.3.418. [DOI] [PubMed] [Google Scholar]
- 40.Voermans N. C., Petersson K. M., Daudey L., Weber B., Van Spaendonck K. P., Kremer H. P., Fernandez G. Neuron. 2004;43:427–435. doi: 10.1016/j.neuron.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez P., Squire L. R. Proc. Natl. Acad. Sci. USA. 1994;91:7041–7045. doi: 10.1073/pnas.91.15.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frankland P. W., Bontempi B. Nat. Rev. Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 43.Nadel L., Moscovitch M. Curr. Opin. Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 44.Stark C. E., Squire L. R. Hippocampus. 2000;10:329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Bosshardt S., Degonda N., Schmidt C. F., Boesiger P., Nitsch R. M., Hock C., Henke K. Hippocampus. 2005;15:1026–1040. doi: 10.1002/hipo.20105. [DOI] [PubMed] [Google Scholar]
- 46.Bosshardt S., Schmidt C. F., Jaermann T., Degonda N., Boesiger P., Nitsch R. M., Hock C., Henke K. Cortex. 2005;41:486–498. doi: 10.1016/s0010-9452(08)70189-8. [DOI] [PubMed] [Google Scholar]
- 47.Henson R. N., Cansino S., Herron J. E., Robb W. G., Rugg M. D. Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- 48.Brozinsky C. J., Yonelinas A. P., Kroll N. E., Ranganath C. Hippocampus. 2005;15:557–561. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- 49.Poldrack R. A., Clark J., Pare-Blagoev E. J., Shohamy D., Creso Moyano J., Myers C., Gluck M. A. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 50.Poldrack R. A., Rodriguez P. Neurobiol. Learn. Mem. 2004;82:324–332. doi: 10.1016/j.nlm.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder J. P., Wingard J. C., Packard M. G. Hippocampus. 2002;12:280–284. doi: 10.1002/hipo.10024. [DOI] [PubMed] [Google Scholar]
- 52.Maguire E. A., Burgess N., Donnett J. G., Frackowiak R. S., Frith C. D., O'Keefe J. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 53.Smith C. Behav. Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 54.Smith C. Sleep Med. Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 55.Borbely A. A., Achermann P. J. Biol. Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]