Abstract
Directional cell migration requires proper cell polarization. The redistribution of the Golgi apparatus is an important event in the polarization and migration of many types of cells, as a polarized Golgi supplies membrane components for leading edge protrusion. Direct current electric fields induce directional cell migration in a wide variety of cells. Here we show that electric fields of 300 mV/mm induce robust Golgi polarization and directional cell migration in CHO cells. Asymmetric Src and PI 3-kinase signalling as well as actin polymerization are essential for electric field-induced Golgi polarization and directional cell migration. The Golgi polarizes at the same time as cells change morphology and migrate directionally in response to an electric field. Golgi polarization in turn significantly reinforces and maintains optimal electrotaxis. It is not known whether electrical signals, when contradicting other directional cues, are still able to polarize cells and direct cell migration. Most strikingly, Golgi polarization and cell migration simply follow the direction of an applied electric field and ignore all other cues generated by wounding a monolayer of CHO cells. Thus, an electric field of 300 mV/mm is the predominant cue to polarize the Golgi and direct cell migration mediated by PI 3-kinase and Src signalling.
Keywords: Golgi polarization, Cell polarity, Directional cell migration, Electric field
Introduction
Cell polarization is important for cell migration. It is often defined by membrane domain segregation, intracellular organelle localization, asymmetric cytoskeletal arrangement and the appearance of polarized cell morphology. In motile cells, these features are closely associated with membrane protrusion and directional cell migration (Kupfer et al., 1982; Kupfer et al., 1983; Etienne-Manneville and Hall, 2001; Etienne-Manneville and Hall, 2003; Nobes and Hall, 1999; Ridley et al., 2003; Raftopoulou and Hall, 2004; Gomez-Mouton et al., 2004). These motile cells have a distinctive leading edge, i.e. lamellipodia or pseudopodia where active membrane protrusion occurs (Ridley et al., 2003; Manes et al., 2003; Jaffe and Hall, 2003; Nabi, 1999; Xu et al., 2003; Devreotes and Janetopoulos, 2003). In Dictyostelium cells and neutrophils, chemoattractant gradients induce polarized G-protein-coupled receptor signalling with PI 3-kinase polarized in the direction of the chemoattractant source and Pten or Rho opposite to it (Parent et al., 1998; Meili et al., 1999; Iijima and Devreotes, 2002; Funamoto et al., 2002; Xu et al., 2003). This polarized cascade results in actin polymerization and membrane protrusion at the leading edge and myosin II redistribution to the rear and eventually directional cell migration towards the chemoattractant.
Golgi polarization is an important feature of cell polarization. This is critically involved in directional cell migration, as the Golgi apparatus plays an important role in anterograde supply of membrane components to the leading edge for membrane protrusion (Bershadsky and Futerman, 1994; Nabi, 1999; Ridley et al., 2003; Prigozhina and Waterman-Storer, 2004). Generally, directional sensing, cell polarization and directional migration are closely associated. This, however, is not always the case. Directional sensing is sometimes distinct from cell polarization in Dictyostelium (Devreotes and Janetopoulos, 2003), and cell polarization indicated by polarization of Golgi apparatus (Golgi polarization) can sometimes be uncoupled from cell migration in fibroblasts (Magdalena et al., 2003a; Magdalena et al., 2003b). Significantly, in addition to secretory traffic directed towards the front of a motile eukaryotic cell, signals from the Golgi matrix play an important role in cell motility and in allowing reorientation of the Golgi towards the direction of movement (Mellor, 2004; Preisinger et al., 2004). Despite significant mechanistic insights gained into the molecular mechanisms of Golgi polarization, it is not fully understood how Golgi polarization regulates directional cell migration.
Many extracellular cues are capable of inducing cell polarization and directional cell migration. These include chemoattractant gradients (Nabi, 1999; Xu et al., 2003; Devreotes and Janetopoulos, 2003), mechanical forces (Li et al., 2002; Decave et al., 2003; Wojciak-Stothard and Ridley, 2003) and gradients of adhesiveness (Libotte et al., 2001). Wounding monolayer cultures is one of the most widely used techniques to study molecular mechanisms of polarization and directional migration of many types cells (Kupfer et al., 1982; Kupfer et al., 1983; Etienne-Manneville and Hall, 2001; Etienne-Manneville and Hall, 2003; Nobes and Hall, 1999; Magdalena et al., 2003a; Magdalena et al., 2003b). In this model, multiple candidate cues are generated for cell polarization and migration into the wound. These include the initial mechanical stimulation, loss of contact inhibition at the wound edge, and chemical gradients formed upon wounding (Martin and Parkhurst, 2004; Singer and Clark, 1999; Grose and Martin, 1999).
Direct current (DC) electric fields (EFs) are able to induce directional responses such as cell migration (galvanotaxis/electrotaxis) and cell division in many cell types (Robinson, 1985; Wang et al., 2000; Zhao et al., 1999b; Song et al., 2002). Therefore EFs have been proposed to play a role in directional cell migration in wound healing and development in vivo (Jaffe and Vanable, 1984; McCaig and Zhao, 1997; McCaig et al., 2002; Nuccitelli, 2003a; Nuccitelli, 2003b; Ojingwa and Isseroff, 2003; Robinson and Messerli, 2003). In addition to any potential physiological roles that endogenous EFs may have in vivo, in vitro application of an EF is a convenient and reliable technique to induce predictable cell polarization and directional migration. Using this as a convenient tool to polarize cells, we report that an EF of 300 mV/mm induces Golgi polarization towards the cathode that is mediated by Src and PI 3-kinase signalling. The Golgi polarizes concomitant with changes in cell morphology and migration towards the cathode. Golgi polarization in turn significantly reinforces and maintains optimal electrotaxis. Using a monolayer wound-healing assay, we show that this large EF is a predominant guidance cue directing both Golgi polarization and cell migration in the scratch-wound model.
Materials and Methods
Chemical agents and cell cultures
Cell culture media and reagents were from Gibco. Brefeldin A (BFA), cytochalasin D (CyD), PP2 (Src inhibitor), Y27632 were from Calbiochem (La Jolla, CA). LS203 (Cdc42 inhibitor) was a kind gift of L. Smith (synthesized and prepared by Proteomics Facility, University of Aberdeen) (Vastrik et al., 1999). CHO cells were grown in NM-F12 medium, with 5% fetal bovine serum (FBS), 50 U/ml penicillin, 50 μg/ml streptomycin. CO2- independent medium was used for experiments performed in room atmosphere. CHO cells were starved for 3 hours in serum-free NM-F12 medium prior to experiments. For wound-healing assays, a confluent monolayer grown in the electrotactic chamber was scratch-wounded using a 10 μl tip. Wounded CHO cells were subject to an EF for 3 hours. All experiments were performed within passage five.
Electric field stimulation and drug treatment
CHO cells were seeded at low density in electrotactic chambers on Falcon tissue culture dishes 16–20 hours before EF exposure. A roof consisting of a No. 1 coverglass was applied and sealed with DC4 on top of the chamber as described (Zhao et al., 1996). The final dimensions of the chamber, through which the electric current was passed, were 40×10×0.3 mm. A DC EF of 300 mV/mm was applied through agar-salt bridges connecting silver/silver chloride electrodes via beakers of Steinberg’s solution to pools of culture medium at either side of the chamber. The dish was placed on a Zeiss Axiovert 100 microscope with temperature control at 37°C. For inhibition experiments, CHO cells were pretreated with PP2 (20 μM), BFA (1 μM, 5 μM), cytochalasin D (0.2 μM), Y27632 (50 μM), Toxin B (10 ng/ml), LS203 (100 ng/ml) or Wortmannin (500 nM) for 2 hours, then exposed to EF in the presence of the same concentration of inhibitor.
Immunofluorescence microscopy
For immunofluorescence staining, CHO cells were fixed in 4% paraformaldehyde (15 minutes), permeabilized (5 minutes in 0.2% Triton X-100) and blocked for 30 minutes with blocking solution (10% goat serum, 1% BSA and 0.02% NaN3 in PBS). A monoclonal antibody against GM130 (1:100, BD Transduction Laboratories) was used to label the Golgi apparatus for 1 hour at room temperature. Monoclonal antibody clone 28 (0.3 μg/ml) kindly provided by M. K. Owada (Kyoto Pharmaceutical University, Kyoto, Japan) (Yamada et al., 2000) was used to label active forms of c-Src tyrosine kinases (pSrc), and anti-phospho-Akt (1:100, Cell Signalling) to stain active Akt (pAkt) for 2 hours at room temperature. Akt is a serine/threonine kinase and its encoding nucleotide sequence was originally identified in the AKT8 virus isolated from a spontaneous thymoma of an AKR mouse (Staal, 1987). It is also called protein kinase B (PKB) (Coffer and Woodgett, 1991; Downward, 1995). Akt/PKB is a downstream signalling target of PI 3-kinase. After washing, the cells were incubated with Texas Red-conjugated secondary antibodies (1:200 Jackson Immuno Research Laboratories) and phalloidin-FITC (1:100 Sigma) for 1 hour at room temperature. Nuclei were stained with DAPI. Images were obtained with a Zeiss inverted fluorescence microscope (Axiovert 100) controlled with MetaMorph software. A 40× Fluor oil-immersion lens was used.
Cell polarization analysis
CHO cells cultured in an EF of 300 mV/mm for 3 hours showed distinct polarized morphology (Fig. 1). Cells with the long axis of the cell body falling in the quadrant between 45° and 315° of the EF direction were scored as polarized in the EF direction (Fig. 1G). This criterion was used for scoring polarization of Golgi, F-actin, pSrc and pAkt. The percentage of polarized cells±s.e.m. was calculated from three separate experiments.
Fig. 1.
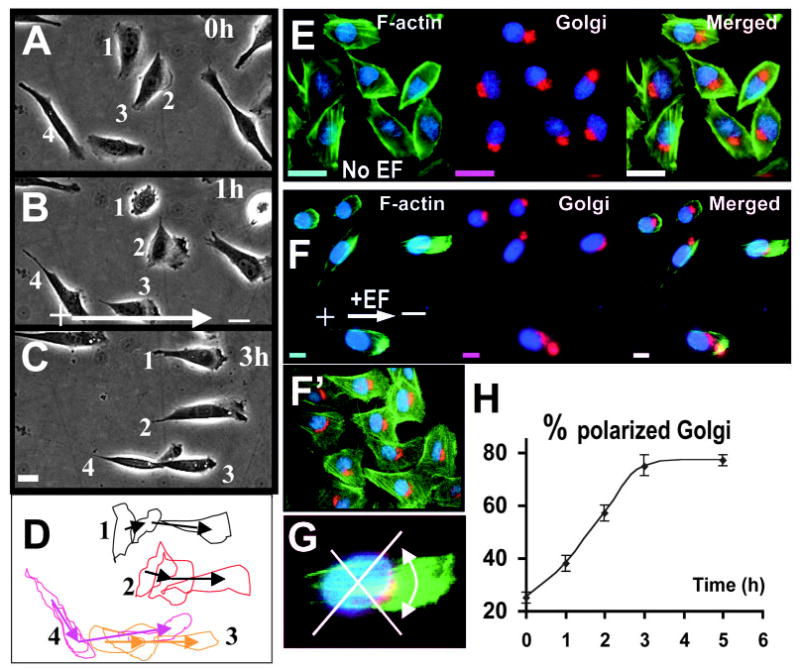
An electric field of 300mV/mm directs CHO cell migration and Golgi polarization. (A–C) Time-lapse images showing morphological polarization and directional migration of CHO cells in a DC EF. (D) Outlines of the labelled cells from A–C highlight morphological polarization and cell migration. (E–F) Golgi (red) polarization and actin (green) distribution in CHO cells cultured in the absence (E) or presence (F) of an EF for 3 hours. The cells were fixed and triple-labelled with GM130 antibody (Golgi marker, red), FITC-phalloidin (F-actin, green) and DAPI (blue). (F′) Golgi polarization in control experiments with cross-current medium flow where chemical gradients, ionic gradients and fluctuation in temperature and pH were eliminated. (G) Cells with the Golgi falling in the indicated quadrant were scored. (H) An EF of 300 mV/mm increased the percentage of cells with the Golgi polarizing towards the cathode with time. Results shown are the mean±s.e.m. For each time point, a minimum of 300 cells was scored from three independent experiments. Bar, 10 μm. See also supplementary material video 1.
Time-lapse video microscopy and quantification of cell migration
Time-lapse images were recorded every 10 minutes and analysed with a MetaMorph system (Universal Imaging Corporation, PA) (Zhao et al., 2002a). Migration directedness (cos θ) shows how a cell migrated directionally within the field, where θ is the angle between the EF vector and a straight line connecting the start and end position of a cell (Zhao et al., 1996). A cell moving perfectly toward the cathode would have a directedness of 1; a cell moving perfectly along the field lines toward the anode would have a directedness of −1. Therefore, the average of directedness values of a population of cells gives an objective quantification of how directionally cells have moved. A group of cells migrating randomly would have an average directedness value of 0. Migration rate was analysed with the following three parameters. Trajectory speed (Tt/T) is the total length of the migration trajectory of a cell (Tt) divided by the given period of time (T). Displacement speed (Td/T) is the straight-line distance between the start and end positions of a cell (Td) divided by the time (T). Displacement speed along the x axis (Dx/T) is a cell’s displacement distance along the x axis (Dx) divided by the time (T).
Fluorescence video imaging of Golgi polarization and cell migration
CHO cells were cultured on glass coverslips coated with fibronectin (5 μg/cm2, Sigma) overnight. After rinsing with HBSS, the cells were incubated for 15 minutes at 37°C with 2.5 μM BODIPY FL ceramide-BSA complex (Molecular Probes). The cells were allowed to recover in fresh NM-F12 medium (5% FBS) for 1 hour before time-lapse recording of bright field and fluorescence images with MetaMorph system. A 40× Fluor oil-immersion objective was used. Statistical analyses were made using unpaired, two-tailed Student’s t-test. Data are expressed as mean±s.e.m.
Results
Electric field-directed cell polarization and migration
In an applied EF of 300 mV/mm, CHO cells elongated and polarized with distinct lamellipodia facing the cathode (Fig. 1A–D, supplementary material video 1). Cells extended lamellipodia and started to migrate towards the cathode within 10–30 minutes of EF application. Simultaneously the cells reoriented and elongated to lie parallel to the field vector (Fig. 1A–D). Electric stimulation increased the number of cells with a polarized morphology consisting of a leading edge lamellipodia and a trailing tail. Exposure to an EF of 300 mV/mm for 3 hours resulted in 76% of the cells showing distinct polarized and elongated morphology, significantly higher than that in a control culture without an applied EF (19%) (Fig. 1C, Table 1). It is interesting to note that CHO cells aligned with the long axis of cell body parallel to, instead of perpendicular to the EF vector, which is very different from other cell types.
Table 1.
Percentage of cells with polarized morphology and Golgi apparatus
| EF
|
||||||||
|---|---|---|---|---|---|---|---|---|
| No EF | No drug | BFA | CyD | PP2 | Toxin B | Y27632 | LS203 | |
| Golgi polarization | 24±2 (204) | 75±4* (198) | 30±2† (199) | 18±3† (201) | 31±3† (199) | 53±2*,† (202) | 49±2*,† (190) | 61±2*, † (195) |
| Morphology polarization | 19±1 (105) | 76±3* (101) | 35±4† (115) | 5±1*,† (110) | 34±7*,† (100) | 60±2*,† (101) | 51±2*,† (105) | 66±3*, † (108) |
CHO cells were starved and pretreated with inhibitors for 2 hours, then subjected to an EF of 300 mV/mm for 3 hours. Data are presented as mean±s.e.m. of three independent experiments. The total numbers of cells are in brackets.
P<0.01 when compared with the no EF value, P<0.01 when compared with the EF no-drug value.
In an applied EF of 300 mV/mm, CHO cells migrated directionally towards the cathode with a directedness value of 0.93±0.01 (mean±s.e.m. n=45). A directedness value of 1 represents 100% of the cells moving with a net displacement in the direction of the cathode. Thus, a directedness value of 0.93±0.01 indicates that nearly all of the cells migrated directionally towards cathode. The trajectory and displacement speeds increased significantly by about two- and threefold respectively, compared to the no EF control data (P<0.001) (Table 2). CHO cells cultured in the electrotactic chamber without an applied EF moved randomly, with an average directedness of 0.06±0.10 (mean±s.e.m., n=45) and a displacement speed of 4.4±0.4 μm/hour (n=45).
Table 2.
Migration rates (μm/hour) of CHO cells
| EF
|
||||||||
|---|---|---|---|---|---|---|---|---|
| NO EF | No drug | BFA | CyD | PP2 | Toxin B | Y27632 | LS203 | |
| Trajectory speed | 7.9±0.6 | 16.6±0.9* | 10.0±0.4† | 3.6±0.2*,† | 7.7±0.6† | 11.5±0.5*,† | 11.3±0.3*,† | 13.0±0.4*,† |
| Displacement speed | 4.4±0.4 | 14.3±0.6* | 6.4±0.4† | 1.7±0.1*,† | 6.0±0.6† | 8.5±0.5*,† | 7.7±0.3*,† | 11.2±0.4*,† |
| Displacement speed along x-axis | 0.1 ±0.5 | 13.5±0.7* | 2.5±0.5† | 0.1±0.1† | 4.0±0.7*,† | 6.7±0.5*,† | 5.8±1.1*,† | 9.0±0.5*,† |
| Directedness (average cosθ) | 0.06±0.03 | 0.93±0.02* | 0.28±0.07† | 0.01±0.08† | 0.40±0.03*,† | 0.73±0.04* | 0.62±0.01*,† | 0.78±0.03* |
| n (cell number) | 45 | 45 | 90 | 45 | 60 | 90 | 90 | 90 |
Definitions of the parameters are given in the Materials and methods. CHO cells were starved and pretreated with inhibitors for 2 hours, then subjected to an EF of 300 mV/mm for 3 hours. Data are presented as mean±s.e.m. of three independent experiments.
P<0.01 when compared with the no EF value
P<0.01 when compared with the EF no-drug value.
An EF of 300 mV/mm represents a 7.5 mV drop across a CHO cell with average diameter of ~25 μm. CHO cells have a membrane potential of 73 mV (Rotoli et al., 1991). Thus the theoretical perturbation of the membrane potential is only about 5% of the resting potential.
Electric field-directed Golgi polarization and asymmetric actin polymerization
In CHO cells cultured in an applied EF of 300 mV/mm both the Golgi apparatus and actin filaments polarized towards the cathode (Fig. 1F–H), whereas in cells cultured without an applied EF, the Golgi apparatus and actin filaments polarized randomly (Fig. 1E). The percentage of Golgi polarized towards the cathode (Fig. 1G) increased gradually during the first 3 hours in an EF. Additional exposure to EF did not increase the value further (Fig. 1H). Exposure to an EF polarized the distribution of actin filaments towards the cathode in 62.7±4.5% cells (n=161) (Fig. 1F; 300 mV/mm for 2 hours), whereas very few cells (~4%) showed F-actin polarization when cultured in the absence of an EF (Fig. 1E).
Golgi polarization is the direct effect of the applied electric fields
To exclude the possibility that Golgi polarization might be an indirect effect mediated by chemical or ionic gradients, temperature or pH variation, we conducted a more stringent control using a cross-current medium flow chamber modified from that described (Erickson and Nuccitelli, 1984; Bai et al., 2004). In this experimental set-up, the electrotactic chamber was perfused constantly with the culture medium in a direction perpendicular to the EF vector. A continuous flow of copious culture medium through the electrotactic chamber was maintained with a peristaltic pump during the entire experimental period (24 ml/hour through a 0.2 mm2 channel). This eliminated possible build up of chemoattractant or ionic gradients and maintained a stable temperature and pH across the chamber during experiments. The Golgi polarization was virtually the same as in experiments performed in the absence of medium flow (Fig. 1F′, compared with Fig. 1F) (64.8±2%, n=583 cells from three independent experiments in cross-current fluid flow experiments). Therefore, Golgi polarization in single CHO cells is mainly due to the direct effects of the applied EF, rather than indirectly by chemical gradients caused by the applied EFs.
Actin polymerization is required for Golgi polarization
To test whether EF-directed Golgi polarization requires actin polymerization, CHO cells were treated with cytochalasin D. Cell morphology and Golgi polarization was assessed quantitatively using the same method as Fig. 1G. Cytochalasin D completely inhibited cell morphology changes, cell polarization, cell migration and Golgi polarization (Fig. 2A,B).
Fig. 2.
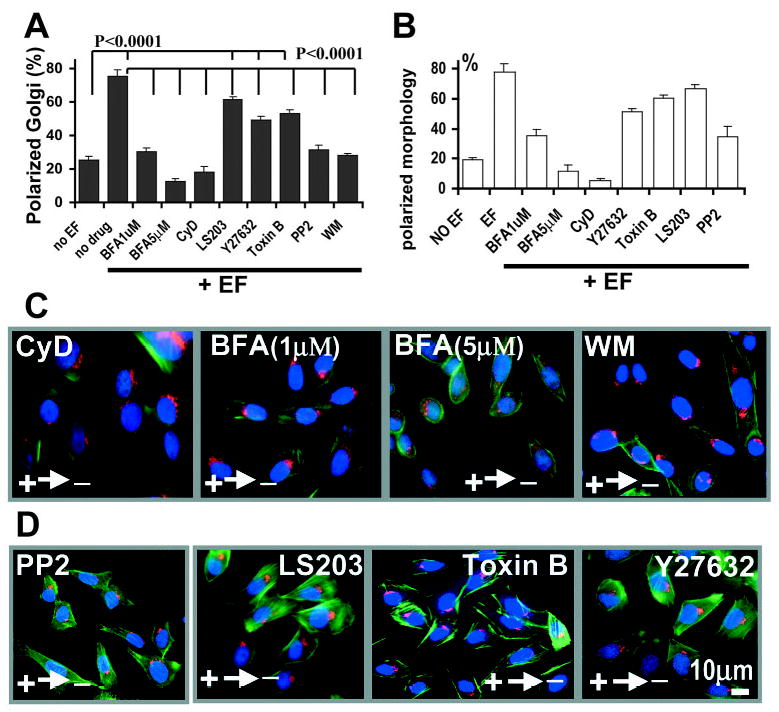
Cathodal polarization of Golgi requires actin polymerization and signalling of Src and PI 3-kinases. (A,C,D) EF-induced Golgi polarization was abrogated by incubation of the cells with Brefeldin A (BFA), Cytochalasin D (CyD), Wortmannin (WM, PI 3-kinase inhibitor) and PP2 (Src inhibitor). Toxin B, LS203 (cdc42 inhibitor) and Y27632 (ROCK inhibitor) decreased Golgi polarization significantly (P<0.0001), but not completely (P<0.0001) (A). (B) The percentage of cells with their morphology polarized towards the cathode was also scored. For drug treatment, CHO cells were pretreated with PP2 (20 μM), BFA (1 μM, 5 μM), CyD (0.2 μM), Y27632 (50 μM) or LS203 (100 ng/ml) for 2 hours, then subjected to an EF of 300 mV/mm for 3 hours, fixed and triple-stained with phalloidin (green), GM130 (red) and DAPI (blue). Golgi polarization was scored as in Fig. 1G. More than 200 cells from three independent experiments were scored for each condition. Bar, 10 μm.
Golgi polarization versus Golgi dispersal and directional cell migration
Brefeldin A (BFA) prevents the assembly of cytosolic coat proteins onto Golgi membranes, resulting in the formation of Golgi tubules and prevents tubule detachment from the Golgi structure which then fuses with the endoplasmic reticulum (ER). This leads to rapid diffusion of Golgi membrane into the ER and disruption of Golgi. BFA at high concentrations of 2–5 μg/ml (~18 μM) causes this rapid dispersal of the Golgi within 5–8 minutes after addition of the drug (Presley et al., 1998; Sciaky et al., 1997; Hirschberg et al., 1998). We used a similar concentration of BFA (15 μM) and found the same quick dispersal of the Golgi apparatus. This treatment resulted in discoid cell morphology. It is impossible to analyse Golgi polarization and cell migration as there is no visible Golgi staining with BODIPY FL ceramide and the cells do not move.
In order to analyse how Golgi polarization regulates cell migration, we chose much lower concentrations of BFA (1 μM and 5 μM). These concentrations represent only 5.6% and 28% of the concentration (5 μg/ml) used to induce dramatic Golgi dispersal (Sciaky et al., 1997; Presley et al., 1998; Hirschberg et al., 1998). BFA at 5 μM caused significant dispersal of Golgi (Fig. 2C), which significantly decreased Golgi polarization and the morphologic polarization induced by an EF (Fig. 2A,B). A lower concentration of BFA (1 μM) was able to abolish EF-induced Golgi polarization completely whereas Golgi staining was unambiguous for polarization scoring (Fig. 2A,C). We therefore chose this concentration for further experiments. BFA at 1 μM significantly inhibited EF-directed cell migration (Figs 4, 5). This indicates that BFA at lower concentrations (1 μM) did cause derangement of Golgi complex reorientation, although did not cause complete dispersal of Golgi. This derangement in Golgi reorientation significantly decreased EF-directed cell migration (see the following section).
Fig. 4.
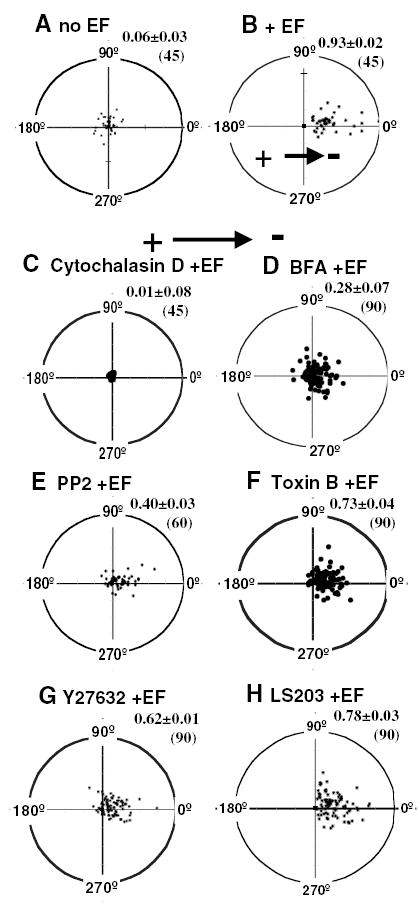
The net translocation of CHO cells in an EF. Cells were followed for a 3-hour period in an EF (B–H) or without an EF (A). Each cell’s position at t=0 is represented by the origin (0,0), with the final position of each cell at 3 hours plotted as a single point on the graph. The radius of each circle represents 100 μm (the average cell length is ~25 μm). n, the total number of cells at a given condition. Values of directedness (the average cosine of the distribution) ±s.e.m. are indicated in the upper right corner of each plot. (C–H) Treated with different inhibitors then in an EF with continuous presence of the same drug. EF, 300 mV/mm.
Fig. 5.
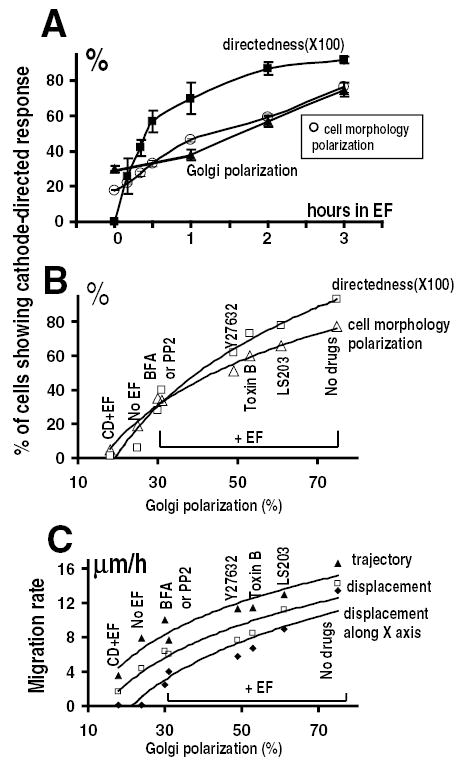
Cathodal Golgi polarization and electrotaxis of CHO cells in an EF. (A) Time course of directional cell migration, morphologic polarization and Golgi redistribution. (B) Correlation of Golgi polarization with morphologic polarization and directedness of cell migration. (C) Correlation of Golgi polarization with migration rates in an EF. In B and C, CHO cells were pretreated as indicated with BFA (1 μM, 5 μM), CyD (0.2 μM), PP2 (20 μM), Y27632 (50 μM), Toxin B (10 ng/ml) or LS203 (100 ng/ml) for 2 hours and subjected to an EF for 3 hours with presence of the drug. Cell migration, morphologic polarization and Golgi polarization were analysed as in Materials and methods. Each data point in A represents mean±s.e.m. from at least 200 cells from three independent experiments.
Treatment with either CyD or BFA completely abolished Golgi polarization in an EF. These data indicate that cathodal redistribution of Golgi and actin are interdependent, as inhibition of actin polymerization completely inhibited Golgi polarization, and inhibition of Golgi redistribution abrogated the polarization of cell morphology and F-actin (Fig. 2).
Essential role of PI 3-kinase and Src signalling in Golgi polarization
To identify signalling elements involved in Golgi polarization, we chose PI 3-kinase as a target. PI 3-kinase signalling is the compass mechanism in chemotactic directional sensing in Dictyostelium, neutrophils and fibroblasts (Ilijima and Devreotes, 2002; Servant et al., 2000; Xu et al., 2003; Firtel and Chung, 2000; Haugh et al., 2000). Treatment with wortmannin completely abolished EF induced Golgi polarization and the changes in cell morphology (Fig. 2). In fibroblast cells, Src tyrosine kinase activity is required for cell polarity establishment via small GTPases, the Rho family members Racl and Cdc42 (Timpson et al., 2001). PP2, a specific inhibitor for Src tyrosine kinases also completely inhibited Golgi and cell morphologic polarization (Fig. 2). More importantly, Akt and Src were activated preferentially at the cathodal side of cells (Fig. 3). In an EF of 300 mV/mm for 1 hour, significant proportion of cells showed redistribution of activated Akt (pAkt) (56±5%, n=154) and Src (pSrc) (62±6%, n=156) towards the cathode (Fig. 3B,D) whereas control cells without EF did not show this polarization (Fig. 3A,C). This polarized signalling is similar to that underlying directional sensing in chemotactic cells (Parent et al., 1998; Firtel and Chung, 2000; Servant et al., 2000; Haugh et al., 2000; Gomez-Mouton et al., 2004).
Fig. 3.
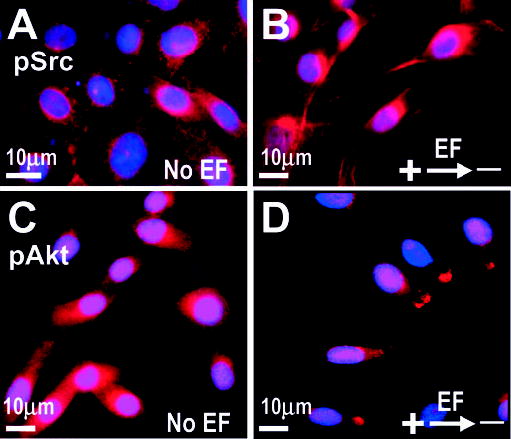
Phospho-Akt and phospho-Src are distributed to the cathodal facing side of CHO cells in a DC EF. CHO cells were fixed after 1 hour in an EF of 300 mV/mm (B,D) or without an EF (A,C) and labelled with anti-phospho-Src (pSrc, red, A,B) or anti-Phospho-Akt antibody (pAkt, red, C,D). Nuclei were labelled with DAPI (blue).
Rho family GTPase and EF-induced Golgi polarization
ROCK (Rho-associated protein kinases) and cdc42 are required for Golgi polarization in fibroblast 3T3 cells and in primary rat astrocytes (Magdalena et al., 2003a; Nobes and Hall, 1999; Etienne-Manneville and Hall, 2003). Y27632 was used to inhibit p160ROCK and Toxin B was used as a general inhibitor of Rho, Rac and Cdc42. Y27632 (50 μM) or Toxin B (10 ng/ml) significantly (P<0.0001) decreased the polarization of both Golgi and cell morphology in an EF (Fig. 2). We used LS203 (a synthesized short peptide) to verify these results. LS203 inhibits cdc42 signalling by binding to the binding motifs of PAK and N-WASP for cdc42/Racl (Vastrik et al., 1999). LS203 reduced the polarization of Golgi and cell morphology to a similar extent as observed with Toxin B treatment (Fig. 2). However, cells treated with all three inhibitors still showed significant (P<0.0001) Golgi and morphologic polarization (Fig. 2). This indicates that the Rho family of small GTPases play an important role in EF induced cell polarization, but they are only partially involved.
Golgi polarization correlates well with but lags slightly behind electrotaxis of CHO cells
To establish the role of Golgi polarization in EF-directed cell migration, we correlated Golgi polarization to the electrotactic response of CHO cells treated with different drugs (Figs 4, 5). In control experiments in the absence of an EF the cells migrated randomly (Fig. 4A). When subjected to an EF of 300 mV/mm, cells migrated toward the cathode (Fig. 4B). Inhibition of actin polymerization using Cytochalasin D completely inhibited the migration of CHO cells (Fig. 4C). BFA (1 μM) significantly decreased the migration rate and directedness (Table 2, Fig. 4D). PP2 abolished Golgi polarization and decreased trajectory speed to the level of non-EF treated cells (Tables 1, 2; Fig. 4E). Inhibition of Rho family small GTPases with Y27632, Toxin B or LS203 (cdc42 inhibitor) decreased the migration rate to a greater extent than they decreased the directedness of migration (Fig. 4F–H).
In an EF, the directedness value of cell migration rose more rapidly than the percentage of cells showing cathodally directed Golgi polarization in the first hour (Fig. 5A). Subsequently the percentage of cells with polarized Golgi and morphology gradually caught up with the migration directedness (Fig. 5A).
Inhibition of Golgi polarization with different drugs accordingly inhibited the EF-induced directional migration and polarized morphology. The directedness of cell migration and cell morphologic polarization correlated significantly (P<0.0001) with Golgi polarization (Fig. 5B). Correlation coefficients of 0.98 and 0.99 indicate the linear regression of cell morphologic polarization and migration directedness on the percentage of Golgi polarization in an applied EF (300 mV/mm for 3 hours).
Golgi polarization also correlated linearly with the migratory indexes. The correlation coefficients of Golgi polarization to displacement speed along the x axis, displacement speed and trajectory speed are 0.97, 0.94 and 0.92 respectively (Fig. 5C).
Golgi polarization reinforces electrotaxis of CHO cells
BFA (1 μM) inhibited Golgi polarization completely (Fig. 2A), but significant electrotaxis remained (Fig. 4D). This suggests that Golgi polarization is not essential for electrotaxis. However, BFA treatment did significantly inhibit electrotaxis by reducing the directedness value from 0.93 in to 0.28 (compare Fig. 4B with4D) (P<0.0001). To further test the role of Golgi polarization in the electrotactic response of CHO cells, we did multichannel time-lapse video imaging (Fig. 6). Active membrane protrusion occurred at the cathodal side ~30 minutes after application of an EF of 300 mV/mm (Fig. 6B). In most cases, only the protrusion next to the Golgi persisted (Fig. 6C). Then the cell changed morphology, moved and polarized towards the cathode. At the same time, the Golgi moved to polarize in the same direction (Fig. 6D). This showed that Golgi polarization lagged slightly behind membrane protrusion but occurred concomitantly with cell morphologic polarization and cell movement toward the cathode. At this stage, electrotactic response, the directional cell movement towards the cathode (along the x axis in EF direction), was slow and cells moved preferentially along the y axis (compare Fig. 6H to 6J). Following Golgi polarization in the direction of the EF, net migration towards the cathode significantly increased, and little movement along the y axis was observed (Fig. 6J). Trajectory speed, displacement speed, x axis displacement speed and directedness of migration increased significantly after the Golgi polarized in the direction of the EF (Fig. 6K-L; P<0.05). These results show that although Golgi polarization is not essential for the electrotaxis of CHO cells (Fig. 4D), it can significantly enhance directional migration when it is polarized in the direction of the EF (Fig. 6).
Fig. 6.
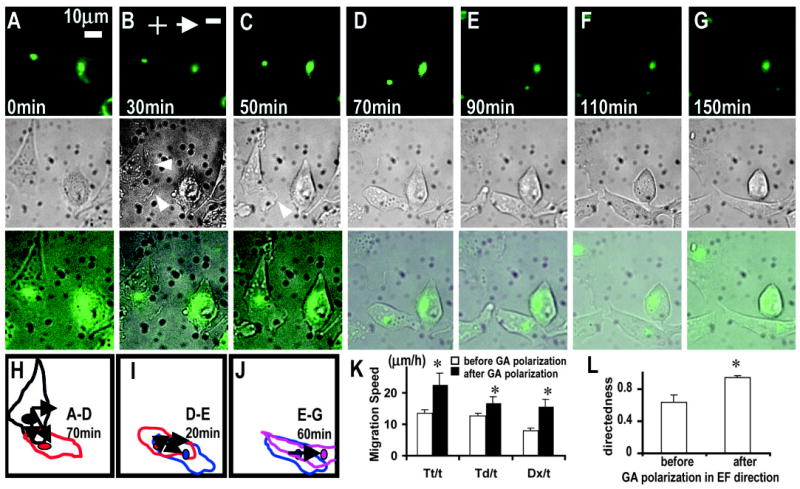
Golgi polarization lags behind membrane protrusion, but can significantly enhance directional cell migration when Golgi polarizes in the EF direction. (A) Both the cell morphology and the Golgi of the cell at the left polarized downward before the onset of EF. (B) Active membrane protrusion happened at the cathodal side 30 minutes after onset of the EF (arrowheads). (C) Only the protrusion next to the Golgi persisted (arrow head). (D) The cell then moved and polarized as well as having the Golgi polarized towards the cathode. After this, net migration towards the cathode increased markedly. The directedness value and displacement speed along the x axis were significantly higher than that before the Golgi polarized in the EF direction (compare J with H and I). Note that the cell on the right did not move and the Golgi did not repolarize. (K,L) Golgi polarization towards the cathode significantly increases the trajectory speed, displacement speed and x-axis displacement speed, and directedness (*P<0.05). n=8 from three independent experiments. See also supplementary material video 2.
An applied EF of 300 mV/mm is a predominant cue in directing Golgi polarization
Scratch wounding of a monolayer culture induces robust polarization of the cells towards the wound in many cell types. Golgi polarize towards the wound in CHO cells at the wound edge (Fig. 7A). Directional cues such as mechanical stimulation, wound void and chemical gradients are present in this model. We tested the hierarchical order of the electrical signal as a directional cue in relation to the other cues. When an EF of 300 mV/mm was applied with the field vector in the normal healing direction, the Golgi polarized more directionally towards the wound (Fig. 7B). Remarkably, when an EF was applied opposite to the normal healing direction, Golgi polarized away from the wound (Fig. 7C). This is against the direction of other coexisting cues. EFs applied with the field vector in or opposite to the normal healing direction resulted in the same percentage of cells showing Golgi polarization towards or away from the wound, respectively (Fig. 7G). The mechanical signals were proposed to provide a crucial directional cue (Martin and Parkhurst, 2004). Clearly, in our experimental system the mechanical cues were present throughout and were being overridden by the applied electrical signals (Fig. 7C). What we do not know at this stage is whether the chemical gradients formed at the wound were affected by the large electric field applied. Chemical gradients inevitably form at the wound (Hansen et al., 1993; Sammak et al., 1997; Klepeis et al., 2001). It is likely that the strong EF may influence the chemical gradients and thus may mediate the directional response.
Fig. 7.
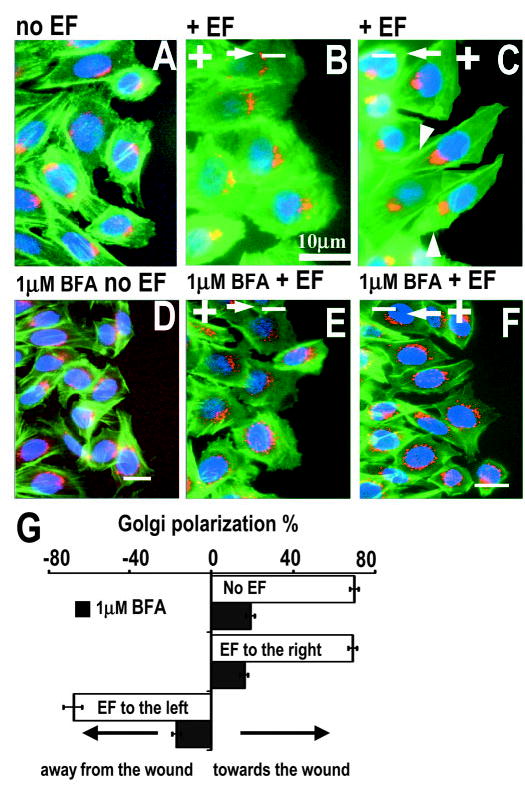
An electric field of 300 mV/mm predominates over other directional cues in directing Golgi polarization in the scratch-wound model. Monolayer culture of CHO cells were wounded and allowed to heal for 4 hours (A) without an EF; (B) with an EF applied in the normal healing direction, or (C) opposite to the normal healing direction (the EF was applied 1 hour after wounding for 3 hours). (C) An EF applied against the default healing direction polarized the Golgi away from the wound, completely ignoring the wound direction. Some degree of F-actin polarization in EF direction can be seen (C, arrowheads). The three cells on the right in C have been focally enhanced to show clearer cell contours. BFA completely inhibited both scratch wounding and EF-induced Golgi polarization (D–G). The cells were triple-labelled with F-actin (green), GM130 (red) and DAPI (blue). Cells along the wound edge were scored as polarized if the Golgi was orientated to the right (for A,B,D,E) or to the left (for C,F) as in Fig. 1G. The percentage of polarized cells±s.e.m. was calculated from the means of three independent experiments from 255~595 cells (G). Bar, 10 μm.
Therefore, an EF of 300 mV/mm predominated over and/or modified other coexisting cues in this scrape-wound model in directing Golgi polarization. In some cells actin filaments appeared to polarize in the direction of the EF against the normal healing direction (Fig. 7C). BFA at a very low concentration of 1 μM completely abolished the Golgi polarization induced by wounding and application of an EF (Fig. 7D–G).
An applied EF is a predominant cue in directing CHO cell migration
When applied opposite to the normal healing direction, an EF of 300 mV/mm directed cells at the wound edge to migrate away from the wound (Fig. 8A–C,G). The cells migrated in the same direction as the Golgi polarized (Fig. 7). This indicates that an EF of 300 mV/mm is able to predominate over and/or modify other coexisting cues in directing cell migration as well as polarizing Golgi in this scratch-wound model.
Fig. 8.
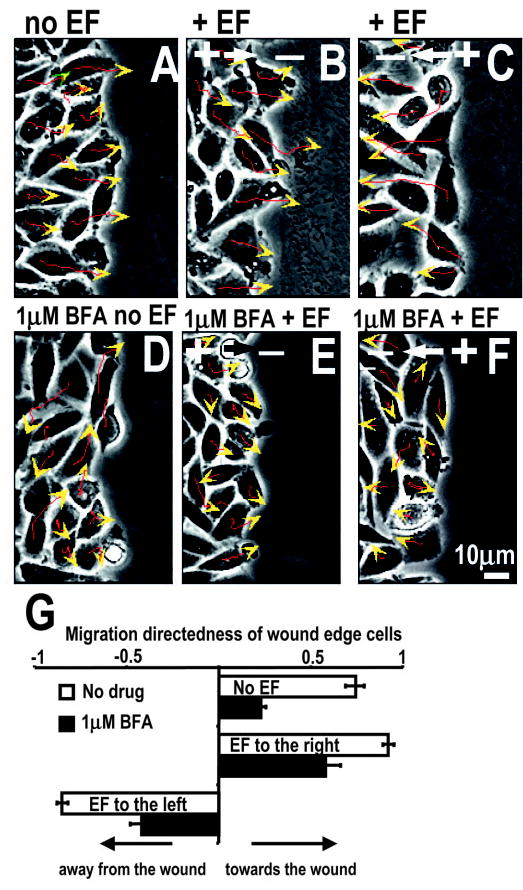
An electric field of 300 mV/mm predominates over other directional cues in directing cell migration in the scratch wound model. Migration trajectories of individual cells are indicated by red lines and direction and endpoint of a 3-hour experiment indicated by arrows. (A) No EF control, wound edge moved into the wound. (B) an EF applied with the field vector in normal healing direction enhanced cell migration into the wound (P<0.001). (C) An EF applied with the field vector opposite to the normal healing direction directed the cells at the wound edge to migrate away from the wound. BFA significantly decreased but did not completely abolish directional cell migration induced by wounding and/or an applied EF (D–G). Directness was calculated from 60 cells collected from three independent experiments (G). See also supplementary material video 3.
BFA completely abolished Golgi polarization induced by wounding and/or an applied EF (Fig. 7D–F). Accordingly, directional migration of wound edge cells was significantly inhibited. Nevertheless, definitive directional migration remained (Fig. 8D–G). Cell tracings demonstrated significantly improved directional cell migration when Golgi polarized in the migration direction (Fig. 8A–C) compared to that when Golgi polarization was inhibited (Fig. 8D–G). This supports the notion that Golgi polarization is not essential for the EF-directed cell movement, but rather permits optimal directional migration in this large electric field.
Discussion
EFs induce persistent and predictable cell polarization and directional cell migration. It is a useful and convenient tool to dissect the intracellular mechanisms of directional polarization and migration (Zhao et al., 2002a; Zhao et al., 2002b; Finkelstein et al., 2004). As EFs direct both Golgi polarization and cell migration, we are able to dissect the relationship between the two events in dissociated cells and in monolayer wounds where multiple cues are present. Using CHO cells, we have shown that: (1) an electrical signal induces pronounced Golgi polarization towards the cathode; (2) Src and Akt are activated at the cathodal side of the cells in an EF and are essential for EF induced polarization of the Golgi apparatus and cell morphology; (3) the Rho family of small GTPases play an important role in EF-induced Golgi polarization, but their inhibition does not completely abolish Golgi polarization; (4) EF-induced Golgi polarization is not essential for electrotaxis, however, Golgi polarization reinforces and maintains an optimal electrotactic cell migration; (5) Most significantly, an EF of 300 mV/mm appears to be a predominant cue directing Golgi polarization and cell migration in the monolayer scratch-wound model. This overriding effect is likely to be mediated through direct and indirect mechanisms.
Parallel orientation of CHO cells in an electric field
Mammalian cells, including human cornea epithelial cells and keratinocytes migrate directionally towards the cathode. Endothelial cells migrate to the anode. Migrating either toward the cathode or anode, those cells align with the long axis of cell body perpendicular to the EF vector (Robinson, 1985; Zhao et al., 1996; Zhao et al., 1997; McCaig and Zhao, 1997; Zhao et al., 2004). CHO cells however polarize very differently; they align their cell bodies in parallel with the EF line with distinct lamellipodia in the direction of migration towards the cathode (Fig. 1C). This orientation of polarized cell morphology is completely different from most other types of cells cultured in an EF. It has been proposed that cells align perpendicular to an EF to minimize the stress of the voltage drop across the cells (Robinson, 1985). CHO cells and one other type of cells we tested, non-small-cell lung cancer cells (A549), are two exceptions. Instead of aligning perpendicular to the field vector, A549 cells align parallel when cultured in an EF (B. Song, J. P. and M.Z., unpublished data). Dissociated CHO cells in our culture system are normally ~25 μm in diameter (cells 1, 2 in Fig. 1A). This produces a voltage drop of about 7.5 mV per cell diameter. When they elongate in an EF of 300 mV/mm, the length of cell along the field vector increased significantly (more than doubled) (compare cells 1, 2 in Fig. 1A with cells 1, 2 in 1C). Thus the voltage drop across the cell doubled (>15 mV). Therefore, minimizing the voltage drop across the cell might not explain why the cells assume a perpendicular orientation in an EF.
Direct versus indirect effects of EFs on Golgi polarization
To exclude possible effects of gradients of chemoattractants, ions and pH on cell polarization, we performed cross-current fluid flow experiments (Erickson and Nuccitelli, 1984; Bai et al., 2004). Golgi polarized to the same extend in experiments where continuous flow of copious culture medium perpendicular to the EF direction was maintained throughout the experiments (Fig. 1F′). Therefore, Golgi polarization in single cells should be mainly due to the direct effects of the applied EF, rather than indirect means.
In the monolayer wound-healing model however, chemical gradients would inevitably form (Hansen et al., 1993; Sammak et al., 1997; Klepeis et al., 2001). Such chemical gradients may be strongly affected by the large EF. It is not yet known whether the chemical gradients play a role in Golgi and cell polarization and cell movement into this wound model. If so, it is likely that the applied EF may induce cell polarization and migration indirectly through modification of the chemical gradients, at least partially. How the applied EFs affect the chemical gradients and what effects this has on cell polarization and migration in the scratch-wound model needs further investigation.
Golgi polarization versus Golgi dispersal and directional cell migration
BFA, at high concentrations causes rapid dispersal of Golgi apparatus into endoplasmic reticulum (ER) (Presley et al., 1998; Sciaky et al., 1997; Hirschberg et al., 1998). In order to analyse how Golgi polarization affects cell migration, we chose a much lower concentration of BFA (1 μM). BFA at 1 μM abolished EF induced Golgi polarization (Fig. 2A) and significantly inhibited EF-directed cell migration (Figs 4, 5, 7, 8). This indicates that BFA at lower concentrations (1 μM) does cause upset in Golgi complex reorientation and inhibits cell migration, although it does not cause complete dispersal of the Golgi. Therefore, the effects of BFA on Golgi polarization and dispersal are highly concentration dependent.
Golgi polarization in response to extracellular cues
Scratch-wounding monolayer cultures of fibroblast, astrocytes and endothelial cells generates reproducible and consistent cues to induce Golgi polarization (Etienne-Manneville et al., 2001; Etienne-Manneville et al., 2003; Nobes and Hall, 1999; Magdalena et al., 2003a; Magdalena et al., 2003b). It is assumed that there are two categories of directional cues: mechanical and chemical. An EF applied in contradiction to these cues in the model overrode their effects on Golgi polarization and directional migration. Golgi polarization and cell migration simply followed the field direction and ignored other cues (Figs 7, 8). Mechanical cues were present in the experiments and were clearly overridden by the strong electric field applied. However, it is very likely that chemical cues might be strongly affected by the large applied EF. As chemical gradients inevitably form at the wounds, perhaps part of the overriding effect is mediated by local chemical gradients modulated by the electric field.
Intracellular signalling mechanisms of EF-induced Golgi polarization and electrotaxis
PI 3-kinase and Src are required for Golgi polarization in the directional cell migration of fibroblast cells (Haugh et al., 2000; Magdalena et al., 2003a). The two kinases are activated at the leading edge of fibroblasts responding to platelet-derived growth factor gradients and keratinocytes in the scratch-wound model (Haugh et al., 2000; Yamada et al., 2000). An applied EF redistributes activated PI 3-kinase and Src towards the cathode (Fig. 3). We have shown that in cornea epithelial cells, applied EFs asymmetrically redistribute membrane receptors (EGF receptor) and activate its downstream signalling target, extracellular regulated kinase (ERK1/2), in a polarized manner towards the cathode (Zhao et al., 1999a; Zhao et al., 2002b). This may finally lead to asymmetric actin polymerization and directional migration towards the cathode.
Rho family members of small GTPases, mainly Rho, Rac1 and cdc42, are important for cell polarity establishment. However, different directional cues and different types of cells appear to involve different Rho family members. In response to scratch wounds, astrocytes and fibroblasts polarize the Golgi into the wound, which requires cdc42 (Etienne-Manneville and Hall, 2001; Etienne-Manneville and Hall, 2003; Nobes and Hall, 1999). Polarization of endothelial cells in response to shear stress, however, is not mediated by cdc42 or PI 3-kinases, but by Rho and Rac (Wojciak-Stothard and Ridley, 2003). EF-induced directional responses involve these three major Rho family members but substantial polarized morphology and Golgi remained (Fig. 2). This suggests that EF-induced Golgi polarization may involve some different signalling pathways partially bypassing Rho small GTPases.
Golgi polarization and directional cell migration
Cell polarization is believed to be a prerequisite for directional cell migration. In chemotaxis, directional sensing mechanisms are activated first, cells then polarize in the right direction and migrate directionally in chemoattractant gradients. During the first hour in an EF, the rate of polarization of the Golgi was slower than both the appearance of a polarized morphology and the increase in the directedness of cell migration (Fig. 5A). The earliest response (<10 minutes) to an applied EF is the membrane protrusion on the cathodal side of the cells (Fig. 6 and supplementary material video 2). Not all the protrusions persisted. Most often, only the protrusion next to the Golgi remained. Polarization of cell morphology, Golgi and cell migration happened concomitantly (Fig. 6).
Before Golgi polarization in the EF direction, cells moved more in the direction Golgi polarizes than towards the cathode. Nevertheless movement of the cells and appearance of a polarized morphology facing the cathode were evident (Fig. 6H). This suggests that although Golgi polarization, morphology polarization and long-lasting cell migration are linearly correlated (Fig. 5B,C), Golgi polarization did not causally precede the appearance of the polarized morphology and directional cell migration in an EF. Golgi polarization however did reinforce and maintain electrotaxis of CHO cells as failure of Golgi to polarize or disruption of Golgi significantly decreased electrotactic responses (Tables 1, 2; Figs 5–8).
In summary, we have shown that an EF of 300 mV/mm is a predominant directional cue sustaining Golgi polarization and electrotaxis. An EF activates Src and Akt asymmetrically, which mediates Golgi polarization and directional cell migration. Golgi polarization is not a causal prerequisite for electrotactic migration, but its polarization reinforces and maintains directional cell migration. Application of an EF offers an easy and useful laboratory technique to dissect out the cellular and molecular mechanisms of directional cell behaviour. Given its predominant directional effects, electrical signals may have potential use in directing cell polarization and migration in cell and tissue engineering.
Acknowledgments
We thank the Wellcome Trust for continuous support. M.Z. holds a Wellcome Trust University Award (058551). The work was also supported by the Wellcome Trust grant (068012). We thank Colin McCaig and Christine Pullar for their critical reading of the manuscript and Lynne Shanley for her comments.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/118/6/1117/DC1
References
- Bai H, McCaig CD, Forrester JV, Zhao M. DC electric fields induce distinct preangiogenic responses in microvascular and macrovascular cells. Arterioscler Thromb Vase Biol. 2004;24:1234–1239. doi: 10.1161/01.ATV.0000131265.76828.8a. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Futerman AH. Disruption of the Golgi apparatus by brefeldin A blocks cell polarization and inhibits directed cell migration. Proc Natl Acad Sci USA. 1994;91:5686–5689. doi: 10.1073/pnas.91.12.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffer PJ, Woodgett JR. Molecular cloning and characterisation of a novel putative protein-serine kinase related to the cAMP-dependent and protein kinase C families. Eur J Biochem. 1991;201:475–481. doi: 10.1111/j.1432-1033.1991.tb16305.x. [DOI] [PubMed] [Google Scholar]
- Decave E, Rieu D, Dalous J, Fache S, Brechet Y, Fourcade B, Satre M, Bruckert F. Shear flow-induced motility of Dictyostelium discoideum cells on solid substrate. J Cell Sci. 2003;116:4331–4343. doi: 10.1242/jcs.00726. [DOI] [PubMed] [Google Scholar]
- Devreotes P, Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- Downward J. Signal transduction. A target for PI (3) kinase. Nature. 1995;376:553–554. doi: 10.1038/376553a0. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Nuccitelli R. Embryonic fibroblast motility and orientation can be influenced by physiological electric fields. J Cell Biol. 1984;98:296–307. doi: 10.1083/jcb.98.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-Mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCζ. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Finkelstein E, Chang W, Chao PH, Gruber D, Minden A, Hung CT, Bulinski JC. Roles of microtubules, cell polarity and adhesion in electric-field-mediated motility of 3T3 fibroblasts. J Cell Sci. 2004;117:1533–1545. doi: 10.1242/jcs.00986. [DOI] [PubMed] [Google Scholar]
- Firtel RA, Chung CY. The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. Bioessays. 2000;22:603–615. doi: 10.1002/1521-1878(200007)22:7<603::AID-BIES3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Mouton C, Lacalle RA, Mira E, Jimenez-Baranda S, Barber DF, Carrera AC, Martinez-A C, Manes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004;164:759–768. doi: 10.1083/jcb.200309101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose R, Martin P. Parallels between wound repair and morphogenesis in the embryo. Semin Cell Dev Biol. 1999;10:395–404. doi: 10.1006/scdb.1999.0326. [DOI] [PubMed] [Google Scholar]
- Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci. 1993;106:995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–1280. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Devreotes P. Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell. 2002;109:599–610. doi: 10.1016/s0092-8674(02)00745-6. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Cell biology. Smurfing at the leading edge. Science. 2003;302:1690–1691. doi: 10.1126/science.1092874. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Vanable JW., Jr Electric fields and wound healing. Clin Dermatol. 1984;2:34–44. doi: 10.1016/0738-081x(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Klepeis VE, Cornell-Bell A, Trinkaus-Randall V. Growth factors but not gap junctions play a role in injury-induced Ca2+ waves in epithelial cells. J Cell Sci. 2001;114:4185–4195. doi: 10.1242/jcs.114.23.4185. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organzing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci USA. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci USA. 2002;99:3546–3551. doi: 10.1073/pnas.052018099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libotte T, Kaiser HW, Alt W, Bretschneider T. Polarity, protrusion-retraction dynamics and their interplay during keratinocyte cell migration. Exp Cell Res. 2001;270:129–137. doi: 10.1006/excr.2001.5339. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Zhao M. Physiological electrical fields modify cell behaviour. Bioessays. 1997;19:819–826. doi: 10.1002/bies.950190912. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Has electrical growth cone guidance found its potential? Trends Neuroscience. 2002;25:354–359. doi: 10.1016/s0166-2236(02)02174-4. [DOI] [PubMed] [Google Scholar]
- Magdalena J, Millard TH, Etienne-Mannevelle S, Launay S, Warwick HK, Machesky LM. Involvement of the Arp2/3 complex and Scar2 in Golgi polarity in scratch wound models. Mol Biol Cell. 2003a;14:670–684. doi: 10.1091/mbc.E02-06-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdalena J, Millard TH, Machesky LM. Microtubule involvement in NIH 3T3 Golgi and MTOC polarity establishment. J Cell Sci. 2003b;116:743–756. doi: 10.1242/jcs.00288. [DOI] [PubMed] [Google Scholar]
- Manes S, Ana Lacalle R, Gomez-Mouton C, Martinez-A C. From rafts to crafts: membrane asymmetry in moving cells. Trends Immunol. 2003;24:320–326. doi: 10.1016/s1471-4906(03)00137-6. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–2105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor H. Cell motility: Golgi signalling shapes up to ship out. Curr Biol. 2004;14:R434–R435. doi: 10.1016/j.cub.2004.05.038. [DOI] [PubMed] [Google Scholar]
- Nabi IR. The polarization of the motile cell. J Cell Sci. 1999;112:1803–1811. doi: 10.1242/jcs.112.12.1803. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003a;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Endogenous electric fields in embryos during development, regeneration and wound healing. Radiat Prot Dosimetry. 2003b;106:375–383. doi: 10.1093/oxfordjournals.rpd.a006375. [DOI] [PubMed] [Google Scholar]
- Ojingwa JC, Isseroff RR. Electrical stimulation of wound healing. J Invest Dermatol. 2003;121:1–12. doi: 10.1046/j.1523-1747.2003.12454.x. [DOI] [PubMed] [Google Scholar]
- Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- Presley JF, Smith C, Hirschberg K, Miller C, Cole NB, Zaal KJ, Lippincott-Schwartz J. Golgi membrane dynamics. Mol Biol Cell. 1998;9:1617–1626. doi: 10.1091/mbc.9.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisinger C, Short B, de Corte V, Bruyneel E, Haas A, Kopajtich R, Gettemans J, Barr FA. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164:1009–1020. doi: 10.1083/jcb.200310061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Curr Biol. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Robinson KR. The responses of cells to electrical fields: a review. J Cell Biol. 1985;101:2023–2027. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR, Messerli MA. Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays. 2003;25:759–766. doi: 10.1002/bies.10307. [DOI] [PubMed] [Google Scholar]
- Rotoli BM, Bussolati O, Dall’Asta V, Gazzola GC. Membrane potential and amino acid transport in a mutant Chinese hamster ovary cell line. J Cell Physiol. 1991;146:417–424. doi: 10.1002/jcp.1041460312. [DOI] [PubMed] [Google Scholar]
- Sammak PJ, Hinman LE, Iran PO, Sjaastad MD, Machen TE. How do injured cells communicate with the surviving cell monolayer? J Cell Sci. 1997;110:465–475. doi: 10.1242/jcs.110.4.465. [DOI] [PubMed] [Google Scholar]
- Sciaky N, Presley J, Smith C, Zaal KJM, Cole N, Moreira JE, Terasaki M, Siggia E, Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Song B, Zhao M, Forrester JV, McCaig CD. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc Natl Acad Sci USA. 2002;99:13577–13582. doi: 10.1073/pnas.202235299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal SP. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci USA. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpson P, Jones GE, Frame MC, Brunton VG. Coordination of cell polarization and migration by the Rho family GTPases requires Src tyrosine kinase activity. Curr Biol. 2001;11:1836–1846. doi: 10.1016/s0960-9822(01)00583-8. [DOI] [PubMed] [Google Scholar]
- Vastrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17–32. Curr Biol. 1999;9:991–998. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- Wang E, Zhao M, Forrester JV, McCaig CD. Re-orientation and faster, directed migration of lens epithelial cells in a physiological electric field. Exp Eye Res. 2000;71:91–98. doi: 10.1006/exer.2000.0858. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang F, van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- Yamada T, Aoyama Y, Owada MK, Kawakatsu H, Kitajima Y. Scraped-wounding causes activation and association of C-Src tyrosine kinase with microtubules in cultured keratinocytes. Cell Struct Funct. 2000;25:351–359. doi: 10.1247/csf.25.351. [DOI] [PubMed] [Google Scholar]
- Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Orientation and directed migration of cultured corneal epithelial cells in small electric fields are serum dependent. J Cell Sci. 1996;109:1405–1414. doi: 10.1242/jcs.109.6.1405. [DOI] [PubMed] [Google Scholar]
- Zhao M, McCaig CD, Agius-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- Zhao M, Dick A, Forrester JV, McCaig CD. Electric field directed cell motility involves upregulated expression and asymmetric redistribution of the EGF receptors and is enhanced by fibronectin and lamin. Mol Biol Cell. 1999a;10:1259–1276. doi: 10.1091/mbc.10.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Forrester JV, McCaig CD. A small, physiological electric field orients cell division. Proc Natl Acad Sci USA. 1999b;96:4942–4946. doi: 10.1073/pnas.96.9.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Jin T, McCaig CD, Forrester JV, Devreotes PN. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis. J Cell Biol. 2002a;157:921–927. doi: 10.1083/jcb.200112070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Pu J, Forrester JV, McCaig CD. Membrane lipids, EGF receptors, and intracellular signals colocalize and are polarized in epithelial cells moving directionally in a physiological electric field. FASEB J. 2002b;16:857–859. doi: 10.1096/fj.01-0811fje. [DOI] [PubMed] [Google Scholar]
- Zhao M, Bai H, Wang E, Forrester JV, McCaig CD. Electrical stimulation directly induces pre-angiogenic responses in vascular endothelial cells by signaling through VEGF receptors. J Cell Sci. 2004;117:397–405. doi: 10.1242/jcs.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]


