Abstract
Background: Nelfinavir, an HIV protease inhibitor with numerous drug–drug interactions, is associated with dyslipidemia. Pravastatin is the preferred statin prescribed for HIV-associated dyslipidemia.
Objective: To examine the effect of nelfinavir on pravastatin pharmacokinetics.
Design: Open-label study in healthy HIV-seronegative adults conducted at the AIDS Clinical Trials Group sites in the United States.
Methods: Subjects received pravastatin 40 mg daily and underwent intensive sampling for pharmacokinetics on day 3. Subjects took only nelfinavir 1250 mg twice daily on days 4–12. On days 13–15, subjects continued nelfinavir and reinitiated pravastatin. Plasma samples were collected over 24 h for the calculation of pravastatin area under the concentration–time curve for 0–24 h on days 3 and 16.
Results: Data from 14 subjects with complete pharmacokinetic samples were available for analysis. The median within-subject percentage change in pravastatin AUC was a decrease of 46.5%. Pravastatin maximum plasma concentrations were also lower when pravastatin was administered with nelfinavir. Median values for the maximum plasma concentrations were 27.9 and 12.4 ng/ml for days 3 and 16, respectively, and the median within-subject decrease was 40.1%.
Conclusions: Coadministration of pravastatin and nelfinavir led to a substantial reduction in pravastatin plasma concentrations. Higher doses of pravastatin may need to be prescribed in order to achieve optimal lipid-lowering activity.
Keywords: HIV infection, statins, pravastatin, nelfinavir, drug interactions
Introduction
Nelfinavir (NFV) is an HIV protease inhibitor (PI) that has demonstrated numerous drug–drug interactions and is also associated with dyslipidemia, which may require lipid-lowering therapy [1]. This therapy uses 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors known as statins. In a previous study, we demonstrated that simvastatin should not be used with ritonavir/saquinavir because of the large increases in plasma concentrations from inhibition of CYP3A4 metabolism [2]. Similarly, atorvastatin exposure was also increased by CYP3A4 inhibitors [2,3]. Pravastatin was likely to be safe when coadministered with ritonavir/saquinavir because, instead of an increase, we observed a 50% decrease in pravastatin area under the concentration–time curve (AUC). Although we also showed that pravastatin did not alter NFV pharmacokinetics, we did not examine the effect of NFV on pravastatin pharmacokinetics. More recently, we have demonstrated that a non-nucleoside reverse transcriptase inhibitor efavirenz, which is a CYP3A4 inducer, also reduced the plasma exposure of pravastatin [4].
The mechanism by which these drugs reduce plasma exposure to pravastatin is unknown, but this drug–drug interaction is of concern because a reduction in pravastatin plasma concentration may adversely affect the drug's ability to lower serum cholesterol effectively. NFV is one of the few PI drugs in clinical use that is not usually combined with ritonavir, but NFV has a similar effect on the CYP enzymes as ritonavir in that it is an inhibitor of CYP3A4 but an inducer of other CYP isoforms. Pravastatin metabolism is complex, utilizing both oxidative and conjugative enzymes for elimination, but CYP3A4 is not a significant enzyme in its metabolism. This study examines the hypothesis that NFV would have a similar effect on pravastatin pharmacokinetics as ritonavir/saquinavir.
Methods
The AIDS Clinical Trials Group (ACTG) study A5108 was a phase I, open-label, multiple-dose, pharmacokinetic drug interaction study. The primary objectives were to examine the effect of efavirenz on the pharmacokinetics of pravastatin, atorvastatin, and simvastatin and the effect of NFV on the pharmacokinetics of pravastatin. The efavirenz–statin interactions were recently published [4]. The data presented here address the effect of NFVon the pharmacokinetics of pravastatin. At the time of this study design, NFV was the most commonly prescribed PI in antiretroviral-naive patients.
Subjects
Healthy HIV-seronegative volunteers were recruited. The planned sample size was 14 subjects. Subjects who did not complete both pharmacokinetics evaluations were replaced. Subjects were accrued to the NFV arm from August through December 2001.
Study design
Subjects self-administered pravastatin 40 mg daily in the morning for the first 3 days and on the fourth day pravastatin was administered under observation. Blood samples were obtained before drug administration as well as 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 h after administration, for pravastatin pharmacokinetics. On days 4 through 12, subjects took NFV 1250 mg twice daily with meals. On days 13 through 15, subjects continued NFV and restarted pravastatin with the morning dose of NFV. On day 16, pravastatin and NFV doses were administered under observation and samples were collected over 24 h for pravastatin pharmacokinetics. Fasting serum total cholesterol, triglycerides, and high density lipoprotein concentrations were measured at baseline and on days 3, 13, and 16 using standard enzymatic assays. Low density lipoprotein (LDL) was calculated using the Friedewald equation. Adherence to study medications was measured using pill counts and questionnaires.
The human subjects committees of each participating institution and the Division of AIDS, National Institute of Allergy and Infectious Diseases, approved this study and informed consent was obtained from all subjects.
Pravastatin assay
Samples for pravastatin plasma concentrations were analyzed by Advion Biosciences Inc. (Ithaca, New York, USA) as previously published [2,4].
Statistical analyses
The primary endpoint was the pravastatin AUC from 0 to 24 h before and after coadministration of NFV. Values for AUC were estimated according to the linear trapezoidal rule [5] implemented using a macro written in SAS version 8.2 (SAS Institute, Cary, North Carolina, USA). There was no extrapolation beyond the dosing interval. The maximum plasma concentration (Cmax), defined as the maximum concentration observed throughout the dosing interval, was also analyzed statistically. If both the 0 and 24 h concentrations or if both study day samples were unavailable for a given subject, pharmacokinetics parameters were not calculated. The Wilcoxon signed rank test [6], applied to within-subject differences in AUC and in Cmax values, was used to test the null hypothesis of no difference in pravastatin exposure before initiation of NFV versus after dosing to steady state. Values of Cmax were of interest because high plasma concentrations of statins may be associated with toxicity [7]. Pravastatin exposure when taken with NFV relative to when taken alone, median within-subject differences, and 90% confidence intervals (CI) around geometric mean ratios are reported. The last are recommended by the US Food and Drug Administration to evaluate bioequivalence and drug interactions [8].
Results
The study enrolled 18 subjects and three discontinued for protocol-defined toxicities: one had grade 2 bilirubin elevation owing to previously undiagnosed Gilbert's disease, one had grade 2 rash, and one had grade 2 creatine kinase elevation. A fourth subject was non-compliant and was discontinued from the study, leaving 14 subjects for whom pharmacokinetics data were available on both study days. Among the 14 subjects, two had grade 2 gastrointestinal complaints, one had grade 3 alanine aminotransferase and grade 2 aspartate aminotransferase on day 16, one had grade 2 fasting LDL elevation on day 13, and one had grade 2 skin eruptions on day 5 deemed unrelated to study medications. The population characteristics were a median age of 29 years, 50% women,79% white, 7% Latino, 7% African-American, and 7% Asian/Pacific Islanders.
Plots of time-specific geometric mean concentrations of pravastatin with and without NFV are shown in Fig. 1. Median pravastatin AUC values on days 3 and 16 were 86.5 and 46.8 ng·h/ml, respectively (Table 1). The P value indicated a high level of statistical significance for this difference. The median within-subject percentage change was a decrease of 46.5%, and the 90% CI around the geometric mean ratio was 0.46–0.65. Pravastatin Cmax values were also lower when pravastatin was administered with NFV. Median Cmax values were 27.9 and 12.4 ng/ml for days 3 and 16, respectively, and the median within-subject decrease was 40.1% (Table 1).
Fig. 1.
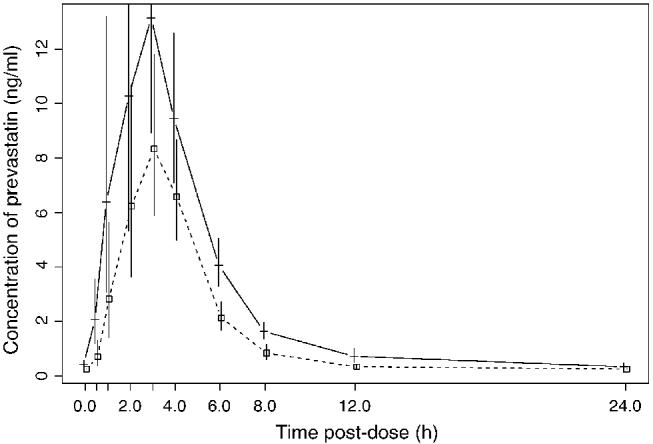
Geometric mean concentration of pravastatin at each scheduled sample time and under the two dosing conditions, without (day 3; —) and with (day 16; - - -) coadminstration of nelfinavir. The 95% confidence intervals around the geometric means are also shown.
Table 1.
Pravastatin pharmacokinetics parameters in the nelfinavir arm (n = 14)a.
| AUC (ng·h/ml) | Maximum plasma concentration (ng/ml) | |
|---|---|---|
| AUC, area under the concentration–time curve. | ||
| Day 3, median | 86.5 | 27.9 |
| Day 16, median | 46.8 | 12.4 |
| Wilcoxon P value | 0.0004 | 0.0023 |
| Median within-subject change [% (range)] | 46.5% (–64.6 to +10.5) | 40.1% (–76.9 to +154.0) |
| 90% confidence interval around the geometric mean ratio | 0.46–0.65 | 0.40–0.70 |
Values presented represent those obtained when pravastatin was administered alone through day 3 and concomitantly with nelfinavir from days 4 to 16. Within-subject changes are those comparing pravastatin concentrations between day 3 and day 16.
The median within-subject change in LDL, from baseline to day 13 (after 9 days of NFV administration), was an increase of 105 mg/l (mean, 144; 95% CI, 65–223). This change was significantly different from zero (P < 0.001,Wilcoxon signed rank test). Median (mean) changes after 3 days of pravastatin administration were –200 mg/l (mean, –195) from day 0 to day 3, and –155 mg/l (mean, –167) from day 13 to day 16, for a median difference in within-subject response to pravastatin of 28 mg/l (P = 0.436, Wilcoxon signed rank). The percentage change in LDL during the two periods was also calculated because the baseline LDL was increased by NFV. After 3 days of pravastatin administration, the median changes were –19.7% (mean, –19.8) from day 0 to day 3, and –11.4% (mean, –14.2) from day 13 to day 16, for a median difference in within-subject response to pravastatin of 6.7% (mean, 5.5). This difference was not statistically significant (P = 0.104, Wilcoxon signed rank test).
Discussion
The precise mechanism of HIV-associated dyslipidemia remains elusive [9]. Whether the dyslipidemia is a consequence of HIV itself, its resultant inflammation, or the adverse effects of medications, the fact remains that dyslipidemia is a common finding in HIV-infected individuals that warrants therapy. Interestingly, even in this study, the short-course administration of nelfinavir in healthy HIV-seronegative subjects was associated with a 105 mg/l median increase in LDL.
Pravastatin has become the preferred statin because of its lack of significant metabolism by P4503A4 and results from studies suggesting that pravastatin is safe and tolerable in patients taking potent antiretroviral therapy [10]. In our previous study, we demonstrated that ritonavir/saquinavir decreased the pravastatin median AUC by 50% [2]. In this study, NFV administration to steady state reduced the exposure to pravastatin by a median of 46.5%. Similarly, we have also demonstrated that efavirenz reduces the AUC of pravastatin [4].This reduction in exposure may compromise the effectiveness of pravastatin in patients taking certain PI drugs or efavirenz. Although our study was not adequately powered nor designed to evaluate pravastatin's effectiveness in lowering LDL, we did note a trend suggesting that its effectiveness was reduced.
The mechanism by which pravastatin concentrations are reduced is not known. The metabolism of pravastatin is complex. Induction of non-CYP3A4 oxidation or glucuronidation may result in more rapid clearance of pravastatin. In addition, hepatic transporter OATP1B1 may participate in increased drug elimination [11–13]. Rifampin, which induces both CYP isoforms as well as some transporters, reduced pravastatin exposure by a mean of 30% without affecting Cmax [14]. However, the CYP3A4 inhibitors itraconazole and mibefradil had no significant effect on pravastatin AUC [15]. Gemfibrozil as well as ciclosporin have been shown to increase pravastatin exposure during concomitant administration, pointing to probable involvement of hepatic transporters in this drug–drug interaction [16,17]. Regardless of the mechanism, the implication is that effectiveness of pravastatin may be reduced.
Clinical studies of pravastatin for the treatment of HIV-associated dyslipidemia have shown mixed results. For example, in a randomized trial of pravastatin versus fenofibrate for combined HIV-associated dyslipidemia, only 36% of subjects receiving 40 mg pravastatin daily met the LDL goal (median 20% decrease) and 18% met the triglyceride goal (median 13% decrease) by week 12 [18]. In contrast, in a randomized, open-label study comparing lipid-lowering therapy (pravastatin or bezafibrate) with a switch from PI drug to non-nucleoside reverse transcriptase inhibitors, Calza and colleagues [19] reported reductions in total cholesterol, LDL, and triglycerides of 45.8%, 39.5%, and 41.2%, respectively, after 20 mg pravastatin daily for 12 months. Interestingly, 55% of subjects receiving pravastatin had normal total cholesterol at 12 months. Benesic and colleagues [20] reported results for patients treated with pravastatin or fluvastatin. In 13 pravastatin-treated subjects (11 taking 10 mg pravastatin), a decrease in total cholesterol levels (from 7.12 to 6.29 mmol/l) after 12 weeks of therapy was reported. The reduction of LDL level was only 14.4% in the pravastatin group, without a significant reduction in triglycerides. None of these studies was specifically analyzed by the PI used to determine whether responses varied with specific PI drugs. It is also possible that dosing differences, adherence, or other factors may account for variations in efficacy reported.
In summary, pravastatin remains the statin of choice in HIV-infected persons taking antiretroviral therapy, given its safety profile compared with simvastatin and atorvastatin. The observed near 50% reduction in the pravastatin median AUC and a trend toward less reduction in LDL when coadministered with NFV suggest that higher doses of pravastatin may need to be prescribed to achieve optimal lipid-lowering activity. It remains unclear what the optimal dose of pravastatin is to achieve maximal lipid-lowering activity safely in HIV-infected individuals taking antiretroviral therapy. It is also possible that greater improvements in lipid levels may be observed if pravastatin doses are adjusted in the setting of agents that are known to induce reductions in concentrations. Further studies exploring the safety, tolerability, and efficacy of higher doses of pravastatin are warranted.
Acknowledgements
The ACTG A5108 team would like to thank the volunteers for participating in this study. Special thanks to F. Aweeka, M. Becker, W. J. Burning, R. Christensen, R. DiFrancesco, E. Ferguson, W. K. Henry, H. Meyers, S. I. Owens, M. Royal, J. Staggers, R. Strada, S. Valle, and, M. J. Werder for their invaluable assistance.
Appendix
Participating ACTG A5108 NFV-pravastatin investigators and sites: J. Norris, S. Valle (Stanford University); T. Powell, D. Daria (University of Cincinnati); and J. Reid, R. Reichman (University of Rochester Medical Center).
Footnotes
Sponsorship: This study was supported by the National Institute of Allergy and Infectious Diseases: AI-38858 to the AIDS Clinical Trials Group, AI-25897 to the University of Cincinnati, AI-27666 to Stanford University, AI-27658 to the University of Rochester Medical Center, AI-27665 to New York University, AI-32770 to the University of Colorado Health Sciences Center, and AI-38855 to the SDAC/Harvard School of Public Health. Additional support provided by Stanford University GCRC grant M01-RR00070 and University of Rochester Medical Center GCRC grant 5-M01-RR0044. Funded in part by Bristol-Myers Squibb Inc. and Pfizer Pharmaceutical.
Note: The results in this study were presented in part at the Second International AIDS Society Conference on HIV Pathogenesis and Treatment. Paris, July 2003.
References
- 1.Bardsley-Elliot A, Plosker GL. Nelfinavir: an update on its use in HIV infection. Drugs. 2000;59:581–620. doi: 10.2165/00003495-200059030-00014. [DOI] [PubMed] [Google Scholar]
- 2.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, for the ACTG 5047 Study Team Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob Agents Chemother. 2001;45:3445–3450. doi: 10.1128/AAC.45.12.3445-3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, Vega JM, Yang A, Alston BL, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immun Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 5.Yeh KC, Kwan KC. A comparison of numerical integrating algorithms by trapezoidal, Lagrange, and spline approximation. J Pharmacokinet Biopharm. 1978;6:79–98. doi: 10.1007/BF01066064. [DOI] [PubMed] [Google Scholar]
- 6.Bergmann R, Ludbrook J, Spooren WPJM. Different outcomes of the Wilcoxon–Mann–Whitney test from different statistics packages. Am Stat. 2000;54:72–77. [Google Scholar]
- 7.Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J. 2004;147:956–965. doi: 10.1016/j.ahj.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Center for Drug Evaluation and Research . Statistical Approaches to Establishing Bioequivalence. US Department of Health and Human Services, Food and Drug Administration; Washington, DC: 2001. FDA Guidance for Industry. [Google Scholar]
- 9.Cespedes MS, Aberg JA. Cardiovascular and endothelial disease in HIV infection. Curr Infect Dis Rep. 2005;7:309–315. doi: 10.1007/s11908-005-0064-3. [DOI] [PubMed] [Google Scholar]
- 10.Dubé MP, Stein JH, Aberg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)–infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 11.Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, et al. Rosuvastatin pharmacokinectics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76:167–177. doi: 10.1016/j.clpt.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, et al. Polymorphism of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: Consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 13.Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, et al. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–350. doi: 10.1016/j.clpt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2004;57:181–187. doi: 10.1046/j.1365-2125.2003.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–1146. doi: 10.1016/j.amjcard.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 16.Kyrklund C, Backman JT, Neuvonen M, Neuvonen PJ. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther. 2003;73:538–544. doi: 10.1016/S0009-9236(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 17.Hedman M, Neuvonen PJ, Neuvonen M, Holmberg C, Antikainen M. Pharmacokinetics and pharmacodynamics of pravastatin in pediatric and adolescent cardiac transplant recipients on a regimen of triple immunosuppression. Clin Pharmacol Ther. 2004;75:101–109. doi: 10.1016/j.clpt.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Aberg JA, Zackin RA, Brobst SW, Evans SR, Alston BL, Henry WK, for the ACTG 5087 team A randomized trial comparing the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses. 2005;21:757–767. doi: 10.1089/aid.2005.21.757. [DOI] [PubMed] [Google Scholar]
- 19.Calza L, Manfredi R, Colangeli V, Tampellini L, Sebastiani T, Pocaterra D, et al. Substitution of nevirapine or efavirenz for protease inhibitor versus lipid-lowering therapy for the management of dyslipidaemia. AIDS. 2005;19:1051–1058. doi: 10.1097/01.aids.0000174451.78497.8f. [DOI] [PubMed] [Google Scholar]
- 20.Benesic A, Zilly M, Kluge F, Weissbrich B, Winzer R, Klinker H, et al. Lipid lowering therapy with fluvastatin and pravastatin in patients with HIV infection and antiretroviral therapy: comparison of efficacy and interaction with indinavir. Infection. 2004;32:229–233. doi: 10.1007/s15010-004-3136-7. [DOI] [PubMed] [Google Scholar]


