Abstract
Only three of the four thyroid hormone receptor (TR) isoforms, α1, β1, and β2, bind thyroid hormone (TH) and are considered to be true TRs. TRα2 binds to TH response elements on DNA, but its role in vivo is still unknown. We produced mice completely deficient in TRα (TRαo/o) that maintain normal serum thyroid-stimulating hormone (TSH) concentration despite low serum thyroxine (T4), suggesting increased sensitivity to TH. We therefore examined the effects of TH (L-3,3′,5-triiodothyronine, L-T3) given to TH-deprived and to intact TRαo/o mice. Controls were wild-type (WT) mice of the same strain and mice resistant to TH due to deficiency in TRβ (TRβ−/−). In liver, T3 produced significantly greater responses in TRαo/o and smaller responses in TRβ−/− as compared with WT mice. In contrast, cardiac responses to L-T3 were absent or reduced in TRαo/o, whereas they were similar in WT and TRβ−/− mice, supporting the notion that TRα1 is the dominant TH-dependent TR isoform in heart. 5-Triiodothyronine (L-T3) given to intact mice produced a greater suppression of serum T4 in TRαo/o than it did in WT mice and reduced by a greater amount the TSH response to TSH-releasing hormone. This is an in vivo demonstration that a TR deficiency can enhance sensitivity to TH. This effect is likely due to the abrogation of the constitutive “silencing” effect of TRα2 in tissues expressing the TRβ isoforms.
Thyroid hormone (TH) action is mediated through specific nuclear TH receptors (TRs) functioning as ligand-dependent transcription factors that increase or decrease the expression of target genes (1–3). There are two TR genes. The TRβ locus generates the β1 and β2 receptors by using two different promoters and alternative splicing. The TRα locus encodes TRα1, a molecule containing 410 aa, and three proteins that do not bind the ligand, triiodothyronine (T3) (4–7) (GenBank accession no. AI322342). TRα2, which results from an alternative splicing of the TRα primary transcript, has 492 aa, the first 370 of which are identical to TRα1. TRΔα1 and TRΔα2, molecules of approximately 154 and 237 aa, respectively, are generated from an internal promoter located in intron 7 of the TRα locus (6). The function of TRα2, which has a wide tissue distribution (8), and that of TRΔα1 and TRΔα2, found mainly in brain, gut, and lung (6), are still unknown. However, all have been shown to inhibit ligand-dependent transactivation of TRβ and TRα1, a phenomenon termed the dominant negative effect (6, 9–11, **).
The relative contribution of the two TR gene products in mediating TH responses is poorly understood because of the paucity of information regarding in vivo function. In vitro DNA binding studies and functional assays in transfected cells have generated conflicting results concerning the specific effect of TR isoforms in gene regulation (12, 13). Interpretation is complicated, however, because data are derived from artificial systems using the overexpression of chimeric gene constructs that may not be faithful models of events occurring in the intact animal. The use of knockout mice that lack a particular TR isoform provides a powerful tool for exploring the relative contribution of each of the TR isoforms to the TH-mediated regulation of various biological processes in different tissues. The specific role of TRα was investigated indirectly in mice deficient in TRβ (14). Results suggested that TRα plays a major role in the regulation of heart rate and basal metabolism (15). This conclusion was confirmed and attributed to the TRα1 isoform (15).
Herein we examined the effects of TH deprivation and treatment with l-3,3′,5-triiodothyronine (L-T3) on the pituitary, liver, and heart of homozygous mice deficient in all forms of TRα (TRαo/o). Compared with those of wild-type (WT) mice, the pituitary gland and liver of TRαo/o mice were more sensitive to TH, whereas the same tissues of TRβ knockout (TRβ−/−) mice, as previously shown, were resistant to TH. In contrast, the heart of TRαo/o mice was hyporesponsive to TH, indicating that TRα is the predominant TR form expressed in this tissue. Given that TRα1 knockout mice have no increased sensitivity to TH (16), our results suggest that TRα2 exerts a dominant negative effect in some tissues in vivo and that the absence of TRα2 enhances sensitivity to TH.
Experimental Methods
Animal Procedures.
As is the case with the TRα−/− mouse (17), the TRαo/o mouse is deficient in both TRα1 and TRα2 encoded by the TRα gene, but, in addition, it lacks the shorter transcripts initiated from the internal promoter located in intron 7 (6). However, the gene sequence for Rev-erbAα, the TR-related protein encoded by the opposite strand of TRα (18, 19), remains intact. In contrast to the TRα−/− mice, the TRαo/o mice survive without rescue by TH treatment. They are fertile but have a slightly slower growth rate. Thus the dose of L-T3 was adjusted to the body weight (BW). Mice resistant to TH due to deletion of the TRβ gene (TRβ−/−) (described in ref. 20) were used for comparison. The TRβ−/− mice were derived after nine crossings of TRβ−/+ heterozygote with WT mice of the C57BL/6J strain before inbreeding. TRαo/+ mice were crossed four times with WT mice of the same strain before heterozygotes were intercrossed. Breeding of the resulting homozygotes TRαo/o and TRα+/+ (WT) mice was used in the grouped experiments.
Mice were weaned in the fourth week after birth and were fed rodent diet containing 0.53 ppm iodine (no. 5053; LabDiet, Brentwood, MO) ad libitum and given tap water. All animal experiments were performed at the University of Chicago according to protocols approved by the Institutional Animal Care and Use Committee.
Because of sex and age differences in serum thyroid-stimulating hormone (TSH) and thyroxine (T4) concentrations, all mice were male and were 60–70 days old at the beginning of each experiment. The BW of TRαo/o mice (mean ± SEM) of 18.4 ± 0.4 g was significantly lower (P < 0.0001) than that of the WT (23.4 ± 0.5 g) and TRβ−/− mice (22.0 ± 0.4 g). Experiments were terminated by exsanguination under methoxyflurane (Pitman–Moore, Mundelein, IL) anesthesia. Livers were completely removed, weighed, cut into 0.2–0.5-g pieces, and frozen on dry ice before storage at −85°C. Atria were removed and heart ventricles were immediately frozen and stored at −85°C.
Induction of Hypothyroidism and Treatment with TH.
TH deficiency was induced in 10–14 male mice of each type (TRαo/o, WT, and TRβ−/−) with a low-iodine diet containing 0.15% 5-propyl-2-thiouracil (PTU), as described (21). On the 11th day, animals of each type were split into two groups. One group received daily i.p. injections of 2 μg of L-T3/100 g BW/day for 4 days, whereas the other group received only the vehicle. The PTU diet was given throughout the L-T3 and vehicle treatment period. The concentration of L-T3 was confirmed by radioimmunoassay. Experiments were terminated 12–16 h after the last injection. In a separate experiment, groups of 4–5 mice were similarly treated and blood was collected for serum T3 determination 24 h after the penultimate L-T3 dose and at 2, 4, 8, and 16 h after the last L-T3 injection. Note that separate animals were used for the 2- and 8-h and 4- and 16-h posttreatment blood sampling.
In a separate experiment, L-T3 was given on the same schedule to groups of mice of each type (10 WT and 7 TRαo/o) with no prior induction of hypothyroidism. Two incremental doses were given consecutively as described (22), 0.2 μg/100 g BW/day, followed by 0.8 μg/100 g BW/day. Blood samples (approximately 300 μl) were obtained from the tail vein before the treatment (baseline) and 12–16 h after the last injection of each incremental L-T3 dose.
The suppressive effect of TH on the response of TSH to TSH-releasing hormone (TRH) was examined in WT and TRαo/o mice. Three groups of 5–6 animals of each type were given the two doses of L-T3 or the vehicle only (basal) for 4 days. On the fifth day, 12–16 h after the last injection, blood samples were obtained before and 15 min after the i.p. administration of 0.275 μg TRH.
Measurements in Serum Samples.
Cholesterol levels were measured with an autoanalyzer as described. Serum TSH was measured with a sensitive radioimmunoassay (23), and results were expressed in bioassayable TSH units. Serum total T4 and T3 concentrations were measured by radioimmunoassays (Diagnostic Products, Los Angeles). The sensitivities of these assays were 0.2 μg T4/dl and 20 ng T3/dl.
Heart Rate.
Electrocardiograms were recorded under chloral hydrate anesthesia (4 mg/10 g BW i.p.) with mice placed in a water bed maintained at 37°-38°C. A Hewlett–Packard monitor/terminal (model 78534AA) with a chart speed of 25 mm/s was used.
Tissue Analyses.
T3 binding to liver nuclei was determined as described (15). Pools of approximately 1 g of frozen liver pieces from at least four animals of each type were used. All animals had received the PTU diet for 14 days and were, thus, hypothyroid before liver collection. T3 binding affinity and maximal binding capacity were determined by the method of Scatchard. The former was corrected for DNA content.
Northern analyses were performed on RNA extracted from tissues of individual animals. Total RNA was prepared by the acid–guanidinium thiocyanate—phenol–chloroform method (24) from livers and heart ventricles frozen 1–3 min after death and kept at −85°C. Fifteen micrograms of total RNA was denatured and fractionated by electrophoresis on 0.8% agarose gel, transferred onto GeneScreen Plus (DuPont/NEN), using VacuGene (Amersham Pharmacia). cDNA probes for iodothyronine type I 5′-deiodinase (5′DI) (25), malic enzyme (ME) (26), and sarcoplasmic reticulum calcium adenosine triphosphatase (SERCA2) [prepared by reverse transcription–PCR with the sense (5′-ACGATCTGTGCTCTGTGTAATGACTCT-3′) and antisense (5′-GCGCGTCGTTCACACCATCACCAGTCA-3′) oligonucleotides] were labeled with [γ-32P]deoxycytidine triphosphate (specific activity 111 Bq/mmol) (DuPont/NEN), using the Random Primed DNA Labeling Kit (Roche Molecular Biochemicals). Hybridization and subsequent washing procedures were carried out as described (27). The quantity of mRNA on radiographs was measured with a molecular imager (Bio-Rad).
To assess the uniformity in RNA transfer among samples, hybridized membranes were reprobed with a cDNA complementary to rRNA. To correct the results of Northern blots, the activity of rRNA probe hybridized to each lane was divided by the average activity of rRNA for the entire group. These ratios were used to correct the activity of the respective mRNA abundance in each lane.
Data Presentation and Statistical Analysis.
Values are reported as mean ± SE. P values were calculated by two-way ANOVA or Student's t test when comparisons were made within the same genotype. Values corresponding to the respective limits of the assay sensitivities were assigned to samples that measured below the detectable range. Outliers were identified by a two-tailed test with a significance level of <0.05 (28, 29). No more than one outlier per treatment group was removed. One TRβ−/− mouse did not achieve a sufficient degree of hypothyroidism with the PTU diet, as determined by a serum TSH level of 725 milliunits/liter and a T4 of 5.3 μg/dl, both 10-fold below and above the group means. All data generated from this mouse were excluded. Statistics were performed using statview 5.0 software (Abacus Concepts, Berkeley, CA).
Results
Thyroid Function Tests and Heart Rate at Baseline in TRαo/o Mice as Compared with WT and TRβ−/− Mice.
Results of thyroid function tests in untreated TRαo/o are compared with those in WT and TRβ−/− mice (Table 1). Despite the similar levels of circulating TSH, TRαo/o mice have significantly lower levels of total T4 (3.29 ± 0.09 μg/dl) compared with WT animals (3.76 ± 0.10 μg/dl, P < 0.005). As previously reported (14, 21), the TRβ−/− animals were resistant to TH and had increased levels of serum T4 and T3 as well as TSH concentration. The mean basal heart rate of TRαo/o mice was significantly lower than that of WT mice, a finding similar to that reported for mice lacking only the TRα1 isoform (16). The higher heart rate in TRβ−/− mice is as reported previously (15, 30) and is consistent with the higher serum TH levels in these animals.
Table 1.
Thyroid function tests and heart rate in adult male TRαo/o mice as compared with WT and Trβ−/− controls
| TSH, mU/liter | T4, μg/dl | T3, ng/dl | Heart rate, beats per min | |
|---|---|---|---|---|
| TRαo/o | 22 ± 3.5 | 3.29 ± 0.09 | 96.1 ± 7.2 | 358 ± 22 |
| (32) | (31) | (11) | (23) | |
| WT | 25 ± 3.0 | 3.76 ± 0.10 | 83.6 ± 3.0 | 470 ± 6 |
| (54) | (47) | (13) | (43) | |
| TRβ−/− | 136 ± 18 | 7.78 ± 0.33 | 142.3 ± 12.4 | 522 ± 8 |
| (42) | (37) | (12) | (48) | |
| P (TRαo/o vs. WT) | NS | <0.005 | NS | <0.0001 |
| P (TRαo/o vs. TRβ−/−) | <0.0001 | <0.001 | <0.005 | <0.0001 |
The number of animals is in parentheses. NS, not significant.
Sensitivity of Serum TSH to the Feedback Regulation by TH.
The observation of a small, but significant, decrease in serum T4 concentration not associated with an alteration in basal serum TSH values could be explained by reduced T4 binding to serum proteins, mild hypothyroidism, or increased sensitivity to TH. To determine the physiological significance of this observation, groups of mice were given two incremental doses of L-T3, and the suppressive effect on serum T4 was examined (Fig. 1). In addition, because the basal serum TSH concentrations were too low for accurate measurement of a decrease induced by L-T3, the TSH response 15 min after the administration of TRH was determined (Fig. 2).
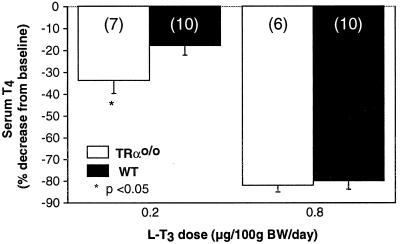
Figure 1.
Effect of TH on the feedback suppression of thyroid function. TRαo/o and WT mice were treated with two incremental doses of L-T3 each for 4 consecutive days. The decrease in serum T4 was expressed as a percentage of the baseline values measured in the same mice before L-T3 treatment. Note the greater suppression of T4 in TRαo/o mice receiving the lower L-T3 dose. The numbers of animals are in parentheses.
Figure 2.
Effect of TH on the TSH response to TRH. Groups of mice from the three genotypes were treated for 4 days with two different doses of L-T3 or with the vehicle only. TSH was measured before and 15 min after the administration of TRH. (A) TSH response to TRH in mice not given L-T3 (basal response). (B) TSH responses to TRH in mice given L-T3, expressed as a percentage of the basal response. Note the paradoxical increase in TRβ−/− mice receiving 0.2 μg L-T3/100 g BW and the greater suppressive effect of 0.8 μg L-T3/100 g BW in TRαo/o mice. In A, the number of animals in each group is in parentheses. In B, data for each bar are derived from five or six animals. P values indicated by * are differences in responses after L-T3 treatment as compared with baseline. P values above bars were obtained by two-way ANOVA of original numerical data.
Treatment with 0.2 μg L-T3/100 g BW brought the serum T4 of TRαo/o animals to 2.26 ± 0.01 μg/dl as compared with 3.01 ± 0.11 μg/dl in WT animals. This represents a reduction of 34.0 ± 5.4% and 18.2 ± 3.7%, respectively (P < 0.05). Treatment with the higher dose of 0.8 μg L-T3/100 g BW/day for 4 more days produced the same degree of serum T4 suppression in the two types of mice (Fig. 1).
The response of TSH to TRH before TH treatment and its suppression after the administration of the same two doses of L-T3 are shown in Fig. 2. In animals not given L-T3, the incremental TSH response to TRH was not different in TRαo/o mice compared with WT mice but was significantly lower in TRβ−/− mice (P < 0.01). The higher dose of L-T3 suppressed the TSH response by 77.3 ± 5.7% in the TRαo/o mice as compared with 42.8 ± 15.8% in WT mice (P = 0.02). Collectively, these data indicate that the feedback regulation of the pituitary–thyroid axis of TRαo/o mice is more sensitive to TH.
Of interest is the lower response of TSH to TRH at baseline in TRβ−/− mice and the 49.2 ± 26.3% paradoxical response in these mice given the lower dose of L-T3. Similar findings were reported 20 years ago with a comparable dose of L-T3 (31) given to humans homozygous for TRβ gene deletion (32).
Thyroid Function in TH-Deprived and TH-Treated Mice.
To investigate the role of TRα in the mediation of TH action, mice were studied during TH deprivation and after supplementation with TH. The dose of 2 μg L-T3/100 g BW (0.5 μg/25 g mouse) has been shown to be effective in bringing the high serum TSH of hypothyroid WT mice to normal, inducing metabolic effects, and producing changes in liver (15, 21, 33). This investigative approach served two purposes. First, it allowed determination of the tissue responses of TRαo/o mice to the same amount of TH as in WT and TRβ−/− mice, which, under basal conditions, have different serum concentrations of TH. Second, observations under the conditions of TH deprivation and full replacement provided a measure of the maximal physiological effect of TH.
The three genotypes that received the PTU diet only had similar serum TSH, T4, and T3 levels (Table 2). L-T3 treatment produced a greater suppression of serum TSH in the TRαo/o mice than in the WT animals, a difference that did not reach statistical significance. As expected, the effect on TRβ−/− mice was lesser, reflecting their decreased sensitivity to TH (Table 2).
Table 2.
Thyroid function tests in TRαo/o, WT and TRβ−/− mice after treatment with PTU and PTU+T3
| PTU*
|
PTU +
T3†
|
|||||
|---|---|---|---|---|---|---|
| TSH, mU/liter | T4, μg/dl | T3, ng/dl | TSH, mU/L | T4, μg/dl | T3, ng/dl | |
| TRαo/o | 10,650 ± 1,900 | 0.71 ± 0.08 | 50 ± 8 | 8 ± 1.5 | 0.37 ± 0.04 | 147 ± 24 |
| WT | 8,700 ± 1,150 | 1.04 ± 0.07 | 48 ± 4 | 18 ± 7 | 0.38 ± 0.05 | 114 ± 23 |
| TRβ−/− | 10,300 ± 1,850 | 0.93 ± 0.11 | 34 ± 11 | 274 ± 83 | 0.95 ± 0.08 | 142 ± 46 |
| P (TRαo/o vs. WT) | NS | NS | NS | NS | NS | NS |
| P (TRαo/o vs. TRβ−/−) | NS | NS | NS | <0.01 | <0.0001 | NS |
NS, not significant.
Fifteenth day of treatment with PTU.
At the termination of the experiment, after 4 days of L-T3 treatment, except for the T3 value measured 24 h after the third and penultimate dose of L-T3.
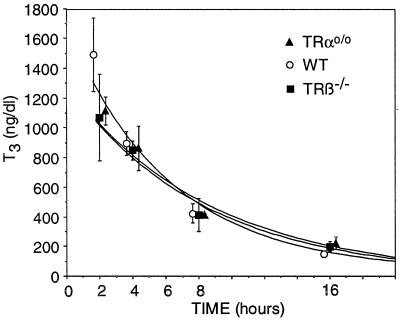
Serum T3 concentrations achieved at different times after the administration of L-T3 were not different among the animals of the three genotypes (Table 2 and Fig. 3).
Figure 3.
Serum T3 concentrations at different times after the administration of L-T3. Blood was obtained at the indicated times after the fourth i.p. dose of L-T3 on the last day of the experiment. Values are not significantly different among the three genotypes at each time point.
Role of TRα in the Mediation of TH Action on Liver.
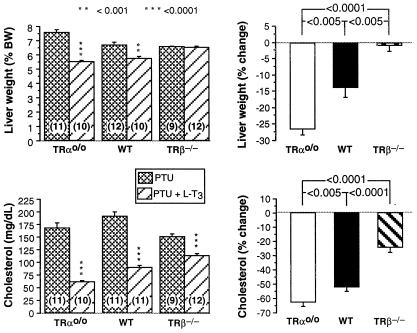
Four known markers of TH action were measured during TH deprivation and after its supplementation as described above. L-T3 treatment reduced the liver weight of WT and TRαo/o mice but, as expected (33), had no effect on the TRβ−/− animals. However, the decrease was greater in TRαo/o mice than in WT mice (P < 0.005) (Fig. 4). TRαo/o mice had a significantly higher reduction in cholesterol after treatment with L-T3 as compared with WT animals (P < 0.005). In contrast, and as expected (15), the T3-mediated reduction in serum cholesterol was significantly smaller in TRβ−/− mice (P < 0.0001) (Fig. 3).
Figure 4.
Effect TH deprivation and L-T3 treatment on liver weight and serum cholesterol. Groups of mice from the three genotypes were TH-deprived (PTU) and then treated with TH (PTU + T3). Shown are measured values (Left) and the percent changes induced by L-T3 treatment (Right). Note the greater relative response to TH of TRαo/o mice.
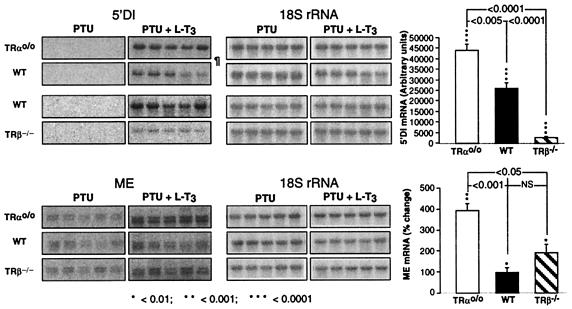
5′DI and ME are well characterized TH-responsive genes, the transcriptional regulations of which have been extensively studied in the rat (3, 34) and have been shown to undergo similar changes in response to L-T3 in the mouse (15, 33). cDNAs of these two genes were used to determine their mRNA content in the liver of TRαo/o, WT, and TRβ−/− mice by Northern blotting (Fig. 5). TH deprivation abolished the ability to detect 5′DI mRNA, even after prolonged exposure of the hybridized membranes. L-T3 treatment produced a significantly greater increase in the abundance of the mRNA of this enzyme in TRαo/o mice as compared with WT animals and a much smaller increase in TRβ−/− mice (P < 0.005 and < 0.0001, respectively). ME mRNA was also significantly (P < 0.001) higher in TRαo/o than in WT mice under L-T3 treatment.
Figure 5.
Effect of TH deprivation and L-T3 treatment on the expression of TH-regulated genes in liver. Mice from the three genotypes were TH-deprived (PTU) and then treated with TH (PTU + L-T3). Liver RNAs were analyzed for their abundance in specific mRNAs by Northern blotting. Radioautographs of membranes hybridized with the two labeled cDNA probes (only five consecutive animals of each group) are shown on the left, and the percent changes induced by L-T3 are plotted on the right. The membrane with RNA from TRαo/o and the corresponding WT mice was exposed for 3 days (¶); those with RNA from the TRβ−/− and another set of RNAs from the same WT mice were exposed for 4 days. The same membranes were rehybridized with an 18S rRNA probe. Note the greater relative response to TH of TRαo/o mice.
Quantification of T3 Binding TR in Liver.
To determine whether the absence of TRα produces a compensatory increase in TRβ, T3 binding studies were carried out in liver nuclei. The maximal T3 binding capacity in three separate experiments averaged 144 fmol/100 μg DNA for livers of WT animals. The corresponding values in TRαo/o and TRβ−/− mice were, respectively, 66% and 21% of the WT. These results indicate that deficiency in one of the TR genes does not produce a compensatory increase in the other. The relative contributions of TRβ and TRα1 in the liver of mice are similar to those reported in the rat (35).
Responses to TH of Heart Rate and TH-Responsive Genes Expressed in Heart.
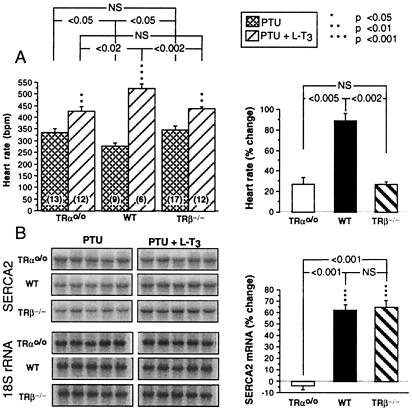
Compared with baseline, TH deprivation decreased the heart rate of both WT and TRβ−/− mice but had no effect on TRαo/o mice (358 ± 22 vs. 335 ± 20 beats/min). L-T3 treatment produced an increase in heart rate in WT and TRβ−/− mice (89 ± 8% and 66 ± 4%) that was significantly higher in relative magnitude than that in TRαo/o mice (27 ± 7%) (Fig. 6A).
Figure 6.
Effect of TH deprivation and L-T3 treatment on heart rate and the expression of a TH-regulated gene in heart ventricle. Mice from the three genotypes were TH-deprived (PTU) and then treated with TH (PTU + L-T3). (A) Mean heart rates (Left) and the percentage changes induced by the treatment with L-T3 (Right). (B) The radioautographs of membranes hybridized with the SERCA2 probe (five consecutive animals of each group) are on the left, and the percentage changes induced by L-T3 are plotted on the right. The same membranes were rehybridized with an 18S rRNA probe. Note the reduced responses of SRECA2 to L-T3 in TRαo/o mice.
Relative to the state of TH deprivation, L-T3 produced a modest increase in cardiac SERCA2 mRNA in the WT and TRβ−/− mice but not in the TRαo/o mice (P < 0.001) (Fig. 6B).
Discussion
TH exerts its effects through interaction with specific nuclear TRs, α1, β1, and β2 (36), that regulate the expression of target genes by binding to cognate TH response elements, either as homodimers or as heterodimers in association with retinoid X receptors (37, 38). Because both TRα and TRβ genes are expressed in most tissues (8), either one or both can be implicated in mediating in vivo the TH-dependent effects.
In our work, we sought to determine the role of TRα by deleting all of its isoforms and examining the resulting tissue responses to TH. Data were compared with those observed in WT mice as well as animals deficient in both TRβ isoforms, manifesting signs of resistance to TH (20). The targeted mutation in the TRαo/o mouse inactivates completely not only the two full-length TRα gene transcripts encoded by the main promoter, but also the two shorter transcripts, TRΔα1 and TRΔα2, derived from the promoter located in intron 7 (6). However, the gene sequence for Rev-erbAα, the TR-related protein encoded by the opposite strand of TRα (18, 19), remains intact.
Similar to the TRα1-deficient mice reported by Wikström et al. (16), TRαo/o mice, of the same age and sex, had significantly lower serum T4 levels and heart rates than the respective WT animals. However, in contrast to the TRα1-deficient mice, the serum TSH concentrations of TRαo/o mice were not different from those of the WT animals, even after the analysis of more than 30 serum samples of each genotype. The maintenance of a normal serum TSH concentration despite a lower concentration of T4 suggested increased sensitivity to TH. Indeed, administration of the same dose of L-T3 to TRαo/o and WT mice had a greater suppressive effect on the serum T4 levels of the former, indicating a greater inhibition of basal TSH, even though the corresponding lower serum TSH values did not reach statistical significance. Evidence for a reduced secretion of TSH in the TRαo/o mice was secured from the greater L-T3-induced suppression of the TSH response to TRH. Together, these results indicate that the feedback regulation of the pituitary–thyroid axis of TRαo/o mice is more sensitive to TH.
We then examined the sensitivity to TH in two peripheral tissues, liver and heart, that express predominantly the TRβ and TRα genes, respectively (15, 39–40, ‡‡). Studies were carried out under the conditions of TH deprivation and TH supplementation, according to a previously tested and standardized protocol (15, 21, 33). This protocol allows the comparison of tissue responses to the same amount of TH in mice that under basal conditions have different concentrations of TH. Moreover, the comparison of measurements obtained under the conditions of TH deprivation and full replacement increases the ability to detect modest effects of the hormone. It should be noted that this method does not achieve complete TH deficiency. However, serum TSH and T4 concentrations were not significantly different among mice of the three genotypes. Furthermore, similar levels of serum T3 were achieved in all genotypes during L-T3 treatment.
Again TRαo/o mice showed evidence for increased sensitivity to TH in yet another tissue, the liver. L-T3 given to TH-deprived animals reduced significantly more the liver weight and serum cholesterol concentration in TRαo/o as compared with WT mice. The former reflects glycogen loss through TH-mediated catabolism and the latter, TH-induced stimulated transcription of low-density lipoprotein receptor and cholesterol 7-α hydroxylase genes, leading to enhanced removal of low-density lipoprotein and cholesterol (41, 42). L-T3 also produced a greater increase in the two TH-inducible liver mRNAs, 5′DI and ME (26, 34, 43, 44), in TRαo/o than in WT animals. It is of interest to note that under the current experimental conditions, TH deprivation reduced the expression of 5′DI mRNA to undetectable levels in animals of the three genotypes, whereas the reduction in ME mRNA was less pronounced. As is the case with the up-regulation of the pituitary TSH gene (20, 21, 45), down-regulation of the liver 5′DI gene, during TH deprivation, does not appear to require the presence of TR.
TRαo/o mice have a significant reduction in heart rate, which increased with the administration of L-T3, as previously reported for TRα1 knockout mice (16). Of interest is our finding that, in contrast to the WT and TRβ−/− mice, the heart rate of TRαo/o mice did not change significantly during TH deprivation. This observation suggests that in absence of TRα the heart rate is in the hypothyroid range but still can be increased by TH, probably through the residual TRβ. Nevertheless, this L-T3-induced increase in the TRαo/o mice was smaller in magnitude compared with the WT and TRβ−/− mice. The effect of L-T3 on SERCA2 mRNA was abolished in TRαo/o mice. Judging from the results obtained with the TRβ−/− mice, the TH-mediated response of SERCA2 is completely TRα-dependent.
The observations made in the TRβ−/− mice are in agreement with those previously reported (15, 22) for TRβ−/− mice produced by a slightly different method of gene disruption (14). A finding not previously reported in the TRβ knockout mouse is the significantly lower response of TSH to TRH at baseline and the paradoxical increase of this response after the administrations of the lower L-T3 dose. Although the reason for this unexpected response cannot be explained, this same effect has been reported in humans genotypically and phenotypically similar to the TRβ−/− mice (31, 32) but not in humans with TH resistance caused by a dominant negative mutant TRβ (46).
Increased sensitivity of TRαo/o mice to TH was demonstrated in the pituitary and liver, both tissues expressing predominantly the TRβ isoform. These mice differ from the TRα1-deficient mice that have persistent expression of TRα2 and, presumably, TRΔα2. Compensatory increases in TRβ expression do not appear to occur at the RNA and protein levels in brain and liver of TRα1-deficient and TRαo/o mice, respectively. Thus, the increased sensitivity of TRαo/o mice to TH cannot be explained by a higher level of TRβ gene expression as observed in livers of mice overexpressing TRβ (33). It is thus likely that the enhanced responsiveness to TH in the TRαo/o mouse is due to the abrogation of the constitutive silencing mediated through TRα2. This ligand-unresponsive TR has been shown to exert a weak dominant negative effect in transfected cells (10, 11). The ubiquitous expression of TRα2 and its presence in tissues with predominantly TRβ-mediated TH action are expected to attenuate the effect of TH, particularly when TH is present in small amounts. The enhanced effect of TH should not be manifested in tissues of TRαo/o mice in which TH action is mediated predominantly through TRα1, as is the case in the heart. It should be noted that our data do not exclude the possible contribution of TRα1 deficiency to the increased sensitivity to TH, by removal of a weaker transactivator that normally competes with the TRβ. The loss of the TRΔα isoforms has no effect on the observed effects on liver and pituitary because they are not expressed in these tissues††. Proof that a deficiency in TRα2 is the basis for the observed hypersensitivity to TH in mice completely deficient of TRα should be contained in the mouse deficient in TRα2 only. Although such mice do exist (47), no information is yet available regarding their precise phenotype.
Acknowledgments
We thank the following investigators for the provision of cDNA probes: 5′DI, P. Reed Larsen; ME, Vera M. Nikodem; and SERCA2, Takashi Nagaya. This work was supported in part by Grant DK 15,070 from the National Institutes of Health to S.R.; the Seymor J. Abrams Thyroid Research Center; a grant from the Ministry of Health and Welfare, Japan, to H.S.; and Grants-in-Aid for Scientific Research (B) 11472225 to Y.M. and 10470226 to H.S. from the Ministry of Education, Science, Sports and Culture, Japan.
Abbreviations
- TH
thyroid hormone
- TR
TH receptor
- TH
thyroid hormone
- TSH
thyroid-stimulating hormone
- T4
thyroxine
- L-T3
L-3,3′,5-triiodothyronine
- BW
body weight
- TRH
TSH-releasing hormone
- 5′DI
iodothyronine type I 5′-deiodinase
- ME
malic enzyme
- SERCA2
sarcoplasmic reticulum calcium adenosine triphosphatase
- PTU
5-propyl-2-thiouracil
- WT
wild-type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
This paper was presented in part at the 82nd Annual Meeting of the Endocrine Society, June 21–24, 2000, Toronto, Canada, and selected as a finalist for the Knoll Award.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.011306998.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.011306998
Kinugawa, K., Minobe, W. A., Wood, W., Ridgway, C. E., Lowes, B. D., Baxter, J. D., Riberio, R. C. J., Long, C. S. & Bristow, M. R. (1999) Circulation 100, Suppl. 18, I–507 (abstr.).
Gauthier, K., Plateroti, M., Chassande, O. & Samarut, J. (2000) in Program, (The Endocrine Society, Toronto), p. 21 (abstr.).
References
- 1.Lazar M A. Endocr Rev. 1993;14:184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- 2.Chin W W. Thyroid. 1994;4:389–393. doi: 10.1089/thy.1994.4.389. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prost E, Koenig R J, Moore D D, Larsen P R, Whalen R G. Nucleic Acids Res. 1988;16:6248. doi: 10.1093/nar/16.13.6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moeller M, Rapoport B, Gavin L A. J Neuroendocrinol. 1989;5:351–356. doi: 10.1111/j.1365-2826.1989.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 6.Chassande O, Fraichard A, Gauthier K, Flamant F, Legrand C, Savatier P, Laudet V, Samarut J. Mol Endocrinol. 1997;11:1278–1290. doi: 10.1210/mend.11.9.9972. [DOI] [PubMed] [Google Scholar]
- 7.Laudet V, Begue A, Henry-Duthoit C, Joubel A, Martin P, Stehelin D, Saule S. Nucleic Acids Res. 1991;19:1105–1112. doi: 10.1093/nar/19.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodin R A, Lazar M A, Chin W W. J Clin Invest. 1990;85:101–105. doi: 10.1172/JCI114398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazar M A, Hodin R A, Chin W W. Proc Natl Acad Sci USA. 1989;86:7771–7774. doi: 10.1073/pnas.86.20.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgos-Trinidad M, Koenig R J. Mol Cell Endocrinol. 1999;149:107–114. doi: 10.1016/s0303-7207(98)00253-6. [DOI] [PubMed] [Google Scholar]
- 11.Tagami T, Kopp P, Johnson W, Arseven O K, Jameson J L. Endocrinology. 1998;139:2535–2544. doi: 10.1210/endo.139.5.6011. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg A N, Monden T, Flynn T R, Boers M E, Cohen O, Wondisford F E. Mol Endocrinol. 1995;9:540–550. doi: 10.1210/mend.9.5.7565802. [DOI] [PubMed] [Google Scholar]
- 13.Feng P, Li Q L, Satoh T, Wilber J F. Biochem Biophys Res Commun. 1994;200:171–177. doi: 10.1006/bbrc.1994.1430. [DOI] [PubMed] [Google Scholar]
- 14.Forrest D, Hanebuth E, Smeyne R J, Everds N, Stewart C L, Wehner J M, Curran T. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss R E, Murata Y, Cua K, Hayashi Y, Seo H, Refetoff S. Endocrinology. 1998;139:4945–4952. doi: 10.1210/endo.139.12.6412. [DOI] [PubMed] [Google Scholar]
- 16.Wikström L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraichard A, Chassande O, Plateroti M, Roux J P, Trouillas J, Dehay C, Legrand C, Gauthier K, Kedinger M, Malaval L, et al. EMBO J. 1997;16:4412–4420. doi: 10.1093/emboj/16.14.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazar M A, Hodin R A, Darling D S, Chin W W. Mol Cell Biol. 1989;9:1128–1136. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spanjaard R A, Nguyen V P, Chin W W. Mol Endocrinol. 1994;8:286–295. doi: 10.1210/mend.8.3.8015547. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier K, Chassande O, Plateroti M, Roux J P, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. EMBO J. 1999;18:623–631. doi: 10.1093/emboj/18.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss R E, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S. Endocrinology. 1997;138:3624–3629. doi: 10.1210/endo.138.9.5412. [DOI] [PubMed] [Google Scholar]
- 22.Weiss R E, Xu J, Ning G, Pohlenz J, O'Malley B W, Refetoff S. EMBO J. 1999;18:1900–1904. doi: 10.1093/emboj/18.7.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohlenz J, Maqueem A, Cua K, Weiss R E, Van Sande J, Refetoff S. Thyroid. 1999;9:1265–1271. doi: 10.1089/thy.1999.9.1265. [DOI] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Berry M J, Banu L, Larsen P R. Nature (London) 1991;349:438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- 26.Magnuson M A, Morioka H, Tecce M F, Nikodem V M. J Biol Chem. 1986;261:1183–1186. [PubMed] [Google Scholar]
- 27.Murata Y, Seo H, Sekiguchi K, Imai T, Lee J, Matsui N. Mol Endocrinol. 1990;4:693–699. doi: 10.1210/mend-4-5-693. [DOI] [PubMed] [Google Scholar]
- 28.Grubbs F. Technometrics. 1969;11:1–21. [Google Scholar]
- 29.Grubbs F E, Beck G. Technometrics. 1972;14:847–854. [Google Scholar]
- 30.Johansson C, Göthe S, Forrest D, Vennsrtöm B, Thorén P. Am J Physiol. 1999;276:H2006–H2012. doi: 10.1152/ajpheart.1999.276.6.H2006. [DOI] [PubMed] [Google Scholar]
- 31.Refetoff S, Degroot L J, Barsano C P. J Clin Endocrinol Metab. 1980;51:41–45. doi: 10.1210/jcem-51-1-41. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Sakurai A, DeGroot L J, Refetoff S. J Clin Endocrinol Metab. 1992;74:49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y, Mangoura D, Refetoff S. Mol Endocrinol. 1996;10:100–106. doi: 10.1210/mend.10.1.8838149. [DOI] [PubMed] [Google Scholar]
- 34.Menjo M, Murata Y, Fujii T, Nimura Y, Seo H. Endocrinology. 1993;133:2984–2990. doi: 10.1210/endo.133.6.8243326. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz H, Lazar M, Oppenheimer J. J Biol Chem. 1994;269:24777–24782. [PubMed] [Google Scholar]
- 36.Lazar M A, Chin W W. J Clin Invest. 1990;86:1777–1782. doi: 10.1172/JCI114906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu V C, Delsert C, Andersen B, Holloway J M, Devary O V, Naar A M, Kim S Y, Boutin J M, Glass C K, Rosenfeld M G. Cell. 1991;67:1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- 38.Kliewer S A, Umesono K, Mangelsdorf D J, Evans R M. Nature (London) 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falcone M, Miyamoto T, Fierro-Renoy F, Macchia E, DeGroot L J. Endocrinology. 1992;131:2419–2429. doi: 10.1210/endo.131.5.1425440. [DOI] [PubMed] [Google Scholar]
- 40.White P, Dauncey M J. J Mol Endocrinol. 1999;23:241–254. doi: 10.1677/jme.0.0230241. [DOI] [PubMed] [Google Scholar]
- 41.Ness G C, Pendelton L C, Zhao Z. Biochim Biophys Acta. 1994;1214:229–233. doi: 10.1016/0005-2760(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 42.Ness G C, Lopez D. Arch Biochem Biophys. 1995;323:404–408. doi: 10.1006/abbi.1995.0061. [DOI] [PubMed] [Google Scholar]
- 43.Zilz N D, Murray M B, Towle H C. J Biol Chem. 1990;265:8136–8143. [PubMed] [Google Scholar]
- 44.Dozin B, Magnuson M A, Nikodem V M. J Biol Chem. 1986;261:10290–10292. [PubMed] [Google Scholar]
- 45.Göthe S, Wang Z, Ng L, Kindblom J M, Campos Barros A, Ohlsson C, Vennström B, Forrest D. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Refetoff S, Weiss R E, Usala S J. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 47.Forrest D, Vennström B. Thyroid. 2000;10:41–52. doi: 10.1089/thy.2000.10.41. [DOI] [PubMed] [Google Scholar]