Abstract
In C4 plants, carbonic anhydrase (CA) facilitates both the chemical and isotopic equilibration of atmospheric CO2 and bicarbonate (HCO3−) in the mesophyll cytoplasm. The CA-catalyzed reaction is essential for C4 photosynthesis, and the model of carbon isotope discrimination (Δ13C) in C4 plants predicts that changes in CA activity will influence Δ13C. However, experimentally, the influence of CA on Δ13C has not been demonstrated in C4 plants. Here, we compared measurements of Δ13C during C4 photosynthesis in Flaveria bidentis wild-type plants with F. bidentis plants with reduced levels of CA due to the expression of antisense constructs targeted to a putative mesophyll cytosolic CA. Plants with reduced CA activity had greater Δ13C, which was also evident in the leaf dry matter carbon isotope composition (δ13C). Contrary to the isotope measurements, photosynthetic rates were not affected until CA activity was less than 20% of wild type. Measurements of Δ13C, δ13C of leaf dry matter, and rates of net CO2 assimilation were all dramatically altered when CA activity was less than 5% of wild type. CA activity in wild-type F. bidentis is sufficient to maintain net CO2 assimilation; however, reducing leaf CA activity has a relatively large influence on Δ13C, often without changes in net CO2 assimilation. Our data indicate that the extent of CA activity in C4 leaves needs to be taken into account when using Δ13C and/or δ13C to model the response of C4 photosynthesis to changing environmental conditions.
Isotope analysis of atmospheric CO2 is an important tool for monitoring changes in the global exchange of CO2 (Flanagan and Ehleringer, 1998; Yakir and Sternberg, 2000). However, to interpret the atmospheric CO2 isotopic signature requires an understanding of the isotopic fractionation steps associated with specific processes during leaf gas exchange (Yakir and Sternberg, 2000). Leaf level models of carbon isotope exchange (Δ13C) in C4 plants have been used for many years to help interpret the response of C4 plants to changing environmental conditions. However, only recently has the genetic manipulation of the C4 photosynthetic apparatus provided an opportunity to reexamine the C4 leaf level models of Δ13C (von Caemmerer et al., 1997a, 1997b).
Most C4 plants utilize a compartmentalized CO2-concentrating mechanism between the mesophyll and bundle sheath cells (BSC) to increase the CO2 partial pressure (pCO2) around the site of Rubisco in the BSC. The first enzymatic step in C4 photosynthesis is the reversible hydration reaction catalyzed by carbonic anhydrase (CA), which converts CO2 to bicarbonate (HCO3−) in the mesophyll cytoplasm. Subsequently, HCO3− is fixed via phosphoenolpyruvate carboxylase (PEPC) into a four-carbon acid that diffuses to the BSC for decarboxylation (Kanai and Edwards, 1999). The specialized biochemistry and leaf anatomy of C4 plants results in a pCO2 around the site of Rubisco severalfold higher than current atmospheric levels, significantly reducing the rates of photorespiration (Hatch, 1987; Kanai and Edwards, 1999).
The carbon isotope discrimination during C4 photosynthesis is determined by the fractionation that occurs during diffusion of CO2 into the leaf, its conversion to HCO3− via CA, and the subsequent carboxylation reactions catalyzed by PEPC and Rubisco (Peisker, 1982; Farquhar, 1983; Peisker and Henderson, 1992; von Caemmerer et al., 1997a). The extent to which Rubisco can fractionate against CO2 is determined by the amount of leakiness (φ), defined as the fraction of CO2 fixed by PEPC that subsequently leaks out of the BSC. If the BSC were gas tight, then all of the CO2 released into the BSC would be fixed by Rubisco and no fractionation would occur at this step. However, CO2 can leak out of the BSC, allowing Rubisco to influence the overall discrimination during C4 photosynthesis (Farquhar, 1983; Peisker and Henderson, 1992).
Differences in the ratio of CO2 partial pressures between the intercellular airspace and the atmosphere (pi/pa) along with φ are the main factors attributed to variation in Δ13C in C4 plants (Farquhar, 1983). The ratio pi/pa is primarily determined by stomatal conductance, whereas φ depends on the physical conductance of the BSC walls and the balance between the C4 and C3 cycles. Little change in φ was determined with gas exchange and Δ13C measurements in various C4 plants under a variety of environmental conditions (Henderson et al., 1992). However, with the use of antisense technologies, it has been shown that Δ13C and φ increase when the capacity of the C3 cycle is reduced relative to the C4 cycle (von Caemmerer et al., 1997a, 1997b). Growth conditions (e.g. elevated CO2 and water stress) have also been reported to influence the balance of the C4 and C3 cycles, leading to an altered isotopic composition of dry matter (Watling et al., 2000; Williams et al., 2001), although the influence on CA was not addressed in these studies.
There is limited research concerning the influence of CA activity on Δ13C in C4 plants. Recent work indicates that CA activity in wild-type Flaveria bidentis is in excess and does not limit CO2 assimilation under normal conditions (von Caemmerer et al., 2004). F. bidentis lines with reduced levels of CA, due to the expression of antisense constructs targeted to a putative mesophyll cytosolic CA, showed that rates of CO2 assimilation were unaffected by a decrease in CA activity until activity was less than 20% of wild type (von Caemmerer et al., 2004). Although large changes in CA activity had little effect on photosynthetic rates, according to the Δ13C theory developed by Farquhar in 1983 (see “Materials and Methods”), the decrease in the hydration reaction of CO2 (Vh) relative to the rate of PEPC carboxylation (Vp) should increase Δ13C potentially without a corresponding change in the rate of net CO2 assimilation (Farquhar, 1983).
In this article, we use F. bidentis plants with low CA activity to examine the influence of the hydration reaction of CO2 on Δ13C during C4 photosynthesis. These results are discussed in relation to measurements of Δ13C made in F. bidentis under various irradiances, as well as plants with reduced levels of Rubisco.
RESULTS
Carbon Isotope Discrimination
Light Response Curves
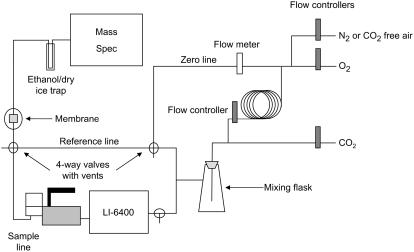
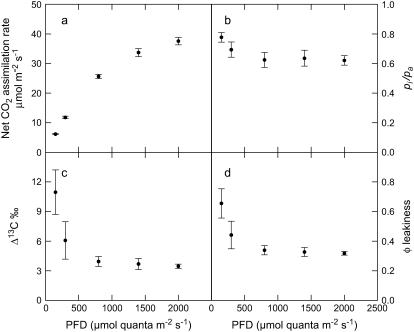
In the mass spectrometric gas-exchange system used here for online Δ13C measurements, the leaf chamber gas outlet of a LI-6400 gas-exchange system (LI-COR) was directly coupled to a mass spectrometer (micromass ISOPRIME; Micromass Ltd.) via a gas-permeable silicone membrane (Fig. 1). This allowed the measurement of the 13C/12C ratio of the CO2 in the airstream without prior purification of that CO2. We measured rates of net CO2 assimilation and Δ13C in F. bidentis wild-type plants in response to photon flux density (PFD) to test our online systems with previously published values of Δ13C from C4 plants (Henderson et al., 1992). A summary of the symbols used in the text are shown in Table I. Net CO2 assimilation increased with PFD to near-saturating rates (Fig. 2a). However, there was little change in Δ13C, pi/pa, and BSC CO2 leakiness (φ), except at the two lowest light levels (Fig. 2, b–d). There was more uncertainty in the Δ13C measurements made at low light because of the higher ratio of the rate of CO2 entry into the chamber to the rate of net CO2 assimilation by the leaf (ξ; see Fig. 2, legend). Leakiness was calculated by rearranging Equation 2 (see equations in “Materials and Methods”) and substituting b4 with Equation 3, with the assumption that the initial CO2 carboxylation reaction catalyzed by PEPC to the rate of CO2 hydration by CA (Vp/Vh) was zero. These gas-exchange and Δ13C measurements are similar to those previously reported for Amaranthus edulis and Zea mays under similar measurement conditions (Henderson et al., 1992).
Figure 1.
Arrangement of the gas flow controllers, the LI-6400 gas exchange system, and the mass spectrometer system used for simultaneous measurements of leaf gas exchange and carbon isotope discrimination. Switching between gas samples was controlled by a manual four-way valve. The zero and reference readings were made before and after each leaf measurement and averaged during the calculations.
Table I.
Symbols used in the text
| Symbol | Description |
|---|---|
| A | Net CO2 assimilation |
| a | Fractionation during diffusion of CO2 from the chloroplast to the atmosphere (4.4‰) |
| al | Fractionation of CO2 diffusion through a liquid (0.7‰) |
| BSC | Bundle sheath cells |
| b3 | Combined discrimination of Rubisco, respiration, and photorespiration (see Eq. 4) |
| b4 | Combined discrimination of PEPC, respiration, and hydration/dehydration of CO2 (see Eq. 3 and Fig. 4) |
| bp | Discrimination by PEPC (2.2‰) |
| Δ13C | Carbon isotope discrimination |
| CA | Carbon anhydrase |
| e | Fractionation during respiration (3‰ or −6‰) |
| es | Fractionation as CO2 dissolves (1.1‰) |
| eb | Equilibrium fractionation factor for the catalyzed hydration/dehydration of CO2 (−9‰) |
| f | Discrimination during photorespiration (10‰ or −6.8‰) |
| φ | The fraction of CO2 fixed by PEPC that subsequently leaks out of the BSC |
| gw | The internal conductance to the diffusion of CO2 between the intercellular air space and the site of carboxylation in the mesophyll cytoplasm |
| h | Catalyzed fractionation during CO2 hydration (1.1‰) |
| kCA | Rate constant of carbonic anhydrase |
| Kc | Michaelis constant of Rubisco for CO2 |
| Ko | Michaelis constant of Rubisco for O2 |
| Kp | Michaelis constant of PEPC for CO2 |
| Md | Rate of mitochondrial respiration |
| Mm | Rate of mitochondrial respiration in the mesophyll cells |
| Ms | Rate of mitochondrial respiration in the BSC |
| pCO2 | Partial pressure of CO2 |
| pe | pCO2 of dry air entering the leaf chamber |
| pi | pCO2 of the intercellular airspace |
| pm | pCO2 of the mesophyll cytoplasm |
| po | pCO2 of dry air leaving the leaf chamber |
| Re | 13C/12C of the air entering the leaf chamber |
| Ro | 13C/12C of the air leaving the leaf chamber |
| PFD | Photon flux density |
| PSII | PSII |
| ξ | pe/(pe − po) |
| s | Fractionation during the leakage of CO2 from the BSC (1.8‰) |
| Vc | Rate of Rubisco carboxylation |
| Vcmax | Maximal rate of Rubisco carboxylation |
| Vh | Rate of CO2 hydration |
| Vo | Rate of photorespiration |
| Vp | Rate of PEP carboxylation (A + Md)/(1 − φ) or (pmVpmax)/(pm + Kp) |
| Vpmax | Maximal rate of PEPC carboxylation |
Figure 2.
a, Net CO2 assimilation rate. b, Ratio of intercellular to ambient CO2 partial pressures (pi/pa). c, Carbon isotope discrimination (Δ13C). d, Bundle sheath leakiness to CO2 (φ) as a function of PFD (μmol quanta m−2 s−1). Measurements were made at a pCO2 of 52 Pa, a pO2 of 4.8 kPa, and a leaf temperature of 30°C. Shown are the means ± the se of measurements made on three to five leaves from two F. bidentis wild-type plants. Values for ξ (Eq. 1) were 29.9 ± 0.75, 15.7 ± 0.54, 7.11 ± 0.24, 5.5 ± 0.23, and 4.9 ± 0.17 at PFDs of 150, 300, 800, 1,400, and 2,000 μmol quanta m−2 s−1. φ was calculated from Equation 5, assuming Vp/Vh = 0.
Rubisco Small Subunit Plants
Net CO2 assimilation in F. bidentis plants with reduced levels of Rubisco caused by antisense RNA constructs targeted to the nuclear-encoded gene for the small subunit of Rubisco (anti-SSu plants) had rates between 40% to 80% of wild-type plants (Table II). Additionally, the ratio of pi/pa, Δ13C, and φ were higher in the anti-SSu-plants as compared with wild-type plants (Table II). The parameter φ was determined from simultaneous gas-exchange and isotope measurements and solving for φ in Equation 2. Our measurements of Δ13C and leaf gas exchange are similar to previously published values by von Caemmerer et al. (1997b). The comparison of our results to previously published Δ13C values shows that our system can accurately and consistently monitor the influence of both environmental conditions and perturbations to the C4 photosynthetic apparatus on instantaneous carbon isotope discrimination.
Table II.
CA rate constant (kCA), net CO2 assimilation rate (A), ratio of intercellular to atmospheric CO2 partial pressure (pi/pa), online Δ13C discrimination, and leakiness of CO2 out of the BSCs (φ) in the anti-SSu plants from the primary transformant 136-13
For calculation of φ, the Vp/Vh ratio was assumed to be zero. Measurements were made at a pCO2 of 52 Pa, a pO2 of 4.8 kPa, a PFD of 2,000 μmol quanta m−2 s−1, and a leaf temperature of 30°C. n = 4 for the wild-type plants.
| kCA | A | pi/pa | Δ13C | Leakiness φ | |
|---|---|---|---|---|---|
| mol m−2 s−1 Pa−1 | μmol m−2 s−1 | ‰ | |||
| 136-13-11#1 | 52 | 17.0 | 0.67 | 6.1 | 0.43 |
| 136-13-12#4 | 88 | 19.5 | 0.64 | 6.5 | 0.45 |
| 136-13-12#3 | 62 | 27.9 | 0.72 | 6.0 | 0.42 |
| 136-13-11#2 | 81 | 29.9 | 0.71 | 5.4 | 0.39 |
| Wild type | 69 ± 2 | 37.6 ± 2.6 | 0.55 ± 0.02 | 2.5 ± 0.4 | 0.25 ± .02 |
CA Plants
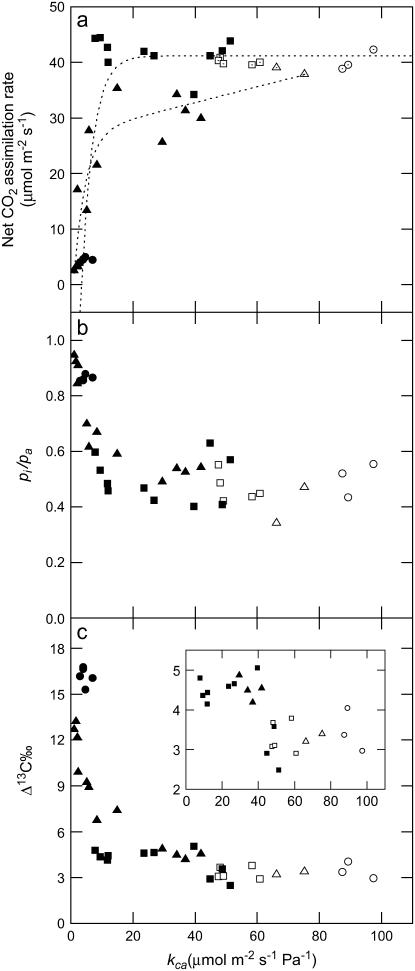
Carbon isotope discrimination (Δ13C) increased as CA activity decreased in the F. bidentis plants containing the antisense RNA constructs targeted to the putative cytosolic CA (anti-CA plants; Fig. 3). CA activity, reported here as a rate constant (kCA μmol m−2 s−1 Pa−1), was determined on leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from doubly labeled 13C18O2 to H216O (see “Materials and Methods”). Interestingly, Δ13C was more sensitive than net CO2 assimilation to changes in CA activity as Δ13C increased in some anti-CA plants, whereas net CO2 assimilation remained similar to wild-type plants (Fig. 3). In these anti-CA plants with reduced CA activity and wild-type rates of net CO2 assimilation, Δ13C increased 1‰ to 2‰, which is a large shift for C4 photosynthesis (Fig. 3c, inset; Table III). In the anti-CA plants, net CO2 assimilation rates and pi/pa were similar to wild-type plants, except when CA activities were less than 20% of wild type (Fig. 3, a and b). Anti-CA plants with extremely low levels of CA activity (<5% of wild type) and low rates of net CO2 assimilation had extremely high values of Δ13C (Fig. 3c).
Figure 3.
Net CO2 assimilation rate, the ratio of intercellular to ambient pCO2 (pi/pa), and carbon isotope discrimination (Δ13C) as a function of the rate constant of leaf CA (kCA μmol m−2 s−1 Pa−1). The inset in c shows the expanded scale of Δ13C where net CO2 assimilation is relatively constant. Each point represents a measurement made on a different plant grown in a glasshouse at ambient CO2 or in a growth cabinet at 1% CO2: wild-type plants grown at ambient CO2 (□); anti-CA plants grown at ambient CO2 (▪); wild-type grown at 1% CO2 (○, ▵); and anti-CA plants grown at 1% CO2 (•, ▴). Measurements were made at 2,000 μmol quanta m−2 s−1, leaf temperature of 30°C, and an inlet CO2 concentration of either 38 Pa in air (▵, ▴) or 52 Pa of CO2 in a 90.5 kPa of N2 and 4.8 kPa of O2 gas mixture (□○, ▪•). The lines represent the best fit for all measurements (wild-type and anti-CA plants) made at either 38 or 52 Pa of CO2.
Table III.
Net CO2 assimilation rate (A), the ratio of intercellular to ambient pCO2 (pi/pa), CAleaf calculated as (kCApm), Δ13C, and Vp/Vh for F. bidentis wild-type plants and anti-CA plants with low CAleaf activity and wild-type-like net CO2 assimilation rates
Measurements were made at a pCO2 of 52 Pa, a pO2 of 4.8 kPa, PFD of 2,000 μmol quanta m−2 s−1, and a leaf temperature of 30°C. Vp/Vh was estimated either by online Δ13C measurements* using Eqs. 3 and 5 (“Materials and Methods”) or estimated from Vp/CAleaf**, where Vp was calculated as (A + Md)/(1 − φ) and CAleaf. gw was assumed to be either 10 or 6 μmol m−2 s−1 Pa−1, and φ was set at either 0.24 or 0.1. Md is the daytime rate of respiration assumed to be 2 μmol m−2 s−1. n = 7 and 5 for anti-CA and wild-type plants, respectively.
| A | pi/pa | CAleaf | Δ13C |
Vp/Vh
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| gw = 10 φ = 0.24 | gw = 6 φ = 0.24 | gw = 10 φ = 0.1 | gw = 6 φ = 0.1 | ||||||
| μmol m−2 s−1 | μmol m−2 s−1 | ‰ | |||||||
| Anti-CA | 42 ± 0.6 | 0.48 ± 0.02 | 228 ± 37 | 4.4 + 0.2 | Δ13C* | 0.46 ± 0.06 | 0.55 ± 0.07 | 0.99 ± 0.08 | 1.1 ± 0.08 |
| In vitro** | 0.29 ± 0.04 | 0.40 ± 0.04 | 0.24 ± 0.03 | 0.30 ± 0.04 | |||||
| Wild type | 40 ± 0.1 | 0.47 ± 0.02 | 774 ± 48 | 3.3 ± 0.2 | Δ13C* | 0.07 ± 0.07 | 0.08 ± 0.08 | 0.61 ± 0.06 | 0.62 ± 0.08 |
| In vitro** | 0.07 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 | |||||
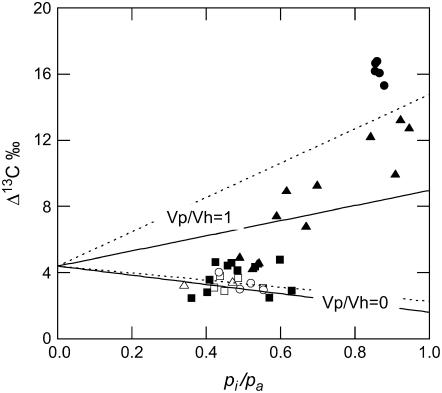
Nearly all of the measured values of Δ13C fall within the theoretical relationship of Δ13C to pi/pa as predicted from the model of C4 carbon isotope discrimination developed by Farquhar (1983; Fig. 4; see “Materials and Methods”). Only in the anti-CA plants with extremely low CA activity do the measured values of Δ13C fall outside the predicted values (Fig. 4). The theoretical relationship of Δ13C and pi/pa was calculated with a φ value of 0.24, and the initial CO2 carboxylation reaction catalyzed by PEPC relative to the CO2 hydration by CA (Vp/Vh) was assumed to be either zero or 1 (as indicated in Fig. 4). The b4 parameter, which is the combined fractionation associated with PEPC, respiration, and the isotopic equilibrium during the dissolution of CO2 and conversion to HCO3−, used in these calculations was determined with either the CA catalyzed (solid lines) or the spontaneous uncatalyzed (dotted lines) CO2 and HCO3− hydration and dehydration fractionation factors (see “Materials and Methods”).
Figure 4.
Carbon isotope discrimination (Δ13C) as a function of the ratio of intercellular to ambient pCO2 (pi/pa). The white symbols are wild-type plants and the black symbols are anti-CA plants. Other symbols and measurement conditions are as described in Figure 3. The lines represent the theoretical relationship of Δ13C and pi/pa, where φ = 0.24, the ratio of the PEPC carboxylation to the CO2 hydration reaction (Vp/Vh) is either 0 or 1, and the b4 parameter is calculated with the catalyzed (solid lines, b4 = −5.7 + 7.9 Vp/Vh) and uncatalyzed (dotted lines, b4 = −4.5 + 12.5 Vp/Vh) CO2 and HCO3− hydration and dehydration fractionation factors. gw was assumed to be large such that pi = pm.
To characterize the influence of CA activity on Δ13C, independent of changes in net CO2 assimilation, we pooled the data of anti-CA plants with reduced CA activity and wild-type-like photosynthetic rates. Δ13C was higher in the anti-CA plants compared to the wild-type plants, whereas pi/pa was unchanged (Table III). The in vivo CA activity (CAleaf), which is the product of kCA and the pCO2 in the mesophyll cytoplasm (pm), was significantly less in the anti-CA relative to the wild-type plants (Table III). The value of pm was calculated with an internal conductance to the diffusion of CO2 between the intercellular airspace and the site of carboxylation in the mesophyll cytoplasm (gw) of 10 mol m−2 s−1 Pa−1. The ratio of Vp/Vh determined from the online measurements of Δ13C was approximately 6 times greater in the anti-CA plants than in the wild-type plants (Table III). It appears that a rather large decrease in leaf CA activity in F. bidentis can maintain the chemical equilibrium between CO2 and HCO3− needed to sustain photosynthesis, but limits the isotopic equilibrium causing Δ13C to increase without changes in φ.
It should be noted that the absolute value of Vp/Vh determined this way is largely influenced by φ and slightly by gw. For example, changing gw from 6 to 10 mol m−2 s−1 Pa−1 shifts calculations of Vp/Vh from 0.08 to 0.07 and 0.55 to 0.46 for wild-type and anti-CA plants, respectively. However, changing φ from 0.24 to 0.10, assuming a constant gw of 10 mol m−2 s−1 Pa−1, causes Vp/Vh to increase from 0.07 to 0.61 in the wild-type plants and from 0.46 to 0.99 in the anti-CA plants (Table III). In the anti-CA plants, which have a reduced capacity to concentrate CO2 within the BSC, it is predicted from the C4 photosynthetic model that φ will decrease relative to the wild-type plants (see below), which would increase the difference of Vp/Vh between the wild-type and the anti-CA plants. Vp/Vh can also be approximated from gas exchange and in vitro CA activity as Vp/CAleaf, where CAleaf is calculated as kCApm and Vp is calculated as (A + Md)/(1 − φ) (von Caemmerer, 2000). The parameter Md is the daytime rate of mitochondrial respiration assumed to be 2 μmol m−2 s−1. The ratio of Vp/Vh determined from the in vitro assays of CA activity was approximately 4 times greater in the anti-CA plants than in the wild-type plants (Table III). The absolute value of Vp/Vh calculated in this manner is also influenced by changes in gw and φ, although neither parameter has a large influence on the relative changes of Vp/Vh between wild-type and anti-CA plants.
Dry Matter δ13C
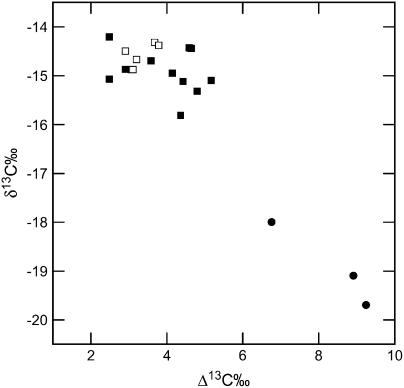
Leaf dry matter δ13C, the ratio of 13C/12C of the sample relative to the standard Vienna Pee Dee Belemnite (VPDB), was lower in plants with low levels of CA and correlated with increases in Δ13C (Fig. 5). Leaf δ13C was determined on plants germinated and grown in a glasshouse. After collecting an entire leaf for δ13C, the three plants with very low CA and photosynthetic rates were transferred after several weeks to the 1% CO2 growth cabinets before leaf gas-exchange measurements were made. Otherwise, the leaf opposite to the one used for gas exchange was sampled for δ13C.
Figure 5.
Leaf dry matter δ13C, determined on the entire leaf opposite to the one used for gas exchange and enzyme analysis, plotted against changes in online carbon isotope discrimination (Δ13C). The white symbols are wild-type plants and the black symbols are anti-CA plants. All plants were germinated and grown in a glasshouse under ambient atmospheric CO2 conditions. The plants with extremely low δ13C values (•) were transferred to the 1% CO2 growth cabinets after tissue was collected for Δ13C analysis.
Photosynthetic and Carbon Isotope Discrimination Models
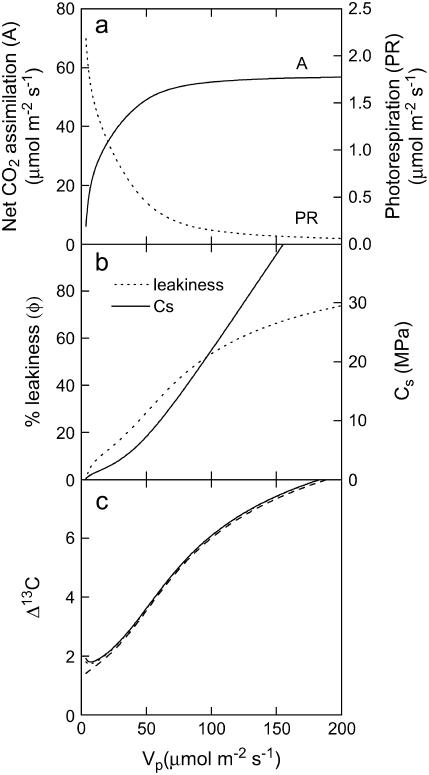
It has recently been shown that low leaf CA activity in F. bidentis reduces the capacity of the C4 cycle by limiting the rate of PEPC carboxylation of HCO3− (Vp) (von Caemmerer et al., 2004). Here, we use the C4 photosynthetic model developed by Berry and Farquhar (1978) and von Caemmerer (2000) to predict the response of net CO2 assimilation, bundle sheath pCO2, photorespiration (Vo), and φ to changes in the activity of PEPC due to a limitation in CA activity. In the C4 photosynthetic model, the CA-mediated hydration/dehydration reaction of CO2 within the mesophyll cytoplasm has not been incorporated. However, manipulating Vp within the model simulates the effect of changing CA activity and leads to a diminished ability to concentrate CO2 within the BSC, which decreases both the photosynthetic rate and φ (Fig. 6, a and b).
Figure 6.
Modeling the response of net CO2 assimilation (a); the pCO2 in the BSC and BSC CO2 leakiness, φ (b); and Δ13C (c) in response to changes in PEPC activity (Vp). The C4 model used a Vcmax of 60 μmol m−2 s−1, a bundle sheath conductance to CO2 per leaf area of 0.03 μmol m−2 s−1 Pa−1, Km of PEPC for CO2 (Kp) of 8 Pa, Km of Rubisco for CO2 (Kc) and O2 (Ko) of 65 Pa and 45 kPa, fraction of PSII in the BSC 0.2 and Rubisco specificity of 2,590 Pa/Pa in the gas phase, and mitochondrial respiration was 2 μmol m−2 s−1, one-half of which was assumed to occur in the mesophyll. The pO2 in the mesophyll was assumed to be 20 kPa. Carbon isotope discrimination was calculated using the C4 photosynthetic model output and a constant pi/pa of 0.4. Vh was set at 2,000 μmol m−2 s−1 and Vp/Vh varied between 0.001 and 0.1, causing only a 0.3‰ shift in Δ13C at a constant φ. The lines for Δ13C represent models determined with Equation 2 by substituting the b4 and b3 factors with Equations 3 and 4, respectively. The lines for Δ13C represent models using different fractionation factors for respiration (3‰ dotted line and −6‰ solid and dashed lines) and photorespiration (10‰ dashed line and −6.8‰ dotted and solid lines).
The outputs from the C4 photosynthetic model, specifically the rates of Rubisco carboxylation (Vc), Vo, Vp, φ, and the pCO2 in the BSC, were then incorporated into the model of C4 carbon isotope discrimination (Δ13C) developed by Farquhar (1983). The Δ13C model was used to determine which photosynthetic parameters would influence Δ13C consistent with our experimental data and to demonstrate the influence of φ on Δ13C independent of changes in Vp/Vh. The model in Figure 6 included sufficient CA activity to keep Vp/Vh close to zero as Vp changes and pi/pa were held constant at 0.4. As shown in Figure 6c, when φ and the pCO2 in the BSC are low, Δ13C decreases as the ability of Rubisco to fractionate is reduced. Additionally, the Δ13C model accounts for the effects of fractionation during respiration (e) and photorespiration (f); however, there is uncertainty in the specific values of factors e and f in the model (Gillon and Griffiths, 1997; Ghashghaie et al., 2003). Therefore, to test the influence of these parameters on the Δ13C model, various values of e (3‰ versus −6‰) and f (−6.8‰ versus 10‰) were used. Even at low CO2 assimilation rates, relatively large changes in e and f had only a small influence on Δ13C (Fig. 6c).
DISCUSSION
Carbon isotope discrimination increased in the short term during leaf gas exchange (Δ13C) and the carbon isotope composition of leaf dry matter (δ13C) decreased in transformants containing reduced levels of leaf CA. As was previously reported (von Caemmerer et al., 2004), leaf CA activity appears to be in excess to maintain steady-state rates of net CO2 assimilation in F. bidentis at high light. A nearly 80% decrease in leaf CA activity was needed before net CO2 assimilation was affected when measured at a CO2 partial pressure (pCO2) of 52 Pa (Fig. 3a). The CA activity required to maintain wild-type-like photosynthetic rates increased when measurements were conducted at a lower pCO2 of 38 Pa (Fig. 3a). This is in agreement with previously published work where reduced leaf cytosolic CA activity affected the initial slope of the CO2 response curve in F. bidentis when the rate of CO2 hydration limited the supply of HCO3− for PEPC carboxylation (von Caemmerer et al., 2004).
Carbon Isotope Discrimination and CA Activity
According to the C4 photosynthetic model (von Caemmerer, 2000), a limitation in the supply of cytosolic HCO3− will lead to a decrease in the initial CO2 carboxylation reaction catalyzed by PEPC and reduce the capacity of the C4 pump to concentrate CO2 within the BSC. A reduced pCO2 in the BSC leads to a decrease in the rate of net CO2 assimilation as well as a lower BSC CO2 leakiness (φ). In the model of C4 carbon isotope discrimination, the main factors that influence Δ13C are changes in the intercellular to ambient CO2 partial pressures (pi/pa) and φ (Farquhar, 1983). When the ratio of PEPC carboxylation to the hydration reaction of CO2 (Vp/Vh) is near zero (i.e. CA activity is high relative to PEPC carboxylation), the C4 carbon isotope model predicts that Δ13C will decrease as φ decreases (Fig. 6). The Δ13C modeling illustrates that when the front end of the C4 cycle is diminished (either by reduced CA and/or PEPC activity or anything else), φ decreases and Δ13C associated with φ also decreases (Fig. 6). However, in the anti-CA plants, which potentially reduced the ability to concentrate CO2 in the BSC, Δ13C increased, which cannot be explained in the model by decreases in φ, but can be explained by changes in Vp/Vh.
Due to the high levels of mesophyll cytoplasmic CA activity in C4 plants, it is generally assumed that CO2 and HCO3− are in close chemical equilibrium. Under such conditions, the ratio of Vp/Vh in Equation 3 approaches zero and can be omitted from the calculation of b4. However, when Vp/Vh tends away from zero, Equation 3 can be expressed with the fractionation factors provided in “Materials and Methods” as b4 = −5.7 + 7.9 Vp/Vh at 25°C. The b4 fractionation factor becomes more positive as Vp/Vh increases and Δ13C increases even without changes in pi/pa and φ. As shown in Figure 4, varying Vp/Vh between 0 and 1 can have a large impact on Δ13C, especially when pi/pa is high. Nearly all the variation in Δ13C in the anti-CA plants can be explained by changes in Vp/Vh (Fig. 4). Only when CA activity and photosynthetic rates decline dramatically do changes in Vp/Vh and pi/pa not accurately predict Δ13C (see below for further discussion).
Variation in the Ratio of PEPC Carboxylation to CO2 Hydration by CA
The large change in Vp/Vh without changes in photosynthesis in the anti-CA plants (Table III) indicates that Δ13C is more sensitive to a reduction in CA activity than net CO2 assimilation. The influence of Vp/Vh on Δ13C is predicted by the C4 photosynthetic model for carbon isotope discrimination developed by Farquhar (1983), and here we demonstrate the influence of CA activity on Δ13C in a C4 plant. In wild-type F. bidentis plants, CA appears to be in excess for supporting photosynthesis and Vp/Vh approaches zero. However, it has been reported that CA activity in most C4 species is only just sufficient to support photosynthetic rates, especially in C4 monocots (Hatch and Burnell, 1990; Gillon and Yakir, 2000, 2001), and the influence of Vp/Vh on Δ13C may be greater in these C4 species. For example, we measured (A.B. Cousins, M. R. Badger, and S. von Caemmerer, unpublished data) CA activity in Z. mays as 266 ± 22 μmol m−2 s−1, which is similar to our anti-CA plants with wild-type photosynthetic rate (see Fig. 3c; Table III). Gillon and Yakir (2001) reported even lower CA activity for a number of C4 grasses, which correspond to the low CA activities we measured in anti-CA plants shown in Figure 3c, inset. The values of Vp/Vh estimated from the CA activity and net CO2 assimilation from this article indicate that Δ13C will differ in C4 plants that have been reported to contain a range of CAleaf activity (2–529 μmol m−2 s−1) with generally similar photosynthetic rates (Gillon and Yakir, 2001). However, a systematic investigation of the influence of Vp/Vh on Δ13C in a range of C4 species needs to be conducted.
Changes in Vp/Vh and its influence on Δ13C also have important implications for interpreting physiological processes responsible for changes in Δ13C and δ13C during C4 photosynthesis, particularly in response to changing environmental conditions. Water stress, reduced nitrogen availability, and atmospheric CO2 availability have all been reported to increase Δ13C in C4 plants by 1‰ to 3‰ (Meinzer et al., 1994; Ranjith et al., 1995; Buchmann et al., 1996; Saliendra et al., 1996; Meinzer and Saliendra, 1997; Meinzer and Zhu, 1998; Watling et al., 2000). Variation in Δ13C in these reports has been interpreted as changes in either pi/pa and/or φ, with the apparent assumption that Vp/Vh remains close to zero in all treatments. However, there are a few reports in the literature that suggest CA activity in C4 plants is also influenced by environmental conditions, including nitrogen status, atmospheric CO2 availability, and salt stress (Cervigni et al., 1971; Burnell et al., 1990; Brownell et al., 1991), implying that environmental conditions may also alter Vp/Vh and thus influence measured Δ13C. Because leaf CA activity in C4 plants is largely dependent on the internal CO2 partial pressures, conditions that influence CO2 availability, such as water stress and growth under elevated atmospheric CO2, will also alter Vp/Vh. C4 photosynthesis generally operates near CO2-saturating conditions at current atmospheric pCO2 such that a reduction in pi due to stomatal closure will cause Vp/Vh to increase. However, under such conditions, the ratio of pi/pa also decreases and the influence of Vp/Vh on Δ13C decreases as shown in Figure 4. Alternatively, it has been shown that, under well-watered conditions, C4 photosynthesis generally does not respond to increases in atmospheric pCO2 (McLeod and Long, 1999; Ghannoum et al., 2000; Wall et al., 2001; Ainsworth and Long, 2005; Leakey et al., 2006). However, because pi/pa is generally constant with changing atmospheric pCO2 and CA has a very high Km for HCO3−, an increase in CO2 availability will increase Vh, whereas PEPC is generally saturated around ambient pCO2 and Vp will not change. This raises the possibility that growth under future atmospheric CO2 conditions will alter Δ13C regardless of other environmental changes if CA is limiting.
Both online and in vitro measurements of Vp/Vh indicated that changes in CA activity have a significant influence on Δ13C without changes in pi/pa and φ. It must be noted that, although changes in gw have a subtle effect on estimates of Vp/Vh, variation in φ can lead to large shifts in the absolute values of Vp/Vh when determined from the Δ13C measurements. There are no direct means of measuring φ, but it can be estimated using Δ13C measurements when Vp/Vh is assumed to be close to zero. The use of antisense technology targeted toward the C4 PEPC enzyme would provide a range of Vp/Vh values and would allow an estimate of φ when Vp/Vh was known to be close to zero.
Low CA and Photosynthetic Mutants
In the majority of CA plants, the increase in Δ13C can be explained by changes in the ratio of Vp/Vh and pi/pa (Fig. 4). However, this explanation does not hold true for plants with very low leaf CA activity and photosynthetic rates. Potentially, the amount of direct fixation of atmospheric CO2 in the BSC, leakage of HCO3− from the BSC, as well as photorespiration and respiration would influence Δ13C especially when net CO2 assimilation is inhibited. Theoretically, CO2 assimilation by direct diffusion of CO2 from the atmosphere into the BSC would increase the Δ13C as the exchange of CO2 between the atmosphere and the BSC would allow Rubisco to fractionate against the heavier carbon isotope. However, a low conductance of CO2 diffusion across the BSC (gw mmol m−2 s−1 Pa−1) is an essential component of the C4 CO2-concentrating mechanism and limits the amount of direct fixation of CO2 under ambient CO2 concentrations (Jenkins et al., 1989; Brown and Byrd, 1993; He and Edwards, 1996; von Caemmerer, 2000; Kiirats et al., 2002). Therefore, even when the initial carboxylation reaction of the C4 pump is limited by low CA activity or low light, there would be little, if any, direct fixation of CO2 in the BSC and minimal influence on Δ13C.
Alternatively, because 13C concentrates in HCO3− and Rubisco preferentially fixes 12C, leakage of HCO3− out of the BSC would change the fractionation factor associated with CO2 leakage from the BSC (s from Eq. 2). However, with the relatively low CA activity in the BSC, it is unlikely that CO2 and HCO3− would be in full isotopic equilibrium and there would be little influence on s (Farquhar, 1983; von Caemmerer et al., 1997a; Ludwig et al., 1998). Additionally, the influence of respiration and photorespiration on the modeled value of Δ13C will increase as the rates of net CO2 assimilation decrease. However, changing the fractionation effect of respiration (e in Eqs. 3 and 4) and photorespiration (f in Eq. 4) to a range of values reported in the literature (Ghashghaie et al., 2003) had only a slight influence on the modeled Δ13C even at low photosynthetic rates (Fig. 6c).
As previously mentioned, Equation 3 simplifies to b4 = −5.7 + 7.9 Vp/Vh at 25°C when the catalyzed fractionation values for eb of −9.0‰ and h of 1.1‰ are used. However, if the interconversion of CO2 and HCO3− occurs via the spontaneous uncatalyzed reaction, eb and h become −7.8‰ and 6.9‰, respectively, and Equation 3 is b4 = −4.5 + 12.5 Vp/Vh, causing the b4 value to become larger, leading to an increase in Δ13C (Fig. 4). The catalyzed and uncatalyzed values of eb and h are taken from previously published work on the hydration and dehydration of CO2 and HCO3− (Mook et al., 1974; Marlier and O'Leary, 1984; Paneth and O'Leary, 1985). The proportion of catalyzed to uncatalyzed hydration/dehydration reactions may have an influence on the Δ13C when the photosynthetic rates are extremely low, such as in the anti-CA plants with extremely low CA activity, but it would have little, if any, influence in wild-type plants.
Carbon Isotope Discrimination Increases at Low Light
The response of Δ13C in C4 plants to various light levels has not been well characterized, but is an important factor to consider when interpreting dry matter δ13C of plants exposed to different light environments or leaves within a canopy. The increase in Δ13C and estimated values of φ in F. bidentis (Fig. 2) are similar to earlier reports that showed that Δ13C generally increases as the PFD decreases (Henderson et al., 1992; Peisker and Henderson, 1992; Tazoe et al., 2005). Buchmann et al. (1996) also showed that Δ13C, calculated from leaf δ13C values, in a number of C4 plants was greater at low PFD. The low conductance of CO2 diffusion across the BSC needed for C4 photosynthesis would limit the direct fixation of CO2 by Rubisco, even under low light, and its influence on Δ13C should be minimal. However, it has been demonstrated with the C4 photosynthetic model that φ increases at low PFD as more electron transport is needed for recycling of photorespired CO2 (von Caemmerer, 2000). The predicted change in φ at low PFD by the C4 photosynthetic model is consistent with our current experimental evidence, as well as earlier published results (Henderson et al., 1992). The evidence from both online and dry matter isotope measurements indicates that growth light conditions need to be considered when interpreting carbon isotope discrimination in C4 plants.
CONCLUSION
CA activity in wild-type F. bidentis appears to be in excess to maintain net CO2 assimilation; however, reducing leaf CA activity had a relatively large influence on Δ13C, often without changes in net CO2 assimilation. The influence of CA activity on Δ13C was also evident in the leaf dry matter δ13C. The model of Δ13C developed by Farquhar (1983) predicted the influence of changes in PEPC carboxylation relative to the hydration reaction of CO2 (Vp/Vh) on Δ13C, except when photosynthetic rates and CA activity were dramatically reduced. It will be important to take the extent of CA activity in C4 leaves into account when using Δ13C and/or δ13C to model leaf level and global C4 photosynthesis in response to changing environmental influences. The influence of environmental conditions on leaf CA activity, Vp/Vh, and thus on Δ13C warrants further investigation.
Additionally, the amount of CA activity in a leaf plays an important role in determining C18OO discrimination during C4 photosynthesis because CA enhances the rate of oxygen exchange between CO2 and leaf H2O and thus determines the extent of isotopic equilibrium. The anti-CA plants will be used to test whether changing leaf CA activity influences C18OO discrimination under similar environmental conditions and whether high CA activity, relative to photosynthetic rates, corresponds to complete isotopic equilibrium between CO2 and leaf H2O as predicted.
MATERIALS AND METHODS
Growth Conditions
Flaveria bidentis plants were previously transformed with antisense RNA constructs targeted to either the nuclear-encoded gene for the small subunit of Rubisco (anti-SSu plants) or a putative cytosolic CA (anti-CA plants; Furbank et al., 1996; von Caemmerer et al., 1997b, 2004). The segregating T1 generations of anti-CA primary transformants with photosynthetic rates similar to wild type were grown during the summer months in a glasshouse under natural light conditions (27°C d/18°C night temperatures). Anti-CA and anti-SSu plants (segregating T2 generation from primary transformant 136-13) with low photosynthetic capacities and wild-type plants were grown under 1% CO2 in a controlled environment growth cabinet at a photosynthetic PFD of 400 μmol quanta m−2 s−1 at plant height and air temperature of 27°C during the day and 18°C at night with a 14-h daylength. Three plants with very low CA and photosynthetic rates were germinated and grown for several weeks in the glasshouse. Subsequently, these plants were transferred to the 1% CO2 growth cabinets before leaf gas-exchange measurements were made. Plants were grown in 5-L pots in garden mix with 2.4 to 4 g Osmocote/L soil (15/4.8/10.8/1.2 N/P/K/Mg + trace elements: B, Cu, Fe, Mn, Mo, Zn; Scotts Australia Pty Ltd.) and watered daily.
Gas-Exchange Measurements
Plants from either the glasshouse or growth cabinet were transferred to the gas-exchange system, where one of the uppermost fully expanded leaves was placed into the leaf chamber of the LI-6400 and allowed to equilibrate at a leaf temperature of 30°C and 2,000 μmol quanta m−2 s−1 for a minimum of 1.5 h. Air entering the leaf chamber was prepared by using mass flow controllers (MKS Instruments) to obtain a gas mix of 90.5 kPa of dry nitrogen and 4.8 kPa oxygen (Fig. 1). A portion of the nitrogen/oxygen mixture was used to zero the mass spectrometer to correct for N2O and other contaminates contributing to the 44 and 45 peaks. Pure CO2 (δ13C = −29‰; VPDB) was added to the remaining airstream to obtain a CO2 partial pressure of approximately 52 Pa. Alternatively, some measurements were made by mixing pure CO2 with CO2-free air and using the CO2-free air as a zero.
The different gas mixtures had no apparent influence on leaf gas exchange or 13C isotope discrimination. Low oxygen (4.8 kPa) was use to minimize contamination of the 46 peak caused by the interaction of O2 and N2 to produce NO2 with the mass spectrometer source element. This was important when looking at C18OO discrimination (A.B. Cousins, M.R. Badger, and S. von Caemmerer, unpublished data). The CO2 used during the gas-exchange measurements had a similar isotopic signature to the CO2 in the high CO2 growth cabinet. This minimized the influence of respired CO2 on the Δ13C measurements in plants with low photosynthetic rates.
The gas mixtures were fed to the inlet of the LI-6400 console and a flow rate of 200 μmol s−1 was maintained over the leaf. The remaining airstream was vented or used to determine the isotopic composition of air entering the leaf chamber (Fig. 1). The efflux from the leaf chamber was measured by either replacing the match valve line with a line connected directly to the mass spectrometer or by placing a tee in the match valve line, allowing flow to both the mass spectrometer and the match valve simultaneously. Gas-exchange parameters were determined by the LI-6400, and pCO2 leaving the chamber was subsequently corrected for the dilution of CO2 by water vapor (von Caemmerer and Farquhar, 1981).
Isotopic Measurements
The efflux from the leaf chamber and the gas mix supplied to the LI-6400 system was linked to a mass spectrometer through an ethanol/dry ice water trap and a thin, gas-permeable silicone membrane, which was housed in a temperature-controlled cuvette. Masses 44 and 45 were monitored continuously and the carbon isotope discrimination during CO2 exchange, Δ13C, was calculated from the ratio of mass 45 to 44 in the reference air, determined before and after each sample measurement, entering the chamber (Re), and the composition of the sample air leaving the leaf chamber (Ro) as described by Evans et al. (1986):
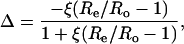 |
(1) |
where ξ = pe/(pe − po), and pe and po are the CO2 partial pressures of dry air entering and leaving the leaf chamber, respectively. A summary of the symbols used in the text is listed in Table I. Zero values for the 44 and 45 peaks were determined before and after the sample measurements were subtracted from both the sample and reference measurements prior to determining the mass ratios. The zero values were typically 1% of the 44 and 45 peaks at 4.8 kPa oxygen and 2% at 20 kPa oxygen.
Calculations of Carbon Isotope Discrimination
The model of C4 carbon isotope discrimination (Δ13C) of Farquhar (1983) was used to determine which factors in the model would influence Δ13C consistent with our experimental data. The simplified model predicts that:
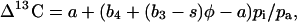 |
(2) |
where a (4.4‰) is the fractionation during diffusion of CO2 and s (1.8‰) is the fractionation during CO2 leakage from the BSCs. The combined fractionation of PEPC, respiration, and the isotopic equilibrium during dissolution of CO2 and conversion to HCO3− (b4) is calculated as (Farquhar, 1983):
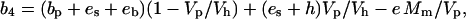 |
(3) |
where bp (2.2‰) is the fractionation by PEPC (O'Leary, 1981), es (1.1‰) is the fractionation as CO2 dissolves (O'Leary, 1984), and eb (−9‰) is the equilibrium fractionation factor of the catalyzed hydration/dehydration reactions of CO2 and HCO3− (Mook et al., 1974). Alternatively, during the hydration/dehydration reactions, the uncatalyzed equilibrium fractionation factor eb = −7.8‰ (Marlier and O'Leary, 1984). The fractionation when CO2 and HCO3− are not at equilibrium is dependent on the rate of CO2 hydration (Vh), the rate of PEPC (Vp), es, and the catalyzed fractionation during CO2 hydration (h). The catalyzed hydration reaction has a fractionation factor of 1.1‰ (calculated by summing the catalyzed CO2 and HCO3− equilibrium fractionation factor −9.0‰ and the catalyzed dehydration fractionation factor 10.1‰; Mook et al., 1974; Paneth and O'Leary, 1985), whereas the uncatalyzed reaction has a 6.9‰ fractionation factor (Marlier and O'Leary, 1984). The fractionation attributed to mitochondrial respiration is e at a rate of mesophyll CO2 release of Mm.
The combined fractionation of Rubisco (30‰), respiration, and photorespiration (b3) can be calculated as:
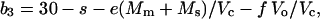 |
(4) |
where Vc is the rate of Rubisco carboxylation reaction, Ms is the rate of BSC mitochondrial respiration, Vo is the rate of photorespiration, and f is the discrimination of photorespiration (Farquhar, 1983).
Equation 2 assumes that the internal conductance to the diffusion of CO2 between the intercellular airspace and the site of carboxylation in the mesophyll cytoplasm (gw) is large, such that pi is equal to the pCO2 at the site of PEPC carboxylation (pc). If gw is low, then Equation 2 can be modified to:
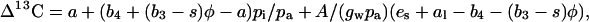 |
(5) |
where A is the net rate of CO2 assimilation and al (0.7‰) is the fractionation of CO2 diffusion through a liquid (O'Leary, 1984).
CA Activity Measurements
CA activity was measured on leaf extracts using mass spectrometry to measure the rates of 18O2 exchange from doubly labeled 13C18O2 to H216O (Badger and Price, 1989; von Caemmerer et al., 2004). Measurements of leaf extracts were made at 25°C with a subsaturating total carbon concentration of 1 mm. The hydration rates were calculated from the enhancement in the rate of 18O loss over the uncatalyzed rate. We then applied this factor to the nonenzymatic first-order rate constant calculated at pH 7.4 appropriate for the mesophyll cytosol (Furbank et al., 1989) and report the CA activity as a first-order rate constant kCA (mol m−2 s−1 Pa−1). kCApm then gives the in vivo CA activity at that particular cytosolic pCO2. Leaf samples were collected after the gas-exchange measurements on the same leaf material and subsequently frozen in liquid nitrogen and stored at −80°C.
Dry Matter δ13C
The opposite leaf to the one used during gas exchange was collected and oven dried at 70°C, and ground with a mortar and pestle. A subsample of ground tissue was weighed and the isotopic composition determined by combustion in a Carlo Erba elemental analyzer; the CO2 was analyzed by mass spectrometry. δ was calculated as [(Rsample − Rstandard)/Rstandard]1,000, where Rsample and Rstandard are the 13C/12C of the sample and the standard VPDB, respectively. Dry matter δ13C was determined on glasshouse-grown plants only because there were large fluctuations in the carbon isotopic composition of the air in the growth cabinets.
Photosynthetic Model
The C4 photosynthetic model developed by Berry and Farquhar (1978) and von Caemmerer (2000) was used to predict the response of net CO2 assimilation, bundle sheath pCO2, pi/pa, photorespiration, and φ to changes in the amount of PEPC activity (Vp). Manipulating Vp within the photosynthesis model was used to simulate the effect of changes in CO2 hydration rates (Vh). The outputs from the C4 photosynthetic model, specifically the rates of Rubisco carboxylation (Vc), Vo, Vp, φ, and the pCO2 in the BSC, were incorporated into the model of C4 carbon isotope discrimination (Δ13C) developed by Farquhar (1983). The Δ13C model was used to determine which photosynthetic parameters would influence Δ13C consistent with our experimental data.
Acknowledgments
We thank Sue Wood for the carbon isotope analysis of dry matter samples and Howard Griffiths for his helpful comments on earlier versions of this manuscript.
This work was supported by a National Science Foundation international postdoctoral fellowship (to A.B.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Susanne von Caemmerer (susanne.caemmerer@anu.edu.au).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.077776.
References
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol 165: 351–371 [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD (1989) Carbonic-anhydrase activity associated with the Cyanobacterium Synechococcus Pcc7942. Plant Physiol 89: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JA, Farquhar GD (1978) The CO2-concentrating function of C4 photosynthesis. A biochemical model. In DO Hall, J Coombs, TW Goodwin, eds, Proceedings of the Fourth International Congress on Photosynthesis. Biochemical Society, London, pp 119–131
- Brown RH, Byrd GT (1993) Estimation of bundle-sheath cell conductance in C4 species and O2 insensitivity of photosynthesis. Plant Physiol 103: 1183–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell P, Bielig L, Grof C (1991) Increased carbonic anhydrase activity in leaves of sodium-deficient C4 plants. Aust J Plant Physiol 18: 589–592 [Google Scholar]
- Buchmann N, Brooks JR, Rapp KD, Ehleringer JR (1996) Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ 19: 392–402 [Google Scholar]
- Burnell JN, Suzuki I, Sugiyama T (1990) Light induction and the effect of nitrogen status upon the activity of carbonic anhydrase maize leaves. Plant Physiol 94: 384–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervigni T, Teofani F, Bassanelli C (1971) Effect of CO2 on carbonic anhydrase in Avena sativa and Zea mays. Phytochemistry 10: 2991–2994 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas-exchange to investigate CO2 diffusion in leaves of higher-plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Farquhar GD (1983) On the nature of carbon isotope discrimination in C4 species. Aust J Plant Physiol 10: 205–226 [Google Scholar]
- Flanagan LB, Ehleringer JR (1998) Ecosystem-atmosphere CO2 exchange: interpreting signals of change using stable isotope ratios. Trends Ecol Evol 13: 10–14 [DOI] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, von Caemmerer S, Jenkins CLD (1996) Antisense RNA inhibition of RbcS gene expression reduces Rubisco level and photosynthesis in the C4 plant Flaveria bidentis. Plant Physiol 111: 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Jenkins CLD, Hatch MD (1989) CO2 concentrating mechanism of C4 photosynthesis—permeability of isolated bundle sheath-cells to inorganic carbon. Plant Physiol 91: 1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannoum O, von Caemmerer S, Ziska LH, Conroy JP (2000) The growth response of C4 plants to rising atmospheric CO2 partial pressure: a reassessment. Plant Cell Environ 23: 931–942 [Google Scholar]
- Ghashghaie J, Badeck F-W, Lanigan G, Nogues S, Tcherkez G, Deleens E, Cornic G, Griffiths H (2003) Carbon isotope fractionation during dark respiration and photorespiration in C3 plants. Phytochemistry Reviews 2: 145–161 [Google Scholar]
- Gillon J, Yakir D (2001) Influence of carbonic anhydrase activity in terrestrial vegetation on the O-18 content of atmospheric CO2. Science 291: 2584–2587 [DOI] [PubMed] [Google Scholar]
- Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20: 1217–1230 [Google Scholar]
- Gillon JS, Yakir D (2000) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against (COO)-O-18 during photosynthesis. Plant Cell Environ 23: 903–915 [Google Scholar]
- Hatch MD (1987) C-4 photosynthesis—a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895: 81–106 [Google Scholar]
- Hatch MD, Burnell JN (1990) Carbonic anhydrase activity in leaves and its role in the first step of C4 photosynthesis. Plant Physiol 93: 825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DX, Edwards GE (1996) Estimation of diffusive resistance of bundle sheath cells to CO2 from modeling of C4 photosynthesis. Photosynth Res 49: 195–208 [DOI] [PubMed] [Google Scholar]
- Henderson SA, von Caemmerer S, Farquhar GD (1992) Short-term measurements of carbon isotope discrimination in several C4 species. Aust J Plant Physiol 19: 263–285 [Google Scholar]
- Jenkins CLD, Furbank RT, Hatch MD (1989) Inorganic carbon diffusion between C4 mesophyll and bundle sheath-cells—direct bundle sheath CO2 assimilation in intact leaves in the presence of an inhibitor of the C4 pathway. Plant Physiol 91: 1356–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Edwards GE (1999) The biochemistry of C4 photosynthesis. In R Sage, R Monson, eds, C4 Plant Biology. Academic Press, San Diego, pp 49–87
- Kiirats O, Lea PJ, Franceschi VR, Edwards GE (2002) Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in a C4 cycle mutant and wild-type Amaranthus edulis. Plant Physiol 130: 964–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140: 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, von Caemmerer S, Price GD, Badger MR, Furbank RT (1998) Expression of tobacco carbonic anhydrase in the C4 dicot Flaveria bidentis leads to increased leakiness of the bundle sheath and a defective CO2-concentrating mechanism. Plant Physiol 117: 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier J, O'Leary M (1984) Carbon kinetic isotope effects on the hydration of carbon dioxide and dehydration of bicarbonate ion. J Am Chem Soc 106: 5054–5057 [Google Scholar]
- McLeod AR, Long SP (1999) Free-air carbon dioxide enrichment (FACE) in global change research: a review. Adv Ecol Res 28: 1–56 [Google Scholar]
- Meinzer FC, Plaut Z, Saliendra NZ (1994) Carbon isotope discrimination, gas-exchange, and growth of sugarcane cultivars under salinity. Plant Physiol 104: 521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Saliendra NZ (1997) Spatial patterns of carbon isotope discrimination and allocation of photosynthetic activity in sugarcane leaves. Aust J Plant Physiol 24: 769–775 [Google Scholar]
- Meinzer FC, Zhu J (1998) Nitrogen stress reduces the efficiency of the C4 CO2 concentrating system, and therefore quantum yield, in Saccharum (sugarcane) species. J Exp Bot 49: 1227–1234 [Google Scholar]
- Mook W, Bommerson J, Staverman W (1974) Carbon isotope fractionation between dissolved bicarbonate and gaseous carbon dioxide. Earth Planet Sci Lett 22: 169–176 [Google Scholar]
- O'Leary M (1981) Carbon isotope fractionation in plants. Phytochemistry 20: 553–567 [Google Scholar]
- O'Leary M (1984) Measurement of the isotope fractionation associated with the diffusion of carbon dioxide in aqueous solution. J Phys Chem 88: 823–825 [Google Scholar]
- Paneth P, O'Leary M (1985) Carbon isotope effect on dehydration of bicarbonate ion catalyzed by carbonic anhydrase. Biochemistry 24: 5143–5147 [DOI] [PubMed] [Google Scholar]
- Peisker M (1982) The effect of CO2 leakage from bundle sheath-cells on carbon isotope discrimination in C4 plants. Photosynthetica 16: 533–541 [Google Scholar]
- Peisker M, Henderson SA (1992) Carbon—terrestrial C4 plants. Plant Cell Environ 15: 987–1004 [Google Scholar]
- Ranjith SA, Meinzer FC, Perry MH, Thom M (1995) Partitioning of carboxylase activity in nitrogen-stressed sugarcane and its relationship to bundle sheath leakiness to CO2, photosynthesis and carbon isotope discrimination. Aust J Plant Physiol 22: 903–911 [Google Scholar]
- Saliendra NZ, Meinzer FC, Perry M, Thom M (1996) Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. J Exp Bot 47: 907–914 [Google Scholar]
- Tazoe Y, Noguchi K, Terashima I (2005) Effects of growth light and nitrogen nutrition on the organization of the photosynthetic apparatus in leaves of a C4 plant, Amaranthus cruentus. Plant Cell Environ doi/10.1111/j.1365-3040.2005.01453.x [DOI] [PubMed]
- von Caemmerer S (2000) Biochemical Models of Leaf Photosynthesis. CSIRO Publishing, Collingwood, Australia
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Ludwig M, Millgate A, Farquhar GD, Price D, Badger M, Furbank RT (1997. a) Carbon isotope discrimination during C4 photosynthesis: insights from transgenic plants. Aust J Plant Physiol 24: 487–494 [Google Scholar]
- von Caemmerer S, Millgate A, Farquhar GD, Furbank RT (1997. b) Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase by antisense RNA in the C4 plant Flaveria bidentis leads to reduced assimilation rates and increased carbon isotope discrimination. Plant Physiol 113: 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Quinn V, Hancock NC, Price GD, Furbank RT, Ludwig M (2004) Carbonic anhydrase and C4 photosynthesis: a transgenic analysis. Plant Cell Environ 27: 697–703 [Google Scholar]
- Wall GW, Brooks TJ, Adam R, Cousins AB, Kimball BA, Pinter PJ, LaMorte RL, Triggs L, Ottman MJ, Leavitt SW, et al (2001) Elevated atmospheric CO2 improved Sorghum plant water status by ameliorating the adverse effects of drought. New Phytol 152: 231–248 [Google Scholar]
- Watling J, Press M, Quick W (2000) Elevated CO2 induces biochemical and ultrastructural changes in leaves of the C4 cereal sorghum. Plant Physiol 123: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DG, Gempko V, Fravolini A, Leavitt SW, Wall GW, Kimball PA, Pinter PJ, LaMorte R (2001) Carbon isotope discrimination by Sorghum bicolor under CO2 enrichment and drought. New Phytol 150: 285–293 [Google Scholar]
- Yakir D, da SL Sternberg L (2000) The use of stable isotopes to study ecosystem gas exchange. Oecologia 123: 297–311 [DOI] [PubMed] [Google Scholar]