Abstract
To gain some insight into the mechanism of plant programmed cell death, certain features of cytochrome c (cyt c) release were investigated in heat-shocked tobacco (Nicotiana tabacum) Bright-Yellow 2 cells in the 2- to 6-h time range. We found that 2 h after heat shock, cyt c is released from intact mitochondria into the cytoplasm as a functionally active protein. Such a release did not occur in the presence of superoxide anion dismutase and catalase, thus showing that it depends on reactive oxygen species (ROS). Interestingly, ROS production due to xanthine plus xanthine oxidase results in cyt c release in sister control cultures. Maximal cyt c release was found 2 h after heat shock; later, activation of caspase-3-like protease was found to increase with time. Activation of this protease did not occur in the presence of ROS scavenger enzymes. The released cyt c was found to be progressively degraded in a manner prevented by either the broad-range caspase inhibitor (zVAD-fmk) or the specific inhibitor of caspase-3 (AC-DEVD-CHO), which have no effect on cyt c release. In the presence of these inhibitors, a significant increase in survival of the cells undergoing programmed cell death was found. We conclude that ROS can trigger release of cyt c, but do not cause cell death, which requires caspase-like activation.
Plant cells can respond to various stimuli, including pathogen attacks, fungal toxins, biotic and abiotic stresses, and chemical agents, by initiating programmed cell death (PCD; McCabe and Leaver, 2000; Rizhsky et al., 2004; van Doorn and Woltering, 2005). However, knowledge of how PCD occurs remains rather obscure, thus necessitating the use of a plant model in which the role of cell components can be investigated in some detail. In this regard, we have shown that, as a result of heat shock (HS) at 55°C, in vitro cultured tobacco (Nicotiana tabacum) Bright-Yellow 2 (TBY-2) cells undergo a process leading to cell death. This process showed several morphological features associated with the better understood phenomenon of PCD in animal systems, namely, cytoplasmic shrinkage, chromatin condensation, and DNA laddering. Hence, although the evidence is not yet complete, it seems very likely that what we were observing in our plant system corresponds to PCD in animals. Moreover, cell death occurring in other plant model systems with the morphological features described above has long been recognized as programmed in a spatial and temporal sense (Balk et al., 1999; McCabe and Leaver, 2000; Tiwari et al., 2002; Rizhsky et al., 2004). With the reservation that final demonstration of the equivalence remains to be firmly established, we use the term PCD to describe the process studied in this work.
In heat-shocked tobacco cells, reactive oxygen species (ROS) are produced with early mitochondrial damage (Vacca et al., 2004). On the other hand, among a variety of events occurring during PCD, the release of cytochrome c (cyt c) has been shown both in mammalian and plant mitochondria. In the former case, it was shown that the released cyt c (1) can work as a ROS scavenger and as an electron donor to cytochrome oxidase, thus driving ATP synthesis, which is necessary for apoptosis to occur (Atlante et al., 2003a, 2003b) and (2) binds to apoptotic protease-activating factor (Apaf-1) protein and, in the presence of ATP/dATP, activates caspase-9, thus triggering activation of a caspase cascade that leads to the morphological changes typical of apoptosis (Li et al., 1997; Zou et al., 1999). In plant cells, cyt c release was already shown in a variety of cases, including menadione-induced death in tobacco protoplasts (Sun et al., 1999), d-Mannose-induced cell death in maize (Zea mays) cells (Stein and Hansen, 1999) during development-associated PCD (Balk and Leaver, 2001), and in response to stress (Balk et al., 1999; Tiwari et al., 2002; Krause and Durner, 2004). However, the possibility that release of cyt c is an obligatory step in plant PCD has been questioned (Xu and Hanson, 2000; Yu et al., 2002; Yao et al., 2004).
This prompted us to investigate whether and how cyt c is released in HS-induced PCD in TBY-2 cells with particular attention to cyt c time-dependent cytosolic and mitochondrial content and to the role of caspase-like protease in causing cyt c degradation and PCD.
We found that cyt c release is dependent on the production of ROS, which cannot trigger PCD. Moreover, after cyt c release, caspase-like activation occurs, leading to cyt c degradation en route to PCD.
RESULTS
Cyt c Release Occurs in HS-Induced PCD of TBY-2 Cells in a ROS-Dependent Manner
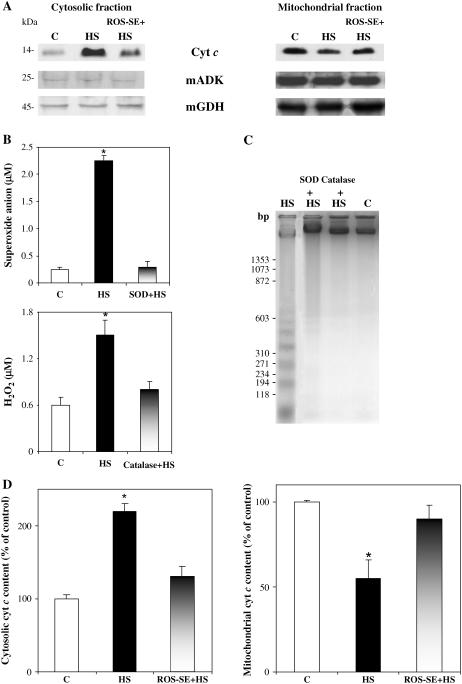
Cyt c release from mitochondria was investigated by immunoblot analysis using a monoclonal antibody against cyt c. Both cytosolic and mitochondrial fractions, obtained from TBY-2 cells subjected to HS (cells in these conditions will be referred to as HS cells), were examined. Typical immunoblots are shown in Figure 1A. Two hours after HS (2-h HS cells), when, due to technical reasons (see “Materials and Methods”), the first analysis could be carried out, an increase of cyt c over control cells was detected in the cytosolic fraction of HS cells. The amount of cyt c in the corresponding mitochondrial fractions, evaluated in the same cellular preparation, decreased with respect to control mitochondria, indicating a release of cyt c from the mitochondria to the cytosol. To verify that release of cyt c takes place in HS cells from intact mitochondria and is not due to mitochondrial damage occurring during the heat treatment, the presence of mitochondrial adenylate kinase isoenzyme 2 (mADK), localized in the mitochondrial intermembrane space, and mitochondrial glutamate dehydrogenase (mGDH), a mitochondrial matrix enzyme, was also checked in the same experiment by immunoblot analysis using the respective antibodies. The amounts of mADK and mGDH found in the cytosolic fraction of the control and HS cells did not differ one from another and were negligible as compared with that of the mitochondrial fraction (Fig. 1A). Hence, we can deduce that the low level of cyt c, as well as of mADK and mGDH, in the cytosolic fraction of control cells is a result of manipulation during the cell fractionation procedure. As a further control, the intactness of the mitochondria was also checked both in control and in 2-h HS cells by measuring the activity of both mADK (Bobba et al., 1999) and fumarase (Balk et al., 1999; Bafunno et al., 2004), a mitochondrial matrix enzyme (Table I). Negligible mADK activity was found in the cytosolic fraction of either control or HS cells and the assay of fumarase activity showed a mitochondrial intactness ranging from 94% to 96%.
Figure 1.
Cyt c release from mitochondria to cytosol in HS TBY-2 cells. The following TBY-2 cell cultures were used: control cells kept at 27°C (C), HS cells (see “Materials and Methods”), cells heat shocked in the presence of either 75 units mL−1 SOD (SOD + HS) or 100 units mL−1 catalase (catalase + HS) or both 75 units mL−1 SOD and 100 units mL−1 catalase, namely, ROS scavengers enzymes (ROS-SE + HS). A, Release of cyt c analyzed by western-blot analysis. Cytosolic and mitochondrial fractions were obtained by a cell fractionation procedure and immunoblot analysis was performed using anti-cyt c antibody (see “Materials and Methods”). The presence of either mADK or mGDH was also checked in the same samples by using the respective antibodies. B, Superoxide anion and H2O2 production. Thirty minutes after HS, 1 mL of cell suspension (0.12 g cells) was taken and superoxide anion concentration and H2O2 were measured in the medium as described in “Materials and Methods.” C, DNA analysis. After HS, cells were kept at 27°C for 72 h. Cells were collected, frozen in liquid nitrogen, and genomic DNA was extracted as described in “Materials and Methods.” Five-hundred nanograms of genomic DNA were separated on a 1.8% (w/v) agarose gel and detected by staining the gel with ethidium bromide. The Mr marker is ΦX174 DNA cleaved with Hae III. D, Statistical analysis of the cytosolic and mitochondrial cyt c content. Cyt c content was expressed, after normalization with mGDH, as the percentage of control cells to which a value of 100 was given. Values represent the mean (±sem) of six independent measurements. The asterisks (*) indicate values that are significantly different from the control (P < 0.01).
Table I.
Detection of mADK activity in the cytosolic fraction and fumarase activity in the mitochondrial fraction of TBY-2 cells
Cytosolic and mitochondrial fractions were obtained by cell fractionation procedure from control (C) and HS cells. mADK activity was measured in the cytosolic fractions spectrophotometrically at 340 nm in the direction of ATP production and the enzymatic activity was expressed as nanomoles NADP+ reduced/min × mg protein. Fumarase activity was measured spectrophotometrically at 240 nm after l-malate addition (50 mm) to isolated mitochondria either in the absence or presence of the detergent TX-100. The enzymatic activity was expressed in nanomoles fumarate formed/min × mg protein (see “Materials and Methods”). Values represent the mean (±sem) of six independent measurements.
| Samples | mADK Activity Cytosolic Fraction | Fumarase Activity
|
|
|---|---|---|---|
| Mitochondrial Fraction | Mitochondrial Fraction + TX-100 | ||
| C | 0.05 ± 0.03 | 12 ± 0.1 | 195 ± 0.1 |
| HS | 0.03 ± 0.02 | 9 ± 0.6 | 190 ± 0.2 |
To ascertain whether such a cyt c release could depend on the production of ROS caused by HS, we investigated the role of ROS production on cyt c release by using ROS scavenging enzymes (ROS-SE), including superoxide dismutase (SOD) and catalase added to the culture medium 30 min before HS (Fig. 1A). No significant release of cyt c from mitochondria to cytosol was found when cell death was induced in the presence of ROS-SE. In a parallel experiment (Fig. 1B), we confirmed that the superoxide anion (O2−.) and hydrogen peroxide (H2O2) are produced in HS cells. O2−. and H2O2 production, as measured 30 min after HS, were, respectively, 10- and 3-fold higher in HS cells than controls. As expected, the presence of either SOD or catalase resulted in an almost complete removal of the ROS produced. That ROS-SE can somehow protect heat-shocked cells against PCD is shown in Figure 1C. DNA laddering (i.e. the DNA cleavage into oligonucleosomal units, typically observed during the apoptosis process and occurring in our system at 72 h after HS), was significantly reduced if cells were subjected to HS in the presence of either SOD or catalase. Prevention of cell death was also consistently found in the presence of ROS-SE. Cell viability measured in 15-h HS cells was 60% ± 2% in the absence of ROS-SE; 90% ± 5% and 80% ± 5% cell survival was found for cells preincubated with SOD or catalase, respectively.
The ability of ROS-SE to prevent cyt c release was investigated statistically as shown in Figure 1D, where a densitometric analysis of the cytosolic and mitochondrial cyt c content is reported as derived from six independent experiments. To account for possible cyt c release due to mitochondrial damage occurring during the cell fractionation procedure, the mGDH amount in each lane was used to normalize the corresponding amount of cyt c revealed on the same filter. Cyt c content in HS cells was found to increase in the cytosolic fraction to 120% ± 10% over control cells; a similar increase over the control was found during cyt c release in development-associated PCD (Yu et al., 2002). A decrease to a value of 55% ± 11% in the mitochondrial fraction with respect to control was consistently detected. However, when PCD was induced in the presence of ROS-SE, the cyt c content in both the cytosolic and mitochondrial fractions was not statistically different from the control value.
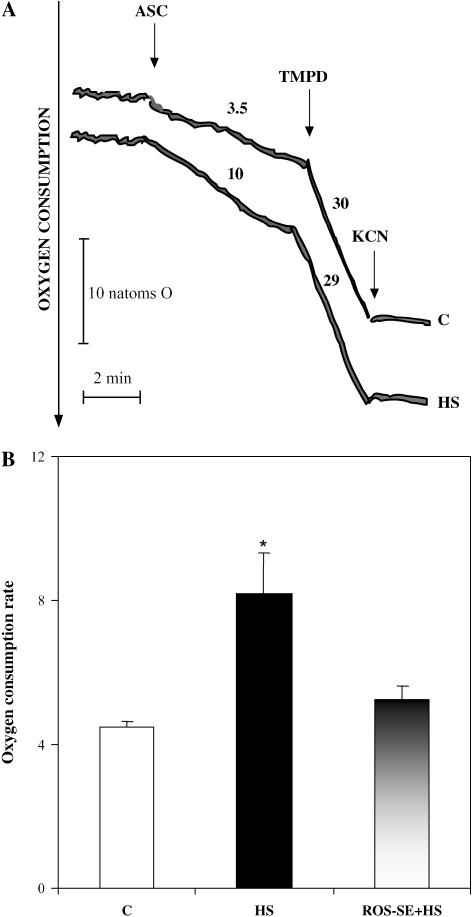
To determine whether the cyt c released into the cytosol was functionally active, we resorted to an experimental approach already reported (Atlante et al., 2003a) in which the ability of cell homogenate of either control or 2-h HS cells to oxidize ascorbate (ASC) was checked. Briefly, because ASC cannot permeate per se the outer mitochondrial membrane (Alexandre and Lehninger, 1984), its oxidation can occur only as a result of the release from mitochondria of a component that can oxidize ASC and then reduce oxygen via cyt c oxidase (i.e. active cyt c; Atlante et al., 2000). Thus, in a typical experiment, the rate of ASC-dependent oxygen consumption was measured polarographically in TBY-2 cell homogenate (Fig. 2). The rate of oxygen consumption, due to 5 mm externally added ASC, was found to be 3.5 and 10 nat oxygen/min × mg cell protein in homogenates from control and 2-h HS cells, respectively. In both cases, externally added N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD; 0.2 mm) was found to strongly stimulate the rate of oxygen uptake that was essentially blocked as a result of the addition of cyanide (KCN; 1 mm), which is a powerful inhibitor of mitochondrial cytochrome oxidase (Fig. 2A). The increase in the rate of oxygen consumption due to added ASC, which mirrors cyt c release, was found to be statistically significant in control and 2-h HS cells. On the other hand, no difference was found when comparing the rates of oxygen uptake due to TMPD addition, which showed a similar capacity of the two samples to support electron flow. In agreement with Figure 1D, a reduction of 46% ± 3% in the rate of oxygen uptake occurred when cell death was induced in the presence of the ROS-SE (Fig. 2B).
Figure 2.
Functional activity of cyt c released from mitochondria of HS TBY-2 cells. The following TBY-2 cell cultures were used: control cells kept at 27°C (C), HS cells, cells heat shocked in the presence of both 75 units mL−1 SOD and 100 units mL−1 catalase (ROS-SE + HS). A, Homogenates (0.2 mg protein) were incubated at 25°C in 1.5 mL of respiratory medium (see “Materials and Methods”) in the presence of rotenone (2 μg), antimycin (2 μg), and myxothiazole (3 μm) in a water-jacketed glass vessel to monitor oxygen consumption polarographically. At the arrows, ASC (5 mm), TMPD (0.2 mm), and KCN (1 mm) were added. Numbers along the traces are rates of oxygen uptake expressed as nat oxygen/min × mg protein. B, Statistical analysis of ASC oxygen consumption rate measured as nat oxygen/min × mg cell protein. Results are means (±sem) of three independent experiments. The asterisk (*) indicates values that are significantly different from the control (P < 0.01).
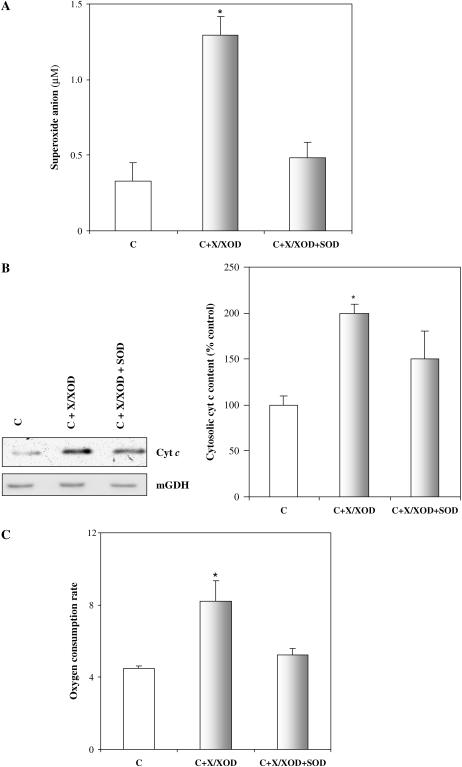
To gain further insight into the ability of ROS to cause cyt c release, another set of experiments incubated normal control TBY-2 cells in the presence of xanthine plus xanthine oxidase (X/XOD), an experimental system already used to produce exogenous ROS in different cell systems (Naveilhan et al., 1994; Satoh et al., 1998; Atlante et al., 2000). The exogenous generated O2−. may dismutate spontaneously to H2O2, which may diffuse inside the cell or directly diffuse across cell membranes, as reported by Mao and Poznansky (1992). The X/XOD system resulted in a 4-fold increase in O2−. production in TBY-2 cells (Fig. 3A). Interestingly, ROS generation with the X/XOD system causes cyt c release in TBY-2 cells as detected by both immunoblot analysis (2-fold increase compared to nontreated cells; Fig. 3B) and polarographic measurement (1.7-fold increase compared to nontreated cells) in cell homogenate (Fig. 3C). Externally added SOD largely prevented both ROS production and cyt c release. In agreement with Delledonne et al. (2001) and de Pinto et al. (2002), where it was shown that exogenous ROS failed to induced cell death both in soybean (Glycine max) and TBY-2 cells in the absence of the simultaneous production of other effectors such as nitric oxide, we found that ROS production by the X/XOD system did not cause death in TBY-2 cells: cell viability was found to be almost unaffected (98% ± 2%) as monitored up to a 72-h period. No DNA laddering was observed at that time (data not shown).
Figure 3.
Cyt c release from mitochondria of control cells consequent on ROS production by an externally added X/XOD system. TBY-2 cells were incubated at 27°C in the absence (C) or presence of 100 μm xanthine plus 1 unit mL−1 xanthine oxidase (C + X/XOD) for 30 min; 75 units mL−1 SOD were present where indicated. A, Superoxide anion production. Thirty minutes after HS, 1 mL of cell suspension (0.12-g cells) was taken and O2−. concentration was measured in the medium. B, Release of cyt c analyzed by western-blot analysis. Cytosolic and mitochondrial fractions were obtained by a cell fractionation procedure and immunoblot analysis was performed using anti-cyt c antibody (see “Materials and Methods”). The presence of mGDH was also checked in the same samples by using the respective antibody. Results are means (±sem) of three independent experiments. C, Oxygen consumption caused by externally added ASC. The measurements were carried out in cell homogenate as reported in Figure 2. The rate of oxygen consumption is expressed as nat oxygen/min × mg cell protein. Results are means (±sem) of three independent experiments. The asterisk (*) indicates values that are significantly different from the control (P < 0.01).
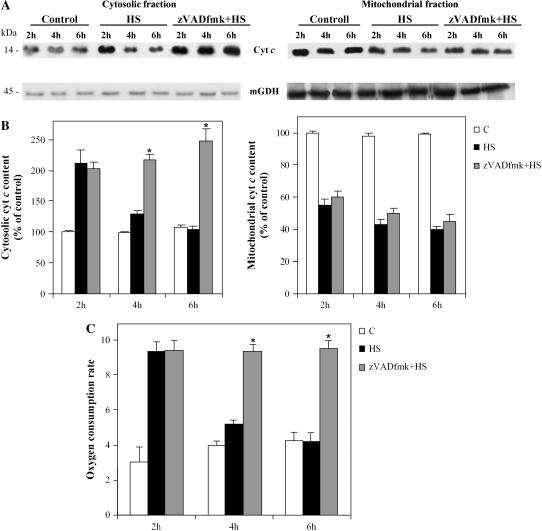
The Cyt c Released in HS-Induced Cell Death in TBY-2 Cells Is Degraded by Caspase-Like Proteases
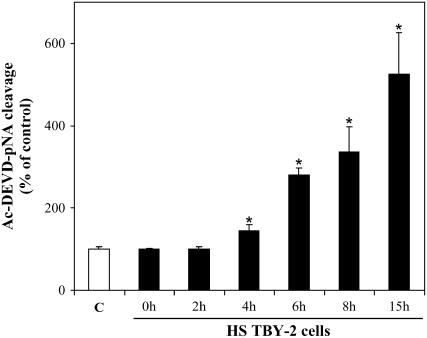
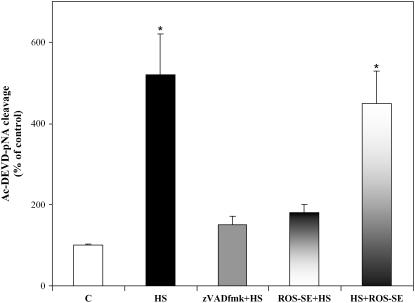
Having ascertained that cyt c was already released in 2-h HS cells, the cyt c content in the cytosolic and mitochondrial fractions was investigated by immunoblot analysis as a function of time after HS. Two hours after HS, the cytosolic cyt c content was approximately twice that in the controls, but it subsequently decreased and, at 6 h, was similar to the controls (Fig. 4, A and B). Conversely, the cyt c content measured in the corresponding mitochondrial fractions was at a minimum after 4 h (43% ± 3% compared to the control in three experiments) and remained constantly low (40% ± 2%) even 6 h after HS (Fig. 4, A and B). The difference in cytoplasmic cyt c content between 2- and 4-h HS cells, and the constancy of the mitochondrial content, are consistent with cytosolic degradation of the released cyt c. To find out whether this derives from activation of caspase-like proteases, as in animal cells (Bobba et al., 1999), we added zVAD-fmk, a broad-range caspase inhibitor (Armstrong et al., 1996), to TBY-2 cells before heat treatment and monitored the cyt c release both by immunoblot analysis (Fig. 4, A and B) and by polarographic measurements in cell homogenate (Fig. 4C). No change in the early cyt c release measured after 2 h was found, which shows that cyt c release is not caspase dependent, as was also found in tobacco protoplasts undergoing menadione-induced apoptosis (Sun et al., 1999). Moreover, in the presence of zVAD-fmk, no decrease of cyt c content was found over a 2- to 6-h period in the cytosolic fraction of HS cells, whereas in the corresponding mitochondrial fraction the cyt c content decreased in 4- and 6-h HS cells as found in untreated HS cells. To substantiate the conclusion that degradation of the released cyt c in the cytosol could arise from activation of caspase-like proteases, we investigate the presence of caspase-3-like protease activity in HS cells up to 15 h (Fig. 5). The caspase-3-like activity was monitored by measuring the hydrolysis of Ac-DEVD-pNA, the caspase-3-like protease-specific substrate (Talanian et al., 1997). Caspase-3-like protease activity was essentially identical to that of the control sister cells in 2-h HS cells, but showed a progressive increase with time after heat treatment, and was 1.45- and 5.25-fold higher than that in control cells in 4- and 15-h HS cells, respectively (Fig. 5). In a parallel experiment, given that caspase-3 is considered a key protease in different cell systems and one of the first effector caspases to be activated in animal cells (Gerhardt et al., 2001), zVAD-fmk and the specific caspase-3 inhibitor (Ac-DEVD-CHO) were compared with respect to their ability to affect cyt c release and cytosolic content during HS-induced PCD (Table II). The inhibitors studied did not differ significantly with respect to their ability to prevent the decrease of cyt c in the cytosolic fraction. In another experiment, we confirmed that zVAD-fmk completely inhibited caspase-3-like activity. A control was also made to show that the presence of ROS-SE in the cell culture resulted in the prevention of caspase-like activation, whereas ROS-SE themselves added in the assay did not affect the caspase activity (Fig. 6).
Figure 4.
Effect of zVAD-fmk on cyt c release from mitochondria of HS TBY-2 cells. The following TBY-2 cell cultures were used: control cells kept at 27°C (C) and HS cells. Where indicated, cells were heat shocked in the presence of 100 μm zVAD-fmk; in the other cultures, the same volume of dimethyl sulfoxide was added. A, Release of cyt c analyzed by western-blot analysis. Cytosolic and mitochondrial fractions were obtained 2, 4, and 6 h after HS and immunoblot analysis was performed by using anti-cyt c and mGDH antibodies. B, Statistical analysis of the cytosolic and the mitochondrial cyt c content. Cyt c content was expressed after normalization with mGDH as a percentage of control cells set at 100. Values represent the mean (±sem) of three independent measurements. C, Oxygen consumption caused by externally added ASC. Homogenates were obtained 2, 4, and 6 h after HS and polarographic measurements were performed as described in Figure 2. Values represent the mean (±sem) of three independent measurements. Asterisks (*) indicate values for zVAD-fmk-treated apoptotic cells that are significantly different from the untreated apoptotic cells (P < 0.01).
Figure 5.
Time-dependent activation of caspase-3-like proteases during HS-induced PCD in TBY-2 cells. Cell cultures were heat shocked and returned to 27°C for a 15-h period. At the indicated time intervals after HS, cells were collected, frozen in liquid nitrogen, and assay for caspase-3-like activity was carried out in 10 μg of cell lysate by following the hydrolysis of the specific caspase-3 substrate Ac-DEVD-pNA. Specific activity is expressed as the percentage of the activity of control cells (C), which was given a value of 100. The variability of caspase-3-like activity in the control cells during the 0- to 15-h period was always less than 5%. Data represent the mean (±sem) of three independent measurements. Asterisks (*) indicate values that are significantly different from the control (P < 0.01).
Table II.
Effect of the specific caspase-3 inhibitor (Ac-DEVD-CHO) and zVAD-fmk on cyt c release during HS-induced PCD
Cell cultures were treated at 55°C and returned to 27°C for a 6-h period. Where indicated, either Ac-DEVD-CHO or zVAD-fmk (100 μm) was added before HS; in the other cultures, the same volume of dimethyl sulfoxide was added. Cytosolic cyt c content was measured by immunoblot analysis of cytosolic fractions from either control (C) or HS TBY-2 cells obtained 2 and 6 h after HS. Cyt c content was expressed, after normalization with GDH, as a percentage of control cells set at 100. Cyt c activity was measured in cell homogenate as oxygen consumption caused by externally added ASC. The rate of the oxygen consumption is expressed as nat oxygen/min × mg cell protein. Values represent the mean (±sem) of three independent measurements.
| Sample | Cytosolic Cyt c Content | Oxygen Consumption Rate |
|---|---|---|
| % of Control | nat Oxygen/min × mg | |
| C2 h | 100 ± 3 | 4.5 ± 1 |
| C6 h | 108 ± 4 | 4.8 ± 0.5 |
| HS2 h | 215 ± 22 | 10 ± 1.5 |
| HS6 h | 105 ± 5 | 5.8 ± 1 |
| Ac-DEVD-CHO + HS2 h | 205 ± 8 | 10 ± 1 |
| Ac-DEVD-CHO + HS6 h | 240a ± 10 | 9.7a ± 0.5 |
| zVAD-kmk + HS2 h | 203 ± 10 | 10.5 ± 1.5 |
| zVAD-kmk + HS6 h | 247a ± 20 | 10a ± 1 |
Values of Ac-DEVD-CHO and zVAD-fmk-treated HS cells that are significantly different from untreated HS cells (P < 0.01).
Figure 6.
Effect of ROS-SE on caspase-3-like activity in HS TBY-2 cells. The following TBY-2 cell cultures were used: control cells kept at 27°C (C), HS cells, cells heat shocked in the presence of zVAD-fmk (100 μm; zVAD-fmk + HS), and cells heat shocked in the presence of both 75 units mL−1 SOD and 100 units mL−1 catalase (ROS-SE + HS). Cells were collected 15 h after HS, frozen in liquid nitrogen, and assay for caspase-3-like activity was carried out in 10 μg of cell lysate following the hydrolysis of the specific caspase-3 substrate Ac-DEVD-pNA. Both 75 units mL−1 SOD and 100 units mL−1 catalase were also added to cell lysate of HS cells (HS + ROS-se). Specific activity is expressed as the percentage of the activity of control cells (C), which was given a value of 100. Asterisks (*) indicate values that are significantly different from the control (P < 0.01).
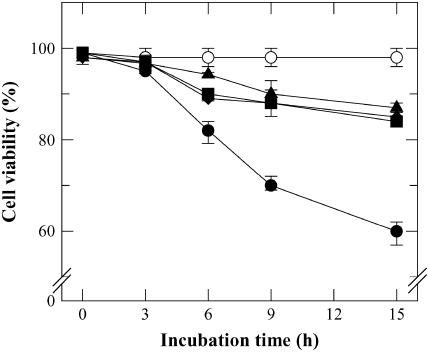
To ascertain whether proteases of the caspase family play a major role in determining HS-dependent cell death, we checked whether incubation of cells in the presence of the broad-range caspase inhibitor zVAD-fmk, the selective inhibitor of caspase-1 (Ac-YVAD-cmk), and that of caspase-3 (Ac-DEVD-CHO), added separately, could prevent cell death. All the checked inhibitors largely prevented HS PCD, cell survival being 87% ± 2.5%, 84% ± 2%, and 85% ± 2%, respectively, 15 h after HS for cells preincubated with either zVAD-fmk or inhibitors of caspase-1 and caspase-3, respectively (Fig. 7). No DNA laddering was observed in the presence of any of the checked caspase inhibitors (data not shown).
Figure 7.
Effect of caspase inhibitors on cell survival in HS TBY-2 cells. TBY-2 cell cultures were heat shocked in the absence (•) or in the presence of different caspase inhibitors (100 μm): zVAD-fmk (▴); Ac-YVAD-cmk (▪); and Ac-DEVD-CHO (♦) and returned to 27°C for a 15-h period. At the indicated time intervals after HS, viability of both control (C) and HS cells was measured as reported in “Materials and Methods.” In both cases, the percentage (±sem) of viable cells was counted in a population of at least 1,000 cells in six separate experiments.
DISCUSSION
We have previously shown that heat treatment of TBY-2 cells in a narrow window around 55°C leads to cell death by a process showing the morphological features of apoptosis (see introduction). At temperatures up to 45°C, cell viability was unaffected, whereas incubation at higher temperatures (60°C–65°C) resulted in rapid cell death not associated with chromatin condensation and DNA laddering (Vacca et al., 2004). Thus, in this work we have used cells heat shocked at 55°C as a model to characterize the apoptosis pathway further.
The release of cyt c from the mitochondrial intermembrane space to the cytosol of cells en route to PCD has been reported in many systems. Although the release of cyt c is considered as a crucial regulatory step in PCD (Kroemer et al., 1997; Bossy-Wetzel et al., 1998; Bobba et al., 2004), the mechanism by which cyt c release occurs is not unique, being dependent on the nature of the cells as well as on how the apoptotic process is induced. In plants, this subject is poorly investigated, and to our knowledge, no detailed investigation of the release mechanism has been to date reported, or on the role and fate of the released cyt c during cell death. In this article, we show that release of cyt c occurs in HS-induced cell death in tobacco cells in a manner in which ROS play a major role and that the released cyt c is degraded en route to cell death in a caspase-dependent manner. Finally, we show that the impairment of caspase activation/function results in strong prevention of cell death. Together, these features further confirm that HS at 55°C results in PCD.
Cyt c Release in HS Cells
At present, the mechanism for release of cyt c in plant PCD remains a matter of speculation. However, our findings rule out the possibility that cyt c release occurs due to damage of the outer membrane because cyt c release occurred under conditions in which mADK was not significantly released. One could argue that using the release of mADK as a control may not be sufficient if the proteins are disparate in size or differentially complexed with other proteins. However, because mADK is a soluble intermembrane enzyme in plants (Igamberdiev and Kleczkowski, 2003) and cyt c is loosely bound to the outer face of the inner mitochondrial membrane, we are encouraged to conclude that mitochondria retaining mADK are essentially intact. Consistently, we can rule out that the release of cyt c can occur in a manner dependent on the mitochondrial permeability transition pore (PTP), which itself allows for mADK release into the extramitochondrial phase. Thus, our system differs from others in which cyt c is released by swollen mitochondria through external membrane rupture (Vander Heiden et al., 1997), by PTP opening (Andrabi et al., 2004; Yao et al., 2004), with a loss of mitochondrial membrane potential (Yang and Cortopassi, 1998), or by formation of a specific channel involving Bcl-2 family protein in the outer mitochondrial membrane (Jürgensmeier et al., 1998). It has also been suggested that mitochondria-mediated ROS generation promoted cyt c release from mitochondria by a PTP-independent process consisting of cyt c dissociation from cardiolipin, followed by permeabilization of the outer membrane by interaction with the voltage-dependent ion-selective channel (Petrosillo et al., 2003).
The Role of ROS in Cyt c Release
In this article, we begin to investigate how ROS production and cyt c release are linked to one another. In HS-induced PCD, we have already reported that a ROS burst takes place in the early phase of the death program (Vacca et al., 2004). Here we found that cyt c release is strongly inhibited by adding ROS-SE, including SOD and catalase, before HS in TBY-2 cells, thus showing that cyt c release depends on the increase in ROS production, which occurs soon after induction of PCD (Fig. 1). In this case, we have to assume that ROS are freely diffusible across the cell membrane so that internal and external ROS are in equilibrium; scavenging of the external ROS will then reduce the internal concentration. In fact, the use of ROS-SE to study the role of intracellular ROS production has been used widely by different investigators (Jiang and Zhang, 2002; Luo et al., 2005; Wang et al., 2005). As a matter of fact, we found that, in the presence of SOD and catalase, the ROS produced independently of the presence of these enzymes were almost totally removed. Interestingly, we also found that exogenous ROS production via the X/XOD system, although not sufficient to induce cell death as found in other exogenous ROS production systems (Delledonne et al., 2001; de Pinto et al., 2002), can cause cyt c release from control cells (Fig. 3). As in Yu et al. (2002), cyt c release proved to be ineffective per se to trigger PCD. Because cyt c release is a consistent feature of PCD, we can speculate that this is not a cause, but a consequence, of the PCD response and both ROS production and cyt c release, although necessary, are not sufficient for PCD to occur. We found that 2 h after HS, the minimal time that can be investigated for technical reasons, when morphological changes due to PCD are still undetected, cyt c release had already occurred (Fig. 1). On the other hand, en route to PCD, other events caused by HS must occur, including activation of transcription and translation and impairment of mitochondrial oxidative metabolism (Vacca et al., 2004). Furthermore, activation of protein factors could also occur as in animal cells, which, binding to cyt c in its new cytoplasmic location, activate a caspase cascade that leads to PCD (Li et al., 1997; Zou et al., 1999).
Our observation that cyt c is released in plant PCD is not unique (see Balk et al., 1999; Stein and Hansen, 1999; Sun et al., 1999; Balk and Leaver, 2001; Tiwari et al., 2002; Krause and Durner, 2004), but because, in addition to immunoblot analysis, we monitor cyt c release by measuring cyt c activity by polarography, this article has the added dimension of showing that cyt c is released from intact mitochondria as a functional protein capable of transferring reducing equivalents from externally added ASC to oxygen (Fig. 2). At present, we do not know whether, as in cerebellar neurons, the cyt c released in plants can work both as a ROS scavenger and as a respiratory substrate for energy production by oxidative phosphorylation (Atlante et al., 2003a, 2003b; Bobba et al., 2004; La Piana et al., 2005) or whether cyt c can transfer electrons from the respiratory chain to the redox enzymes that generate mitochondrial ROS as signaling molecules for apoptosis to occur (Giorgio et al., 2005). Nonetheless, this point is under investigation.
Caspase-Like Proteases Have No Effect on the Release of Cyt c, But Can Degrade It
The role of the released cyt c, and in particular the time course of the changes in cyt c levels both in the cytosolic and mitochondrial fractions, deserves special discussion. At 2 h from the heat treatment, an increase of cyt c content in the cytosolic fraction and a decrease in the mitochondria occur. However, a clear difference is found after longer periods from the treatment when the amount of cyt c remains constant in the mitochondria, even if at a concentration 50% lower than in the control, but is strongly decreased in the cytosol. A corresponding decrease in the cyt c activity was also detected (Fig. 4). These findings demonstrate that cyt c in plant cells is released during the phase of PCD commitment and is degraded during the execution phase. So far, the degradation of released cyt c has been reported in two cell systems of animal origin (i.e. cerebellar granule cells; Bobba et al., 1999, 2004) and superior cervical ganglion neurons (Neame et al., 1998). In both cases, the degradation process was prevented by zVAD-fmk, thus suggesting a primary role for caspases in regulating the cytosolic concentration of cyt c.
In animal systems, specific proteases are activated in the execution phase of PCD, namely, caspases (for review, see Hengartner, 2000). The existence of caspases in plants is controversial (Lam and del Pozo, 2000; Woltering et al., 2002), however, caspase-3-like protease has recently been partially purified and characterized (Chichkova et al., 2004) and proved to be activated during heat-induced apoptosis in tobacco cells (Tian et al., 2000). We have consistently found a time-dependent activation of caspase-3-like protease in HS cells. Caspase-3-like activity was undetectable in 2-h HS, when cyt c is at its maximal level in the cytosolic fraction, whereas activation of caspase-3-like activity occurred 4 to 6 h after HS when the level of cyt c was strongly decreased in the cytosol (Fig. 5). Thus, a caspase-dependent proteolysis of cyt c can be assumed to occur. Such a conclusion is confirmed by the experiments using the broad-range caspase inhibitor zVAD-fmk and the specific caspase-3 inhibitor AC-DEVD-CHO, which gave clear experimental evidence that cyt c released in the cytosolic fraction of HS-TBY-2 cells is not mediated by caspase because neither zVAD-fmk nor Ac-DEVD-CHO can block cyt c release, but both of them can block its degradation (Fig. 4; Table II). It was shown that caspase-specific inhibitors can prevent different forms of plant PCD, suggesting that caspase-like proteases may also be involved in this response in plants (Elbaz et al., 2002; Mlejnek and Procházka, 2002). A further importance of the role of caspase-like protease in plant apoptosis is given by the finding that the broad-range caspase inhibitor zVAD-fmk and the specific caspase-1 and caspase-3 inhibitors largely block HS-induced cell death in TBY-2 cells (Fig. 7).
Furthermore, we show that ROS scavenger enzymes, which per se have no effect on caspase-3-like activity, but, removing ROS, inhibit cyt c release (Fig. 1) and block the caspase-3-like activity in HS cells (Fig. 6). This suggests that the relevance of cyt c release in the PCD mechanism is to take part in activation of the caspase-like cascade.
The picture emerging from our previous studies (Vacca et al., 2004) and from this work could be, then, as follows. In the phase ranging from 0 to 2 h after HS, an immediate production of ROS occurs that promotes cyt c release from intact mitochondria. The caspase-like proteases play no role in this phase, essentially being inactive; later, ranging between 4 to 15 h, the released cyt c contributes to their activation. The increase in caspase-like activity and the resulting caspase-like cascade results in degradation of cyt c and leads to cell death.
MATERIALS AND METHODS
Cell Culture, Growth Conditions, and HS Treatment
The suspension of tobacco (Nicotiana tabacum L. cv BY-2) BY-2 cells was routinely propagated and cultured at 27°C, according to Nagata et al. (1992). In this work, a stationary culture was diluted 4:100 (v/v) and cultured for 4 d. HS at 55°C was induced by transferring the cell suspension into a water bath at 55°C, with a heating time of either 3 or 10 min, depending on the cell suspension volume (10 or 100 mL, respectively). After HS, the cells were returned to 27°C. Cell viability was measured using trypan blue staining as described in de Pinto et al. (1999). At the indicated times, aliquots of cells were collected for analysis either by filtration on Whatman 3M paper or by centrifugation at 1,000g for 10 min at 25°C.
Preparation of Cell Homogenate, Mitochondria, and Cytosolic Fractions
Mitochondria and cytosolic fractions were isolated from lysed protoplasts essentially as described in de Pinto et al. (2000), with some modifications. Briefly, TBY-2 cells (0.5–10 g) were washed with the preplasmolysis buffer consisting of 0.65 m mannitol and 25 mm Tris-MES, pH 5.5. The cells were then incubated with 2% (w/v) caylase (Cayla) and 0.1% pectolyase (Sigma) in the same medium. The digestion of the cell wall proceeded for 1 h, in the dark, at 30°C. The protoplasts were sedimented at 200g for 5 min at 25°C and washed three times with preplasmolysis buffer adjusted to pH 6.5. Because the experimental procedure described takes 2 h from HS, analysis is not possible before this time. The protoplasts were then suspended in the ice-cold lysis buffer (5 mL/g of cells) consisting of 0.3 m Suc, 10 mm EDTA, 10 mm potassium phosphate (pH 7.4), 2 mm Cys, and 0.2% bovine serum albumin (BSA) and lysed on ice by homogenizing them in a Potter, the pestle of which was connected to a motor-driven grinder. Cell homogenate was obtained by centrifuging the suspension twice at 1,500g for 5 min at 4°C to remove cell debris.
The homogenate was centrifuged at 10,000g for 15 min at 4°C, the resulting pellet was rehomogenized in isolation buffer (0.3 mm Suc, 0.2% BSA, and 10 mm potassium phosphate, pH 7.5), and the supernatant was kept on ice. Mitochondria were precipitated at 17,000g for 15 min at 4°C and gently suspended in 0.1 to 1 mL of isolation buffer, depending on the starting cell weight, to obtain about 4 mg of mitochondrial protein/mL. After the first centrifugation at 10,000g, the supernatant was centrifuged again at 15,000g and the supernatant obtained was utilized as the cytosolic fraction, aliquoted, and stored at −80°C. Cell protein assay was carried out according to Bradford (1976).
mADK and Fumarase Assay
The integrity of mitochondria in homogenates after purification was assessed by measuring the activities of both mADK, an enzyme of the mitochondrial intermembrane space, and fumarase, an enzyme of the mitochondrial matrix. mADK activity was measured spectrophotometrically at 340 nm in the direction of ATP production by using the ATP-detecting system, consisting of Glc (2.5 mm), hexokinase (0.2 enzymic units [EU]), Glc 6-P dehydrogenase (0.1 EU), and NADP+ (0.25 mm). The rate of absorbance increase at 340 nm following ADP addition (1 mm) was measured as the tangent to the initial part of the progress curve and expressed as nanomoles NADP+ reduced/min × mg protein. The value of ɛ340 for NAD(P)H measured under our experimental conditions was 6.3 mm−1cm−1.
Fumarase activity was measured spectrophotometrically at 240 nm as reported in Bergmeyer et al. (1974) by following the rate of absorbance increase at 240 nm after l-malate addition (50 mm) to isolated mitochondria (control and 2-h HS cells) either in the absence or presence of the detergent Triton X-100 0.2% (TX-100). The rate of reaction was measured as a tangent to the initial part of the progress curve and expressed in nanomoles fumarate formed/min × mg protein. The percent integrity of the inner membrane was calculated as 1 − (vo−TX-100/vo+TX-100) × 100. The value of ɛ340 for fumarate measured under our experimental conditions was 2.44 mm−1cm−1.
Oxygen Uptake Studies
Oxygen consumption was measured polarographically at 25°C as in Atlante et al. (2003a) in 1.5 mL of respiratory medium consisting of 210 mm mannitol, 70 mm Suc, 20 mm TRIS-HCl, 3 mm MgCl2, 5 mg/mL BSA, and 5 mm potassium phosphate, pH 7.4, by means of a Gilson 5/6 oxygraph using a Clark electrode. The instrument sensitivity was set to a value that allowed us to follow rates of oxygen uptake as low as 0.5 nat/min × mg protein.
To detect the presence of cyt c in the cytosolic fraction polarographically, the ability of both the control and 2-h HS cell homogenates to oxidize ASC (5 mm) was checked. Because ASC cannot permeate the outer mitochondrial membrane (Alexandre and Lenhinger, 1984), its oxidation can only occur as a result of the release from mitochondria of a component that can oxidize ASC and then reduce oxygen via cyt c oxidase (i.e. cyt c present in the cytosolic fraction; Atlante et al., 2000).
Immunoblot Analysis
Immunoblot analysis was performed on cytosolic and mitochondrial extracts from control and HS cultures. Mitochondria were incubated with 0.2% TX-100 for 5 min on ice and samples were centrifuged at 15,000g for 15 min at 4°C to remove insoluble materials. Supernatants, corresponding to the solubilized mitochondrial fraction, were aliquoted and stored at −80°C. Either 30 μg of cytosolic proteins or 10 μg of mitochondrial proteins were loaded onto a 15% SDS-polyacrylamide gel, separated, and transferred to a polyvinylidene difluoride membrane, which was probed with the monoclonal anti-cyt c antibody (1:500 dilution; Pharmingen). The same filter was stripped and reprobed with either the polyclonal anti-mADK isoenzyme 2 antibody (1:200 dilution; Abgent) or the polyclonal anti-mGDH antibody (1:1000 dilution; provided by Dr. F. Rothe, Institut Medizinische Neurobiologie, University of Magdeburg, Magdeburg, Germany). Immunoblot analysis was performed with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibodies using enhanced chemiluminescence western-blotting reagents (Amersham-Pharmacia Biotech).
Densitometry values for immunoreactive bands were quantified using a GS-700 imaging densitometer (Bio-Rad Laboratories); multiple expressions of the same immunoblots in a linear range were performed. Cyt c protein levels were calculated as a percentage of the control taken as 100 in arbitrary units after normalization using the amount of mGDH in each lane on the same filter. The variability of protein levels in the control cells, measured by comparing the percentage protein level from different control samples on the same filter, was always less than 5%.
Superoxide Anion and H2O2 Determination
H2O2 in the extracellular phase was measured according to Bellincampi et al. (2000). Briefly, 1 mL of cell cultures was harvested by centrifugation (10,000g, 20 s, 25°C) and the H2O2 concentration was measured in the supernatant. An aliquot of supernatant (500 μL) was added to 500 μL of assay reagent (500 μm ferrous ammonium sulfate, 50 mm H2SO4, 200 μm xylenol orange, and 200 mm sorbitol). After 45 min of incubation, the peroxide-mediated oxidation of Fe2+ to Fe3+ was determined by measuring the A560 of the Fe3+ xylenol orange complex.
The detection of superoxide anion in the extracellular phase was performed using the nitroblue tetrazolium method (Murphy et al., 1998). Briefly, 1 mL of cell cultures was harvested by centrifugation (10,000g, 20 s, 25°C) and the O2−. concentration was measured in the supernatant by monitoring the reduction of nitroblue tetrazolium (100 μm) at 530 nm. SOD (100 units) was added in a duplicate cuvette to access the specificity of measured changes of absorbance on O2−. concentration. The amount of superoxide anion was then calculated using ɛ550 nm = 12.8 mm−1cm−1 (Murphy et al., 1998).
DNA Fragmentation Analysis
At 72 h after HS, cells were collected from cell suspension and homogenized in liquid nitrogen. DNA was extracted by the cetyl-trimethyl-ammonium bromide method (Murray and Thompson, 1980). DNA samples were digested with 100 μg mL−1 DNAse-free RNAse for 1 h at 37°C, and DNA fragments were separated by electrophoresis on a 1.8% (w/v) agarose gel, followed by visualization by staining with ethidium bromide.
Measurement of Caspase-3-Like Activity in TBY-2 Cells
At time intervals over a 15-h period following HS, TBY-2 cells were collected from 1 mL of cell suspension by centrifugation (10,000g, 20 s, 4°C), washed once with preplasmolysis buffer, ground in liquid nitrogen, and stored at −80°C. Cells were lysed at 4°C in 100 μL of lysis buffer (BD Biosciences) and 10 μg of lysate containing cytosol and mitochondria were assayed for caspase-3-like activity with 50 μm of Ac-DEVD-pNA as substrate using the caspase colorimetric assay kit, according to the manufacturer's instructions (BD Biosciences). After incubation at 37°C for 1 h, the absorbance of pNA hydolyzed from the peptide substrate was quantified at 405 nm using a microplate reader.
Statistics
The data are reported as the mean ± sem for the indicated experiments. The statistical significance of differences between groups was determined by one-way ANOVA followed by a Student-Newman-Keuls test. Statistical differences between mean values of control and treated cells were determined with the Student's t test. All experiments were repeated at least three times.
Acknowledgments
The authors wish to thank Prof. Shawn Doonan for his critical reading of the manuscript.
This work was supported by the Italian Ministry of Instruction, University and Research, “Contributi straordinari di ricerca/aree obiettivo 1” (to E.M.) and by Programmi di Ricerca a Rilevante interesse Nazionale, “I mitocondri nello stress e nella morte cellulare in sistemi vegetali” (to S.P.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ersilia Marra (e.marra@ibbe.cnr.it).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.106.078683.
References
- Alexandre A, Lehninger AL (1984) Bypasses of the antimycin a block of mitochondrial electron transport in relation to ubisemiquinone function. Biochim Biophys Acta 767: 6627–6636 [DOI] [PubMed] [Google Scholar]
- Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF (2004) Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J 18: 869–871 [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, et al (1996) Fas-induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem 271: 16850–16855 [DOI] [PubMed] [Google Scholar]
- Atlante A, de Bari L, Bobba A, Marra E, Calissano P, Passarella S (2003. a) Cytochrome c, released from cerebellar granule cells undergoing apoptosis or excytotoxic death, can generate protonmotive force and drive ATP synthesis in isolated mitochondria. J Neurochem 86: 591–604 [DOI] [PubMed] [Google Scholar]
- Atlante A, Bobba A, Calissano P, Passarella S, Marra E (2003. b) The apoptosis/necrosis transition in cerebellar granule cells depends on the mutual relationship of the antioxidant and the proteolytic systems which regulate ROS production and cytochrome c release en route to death. J Neurochem 84: 960–971 [DOI] [PubMed] [Google Scholar]
- Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S (2000) Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J Biol Chem 275: 37159–37166 [DOI] [PubMed] [Google Scholar]
- Bafunno V, Giancaspero TA, Brizio C, Bufano D, Passarella S, Boles E, Barile M (2004) Riboflavin uptake and FAD synthesis in Saccharomyces cerevisiae mitochondria: involvement of the Flx1p carrier in FAD export. J Biol Chem 279: 95–102 [DOI] [PubMed] [Google Scholar]
- Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13: 1803–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Leaver CJ, McCabe PF (1999) Translocation of cytochrome c from the mitochondria to the cytosol occurs during heat-induced programmed cell death in cucumber plants. FEBS Lett 463: 151–154 [DOI] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxine-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122: 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU, Gawehn K, Grassl M (1974) Enzymatic assay of fumarase. In HU Bergmeyer, ed, Methods of Enzymatic Analysis, Vol 1. Academic Press, New York, pp 543–545
- Bobba A, Atlante A, de Bari L, Passarella S, Marra E (2004) Apoptosis and cytochrome c release in cerebellar granule cells. In Vivo 18: 335–344 [PubMed] [Google Scholar]
- Bobba A, Atlante A, Giannattasio S, Sgaramella P, Calissano P, Marra E (1999) Early release and subsequent caspase-mediated degradation of cytochrome c in apoptotic cerebellar granule cells. FEBS Lett 457: 126–130 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17: 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Chichkova NV, Kim SH, Titova ES, Kalkum M, Morozov VS, Rubtsov YP, Kalinina NO, Taliansky ME, Vartapetian AB (2004) A plant caspase-like protease activated during the hypersensitive response. Plant Cell 16: 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, Francis D, De Gara L (1999) The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma 209: 90–97 [DOI] [PubMed] [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2000) Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco Bright Yellow 2. Plant Physiol Biochem 38: 541–550 [Google Scholar]
- de Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for programmed cell death activated by nitric oxide and reactive oxygen species in tobacco Bright Yellow 2 cells. Plant Physiol 130: 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interaction between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98: 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz M, Avni A, Weil M (2002) Constitutive caspase-like machinery executes programmed cell death in plant cells. Cell Death Differ 9: 726–733 [DOI] [PubMed] [Google Scholar]
- Gerhardt E, Kugler S, Leist M, Beier C, Berliocchi L, Volbracht C, Weller M, Bahr M, Nicotera P, Schulz JB (2001) Cascade of caspase activation in potassium-deprived cerebellar granule neurons: targets for treatment with peptide and protein inhibitors of apoptosis. Mol Cell Neurosci 1: 7–31 [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, et al (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 22: 221–233 [DOI] [PubMed] [Google Scholar]
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770–776 [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Kleczkowski LA (2003) Membrane potential, adenylate levels and Mg2+ are interconnected via adenylate kinase equilibrium in plant cells. Biochim Biophys Acta 1607: 111–119 [DOI] [PubMed] [Google Scholar]
- Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53: 2401–2410 [DOI] [PubMed] [Google Scholar]
- Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA 95: 4997–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Durner J (2004) Harpin inactivates mitochondria in Arabidopsis suspension cells. Mol Plant Microbe Interact 17: 131–139 [DOI] [PubMed] [Google Scholar]
- Kroemer G, Zamzami N, Susin SA (1997) Mitochondrial control of apoptosis. Immunol Today 18: 44–51 [DOI] [PubMed] [Google Scholar]
- La Piana G, Marzulli D, Gorgoglione V, Lofrumento NE (2005) Porin and cytochrome oxidase containing contact sites involved in the oxidation of cytosolic NADH. Arch Biochem Biophys 436: 91–100 [DOI] [PubMed] [Google Scholar]
- Lam E, del Pozo O (2000) Caspase-like protease involvement in the control of plant cell death. Plant Mol Biol 44: 417–428 [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489 [DOI] [PubMed] [Google Scholar]
- Luo J, Robinson JP, Shi R (2005) Acrolein-induced cell death in PC12 cells: role of mitochondria-mediated oxidative stress. Neurochem Int 47: 449–457 [DOI] [PubMed] [Google Scholar]
- Mao GD, Poznansky MJ (1992) Electron spin resonance study on the permeability of superoxide radicals in lipid bilayers and biological membranes. FEBS Lett 305: 233–236 [DOI] [PubMed] [Google Scholar]
- McCabe PF, Leaver CJ (2000) Programmed cell death in cell cultures. Plant Mol Biol 44: 359–368 [DOI] [PubMed] [Google Scholar]
- Mlejnek P, Procházka S (2002) Activation of caspase-like proteases and induction of apoptosis by isopentenyladenosine in tobacco BY-2 cells. Planta 215: 158–166 [DOI] [PubMed] [Google Scholar]
- Murphy TM, Vu H, Nguyen T (1998) The superoxide synthases of rose cells: comparison of assays. Plant Physiol 117: 1301–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8: 4321–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Hasezawa S (1992) Tobacco BY-2 cell line as the “HeLa” cell in the cell biology of higher plants. Int Rev Cytol 132: 1–30 [Google Scholar]
- Naveilhan P, Neveu I, Jehan F, Baudet C, Wion D, Brachet P (1994) Reactive oxygen species influence nerve growth factor synthesis in primary rat astrocytes. J Neurochem 62: 2178–2186 [DOI] [PubMed] [Google Scholar]
- Neame SJ, Rubin LL, Philpott KL (1998) Blocking cytochrome c activity within intact neurons inhibits apoptosis. J Cell Biol 142: 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, Ruggiero FM, Paradies G (2003) Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 17: 2202–2208 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Shulaev V, Mittler R (2004) Measuring programmed cell death in plants. Methods Mol Biol 282: 179–189 [DOI] [PubMed] [Google Scholar]
- Satoh T, Numakawa T, Abiru Y, Yamagata T, Ishikawa Y, Enokido Y, Hatanaka H (1998) Production of reactive oxygen species and release of L-glutamate during superoxide anion-induced cell death of cerebellar granule neurons. J Neurochem 70: 316–324 [DOI] [PubMed] [Google Scholar]
- Stein JC, Hansen G (1999) Mannose induces an endonuclease responsible for DNA laddering in plant cells. Plant Physiol 121: 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zhao Y, Hong X, Zhai Z (1999) Cytochrome c release and caspase activation during menadione-induced apoptosis in plants. FEBS Lett 462: 317–321 [DOI] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW (1997) Substrate specificities of caspase family proteases. J Biol Chem 272: 9677–9682 [DOI] [PubMed] [Google Scholar]
- Tian RH, Zhang GY, Yan CH, Dai YR (2000) Involvement of poly (ADP-ribose) polymerase and activation of caspase 3-like protease in heat shock-induced apoptosis in tobacco suspension cells. FEBS Lett 474: 11–15 [DOI] [PubMed] [Google Scholar]
- Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128: 1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca RA, de Pinto MC, Valenti D, Passerella S, Marra E, De Gara L (2004) Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tabacco Bright-Yellow 2 cells. Plant Physiol 134: 1100–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91: 627–637 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10: 117–122 [DOI] [PubMed] [Google Scholar]
- Wang X, Takahashi N, Uramoto H, Okada Y (2005) Chloride channel inhibition prevents ROS-dependent apoptosis induced by ischemia-reperfusion in mouse cardiomyocytes. Cell Physiol Biochem 16: 147–154 [DOI] [PubMed] [Google Scholar]
- Woltering EJ, van der Bent A, Hoeberichts FA (2002) Do plant caspases exist? Plant Physiol 130: 1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Hanson MR (2000) Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiol 122: 1323–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Cortopassi GA (1998) dATP causes specific release of cytochrome c from mitochondria. Biochem Biophys Res Commun 250: 454–457 [DOI] [PubMed] [Google Scholar]
- Yao N, Eisfelder BJ, Marvin J, Greengerg J (2004) The mitochondriaon—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40: 596–610 [DOI] [PubMed] [Google Scholar]
- Yu XH, Perdue TD, Heimer YM, Jones AM (2002) Mitochondrial involvement in tracheary element programmed cell death. Cell Death Differ 9: 189–198 [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X (1999) An APAF-1 cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem 274: 11549–11556 [DOI] [PubMed] [Google Scholar]