Abstract
Polarized Th1 and Th2 cells expressing the same TCR produce distinct biochemical responses to ligand engagement. Compared to Th1 cells, Th2 cells show altered substrate tyrosine phosphorylation and a diminished or transient Ca2+ response. Here we demonstrate that agonist stimulation of Th1 cells leads to the predominant appearance of fully phosphorylated (p23) TCR ζ, substantial phosphorylation of zeta-associated protein 70 (ZAP-70), and strong elevation of intracellular Ca2+, whereas agonist stimulation of Th2 cells expressing an identical TCR results in an elevated p21:p23 TCR ζ ratio, little or no detectable ZAP-70 phosphorylation, and a more limited elevation in intracellular Ca2+. Th2 cells consistently had twofold lower surface CD4 expression as compared to Th1 cells with the same TCR. When CD4 levels in Th2 cells were raised to Th1 levels using retroviral gene transfer, the transduced cells showed greater generation of p23 phospho-ζ, measurable phosphorylation of ZAP-70, and increased Ca2+ responses. These findings suggest that the apparent qualitative differences in TCR signaling characterizing Th1 versus Th2 cells are largely the result of modest quantitative variation in CD4 expression, with decreased CD4 expression playing a significant role in attenuating the proximal signaling responsiveness of Th2 cells to TCR ligands.
Keywords: Th1/Th2 cells, T cell receptors, Signal transduction, Cytokines, Protein kinases/phosphatases
Introduction
While it is an oversimplification of in vivo physiology, the Th1–Th2 model is a convenient and generally applicable paradigm for naive CD4+ T cell differentiation [1, 2]. The set of effector cytokines produced by an antigen-activated CD4+ T cell is largely determined by the particular combination of T cell receptor (TCR), cytokine, and costimulatory signals received by the naive T cell. In particular, T cells expressing the same transgenic TCR can be induced to develop along the Th1 (IFN-γ-producing) or the Th2 (IL-4-producing) pathways by varying the strength (intensity/duration) of the TCR signal received by the T cell, provided that the other factors able to influence this fate determination are held constant [3, 4]. Both in vivo and in vitro, low-intensity and/or short-duration TCR signals favor Th2 development from unpolarized precursors [5].
Differential TCR signaling not only characterizes the initial choice of effector lineage by naive cells, it is also a feature of fully differentiated Th1 and Th2 cells. Studies with mouse T cell clones [6, 7] and with cells from TCR-transgenic animals [8] revealed that in comparison to Th1 cells, Th2 cells exhibit lower and/or more transient elevation of intracellular Ca2+ following antigen stimulation. In contrast to Th1 clones, other analyses using mouse T cell clones demonstrated that Th2 cells were poor at phosphorylation of zeta-associated protein 70 (ZAP-70) following TCR ligation [9]. Human Th2 clones were also reported to show less tyrosine phosphorylation of substrate proteins than Th1 clones following anti-CD3 stimulation [10]. A possible molecular explanation for these differences between Th1 and Th2 cells is the reported exclusion of the CD4 coreceptor from the immunological synapse and from lipid rafts upon antigen stimulation [11]. However, whether this completely accounts for the distinct TCR-associated signaling of Th1 vs. Th2 cells is not known [12], as other defects in Th2 cells have been reported [13].
The altered downstream phosphorylation seen in Th2 cells resembles that reported by this laboratory for Th1 cells in which CD4-MHC class II interactions are disrupted [14]. Therefore, we undertook a re-examination of the status of the CD4 molecule in polarized Th2 cells. Here we report that such cells consistently show a lower surface level of CD4 expression than Th1 cells derived from the same precursor pool. This lower CD4 expression could be linked to the altered signaling pattern of the Th2 cells through experiments showing a switch to a more Th1-like pattern of tyrosine phosphorylation and Ca2+ signaling following enhancement of CD4 expression using a retroviral vector. These data provide evidence that a modest quantitative change in expression of a surface receptor can have qualitative effects on signaling in differentiated T cells and that decreased CD4 expression plays a significant role in controlling the antigen responsiveness of polarized Th2 cells.
Results
Differential TCR down-modulation in response to agonist by Th1 vs. Th2 cells expressing identical TCR
To examine in a controlled fashion the origin of the differences previously described as characteristic of the TCR-dependent signaling events in Th1 vs. Th2 cells, we generated polarized effector cells using TCR-transgenic RAG−/− CD4+ T cells. This ensured that all cells being studied expressed the same receptor subunits and differed only in the cytokine environment to which they were exposed as naive cells in vitro. As expected [2], cells experiencing TCR signals in the presence of IL-12 and absence of IL-4 developed uniformly into Th1 cells secreting IFN-γ, whereas those stimulated in the presence of IL-4 and anti-IL-12 became Th2 cells that produced IL-4 (Fig. 1A).
Figure 1.
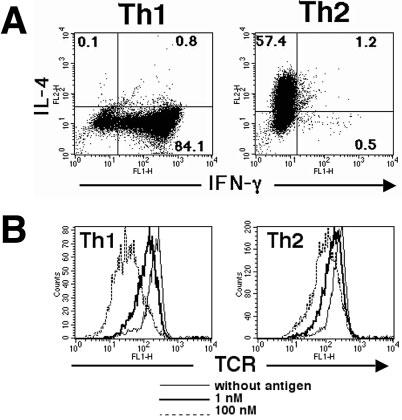
Differential TCR down-modulation among Th1 and Th2 cells expressing the same TCR chains. 5C.C7 TCR-transgenic T cells were polarized in vitro to produce Th1 and Th2 effector cells. Rested Th1 and Th2 cells were stimulated with P13.9 and PCC88–104 (1 μM) for 5 h and then analyzed for cytokine production (A). These Th1 and Th2 cells were then exposed to antigen-presenting cells pulsed with either 1 or 100 nM MCC88-103 peptide and the surface expression of Vβ3-containing TCR was assessed 5 h later by flow cytometry (B). The mean fluorescent intensities for untreated cells, cells exposed to 1 nM pulsed presenting cells, or cells exposed to 100 nM pulsed presenting cells, respectively, were 215, 135, and 47 for Th1 cells and 246, 173, and 120 for Th2 cells. Results are representative of eight experiments.
These differentiated effector cells were then re-exposed to antigen-presenting cells bearing the strong agonist MCC/I-Ek combination and TCR down-modulation was examined after a 5-h incubation period. We have previously shown that the extent of receptor loss from the cell surface closely tracks the quality as well as intensity of TCR signaling as assessed by the nature of TCR ζ chain and ZAP-70 tyrosine phosphorylation [15, 16], and based on previous reports of defects in the latter event in Th2 cells, we anticipated that there would be less TCR lost from the surface of Th2 as compared to the Th1 cells. Fig. 1B shows that indeed, the 5C.C7 Th2 cells have a smaller antigen-induced decrease in surface TCR than Th1 cells under these assay conditions. Thus, even using the MCC88–103 peptide, which induces more vigorous responses by 5C.C7 T cells than the immunogen PCC, TCR down-modulation was limited in Th2 cells as compared to Th1 cells.
Differential TCR-associated tyrosine phosphorylation events in Th1 and Th2 cells
These phenotypic data were consistent with past reports of distinct proximal TCR-induced phosphorylation events in Th1 vs. Th2 effector T cells. To examine this issue directly, polarized 5C.C7 cell lines were incubated with antigen-expressing cells expressing I-Ek plus PCC88–104 for 5 min. ZAP-70 and associated proteins were then precipitated, separated by SDS-PAGE, and analyzed by blotting with anti-phosphotyrosine antibody (Fig. 2). The Th1 cells showed phosphorylation of ZAP-70 and a nearly equal generation of the two main forms of phospho-ζ (p21 and p23), corresponding to the full activation pattern induced by agonist ligands as reported in previous studies of naive or Th1-polarized T cells [17, 18]. In contrast, the Th2 cells showed less overall TCR-associated protein tyrosine phosphorylation and predominant generation of partially phosphorylated (p21) TCR ζ chain in combination with nearly undetectable phosphorylation of ZAP-70. This latter pattern corresponds to that observed using partial agonists or antagonists to stimulate naive or Th1-polarized CD4+ or CD8+ T cells [17, 18]. It also resembles the signaling pattern seen with Th1 cells stimulated under conditions in which CD4-MHC class II interactions are disrupted [14], suggesting a possible role for reduced CD4 function in the signaling behavior of Th2 cells.
Figure 2.
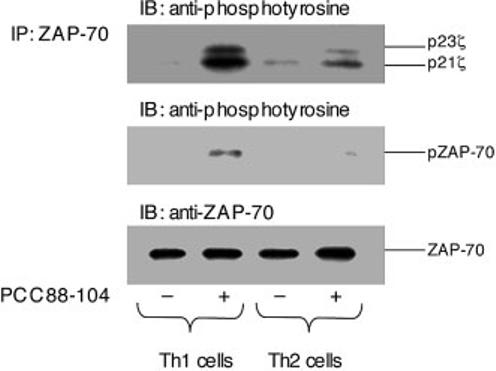
Differential TCR-associated protein tyrosine phosphorylation in Th1 vs. Th2 effector cells. Polarized Th1 and Th2 effector 5C.C7 T cells were exposed to antigen-presenting cells pre-pulsed with 100 μM PCC88–104 peptide. After 5 min of cell contact, the cells were lysed, and equal amounts of protein extract from each sample were subjected to immunoprecipitation (IP) with an anti-ZAP-70 antibody followed by SDS-PAGE analysis and immunoblotting (IB) with monoclonal anti-phosphotyrosine antibody. The same blot was stripped, then immunoblotted with anti-ZAP-70 antibody to demonstrate equal loading in all lanes. Results are representative of three experiments.
To determine whether the Th1-polarized 5C.C7 cells studied here showed the same influence of CD4 function on proximal TCR signaling, we repeated the phosphorylation studies in the presence of anti-CD4 blocking antibody (Fig. 3). Control 5C.C7 Th1 cells showed substantial levels of the p23 phosphorylated form of TCR ζ chain, whereas those exposed to antigen in the presence of anti-CD4 showed little p23 TCR ζ generation and predominant p21 production, consistent with our earlier study. Our previous studies have shown that in addition to CD4-associated Lck, there is a separate pool of Lck associated with the TCR [19], providing a source of kinase for generation of the antigen-induced p21ζ seen in this experiment even when CD4-Lck is not involved.
Figure 3.
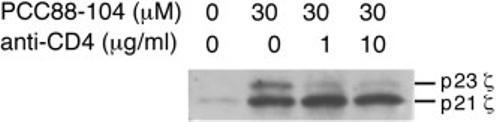
Alteration of ζ chain phosphorylation pattern in Th1 cells with disrupted CD4 function. Polarized 5C.C7 Th1 cells were exposed to antigen-presenting cells pre-pulsed with 30 μM PCC88–104 peptide in the absence or the presence of the indicated amount of anti-CD4 mAb. After 5 min of cell contact, the extent of tyrosine phosphorylation involving the TCR-associated ζ chains was evaluated by immunoblotting after cell lysis and immunoprecipitation with anti-CD3ε antibody. Results are representative of two experiments.
Diminished CD4 expression on Th2 cells and its role in altered signaling
Given the similarity of signaling in Th2 cells and in Th1 cells deprived of full CD4 function, as well as a prior report of defective CD4 localization to the synapse and rafts in Th2 cells [11], we examined surface expression of CD4 and other potentially relevant proteins on Th1 vs. Th2 effectors. CD3, CD28, and an isoform of CD45 (T200) were expressed almost equivalently on both Th1 and Th2 cell surfaces (Fig. 4). However, a striking difference between Th1 and Th2 cells was seen in the expression of CD4. Th1 cells consistently expressed twofold more CD4 than Th2 cells (Th1/Th2 ratio = 2.25±0.62 in ten independent experiments). Similar results were obtained with polarized Th1 and Th2 cells generated from other TCR-transgenic cells, including DO11.10 (Th1/Th2 ratio = 2.04±0.64 in three independent experiments) and HA110–120 specific T cells (Th1/Th2 ratio = 1.57±0.20 in five independent experiments). These results establish that the difference in CD4 expression between the two types of effector T cells was not a peculiar feature of 5C.C7 cells.
Figure 4.
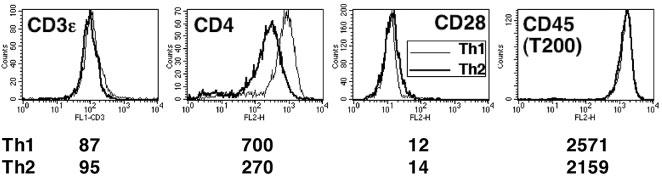
Selective decrease in CD4 surface membrane expression on polarized Th2 cells as compared to Th1 cells. Polarized 5C.C7 T cells were stained for CD3ε, CD4, CD28, and CD45 (T200) expression and analyzed by flow cytometry. The numbers below each panel represent the mean fluorescent intensity. Results for CD4 are representative of ten experiments.
To study whether the quantitative difference in CD4 expression between Th1 and Th2 cells might account for what appeared to be qualitative differences in TCR signaling, we restored CD4 expression in Th2 cells using retroviral gene transfer (Fig. 5A). Retrovirus-infected Th2 cells whose CD4 expression had been restored to the level characteristic of naive or Th1 T cells were isolated by electronic cell sorting based on co-expression of enhanced green fluorescent protein (EGFP). The TCR signaling response of these transduced Th2 cells and of control-infected cells expressing only EGFP to agonist ligands (PCC or MCC peptides) was then examined (Fig. 5B).
Figure 5.
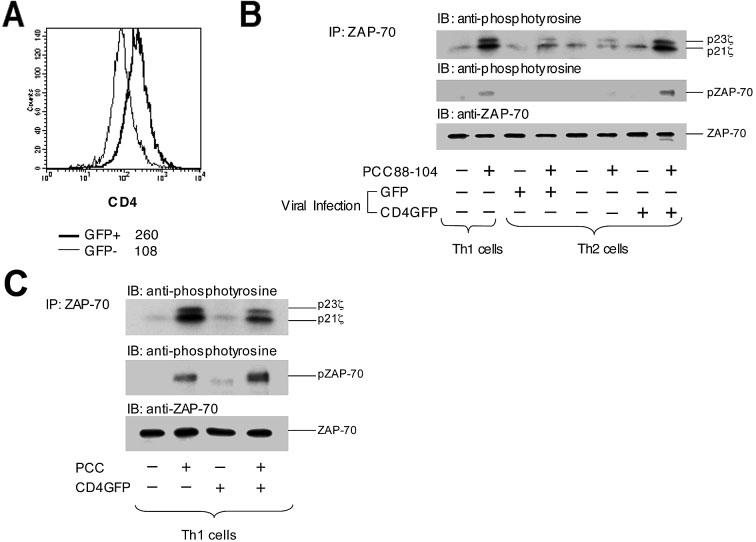
Th1-like pattern of proximal TCR-induced protein tyrosine phosphorylation in Th2 cells with restored CD4 expression. (A) Polarized Th1 and Th2 5C.C7 T cells were infected with recombinant retrovirus encoding either EGFP or a mouse CD4-EGFP fusion protein. The infected cells were then subjected to cell sorting for EGFP-positive cells. Infected cells expressing CD4-EGFP showed restoration of CD4 expression to a level typical of polarized Th1 cells, which is ∼2.5-fold greater than that of the uninfected EGFP-negative Th2 cells from the same culture. (B) Th1 cells, EGFP-negative Th2 cells, and Th2 cells expressing either EGFP or CD4-EGFP were stimulated with antigen-presenting cells pulsed with 100 μM PCC88–104 peptide. Five minutes later, the cells were lysed, and the lysates were subjected to immunoprecipitation and immunoblotting analysis as described in Fig. 2. Results are representative of three experiments. (C) Polarized Th1 cells were either left uninfected or infected with retrovirus encoding CD4-EGFP and sorted for EGFP expression. These cells were then stimulated with antigen-presenting cells pulsed with 100 μM PCC88–104 peptide, lysed, and the lysates subjected to immunoprecipitation with anti-ZAP-70 antibody followed by SDS-PAGE analysis and immunoblotting with either anti-phosphotyrosine or anti-ZAP-70 antibody.
The overall extent of TCR-associated protein tyrosine phosphorylation and the p23/p21 TCR ζ ratio in activated, infected, CD4-restored Th2 cells was close to that seen with antigen-stimulated Th1 cells (Fig. 3), indicating that the quantitative difference in CD4 expression between polarized Th1 and Th2 cells was by itself able to account in large measure for the striking differences in proximal TCR phosphorylation seen in the two differentiated cell populations. Expression of the CD4-EGFP protein in polarized Th1 cells did not change their proximal signaling pattern (Fig. 5C). The p23/p21 ζ chain phosphorylation data from all experiments are summarized in the supplementary information, Table 1. No differences were seen between Th1 and Th2 cells in the expression of the key signaling molecules ZAP-70 (Fig. 5), Lck, or ζ (see supplementary information Fig. 1), consistent with CD4 expression being the key determinant of the distinct proximal signaling in Th2 as compared to Th1 cells.
Cytokine gene expression, especially of IL-4, has been associated with elevation of intracellular Ca2+ that affects the nuclear translocation of NFAT transcription factors via activation of the phosphatase calcineurin [20]. The previously reported differences in Ca2+ signaling by Th1 and Th2 cells were reproduced here with polarized 5C.C7 cells (Fig. 6A). Restoration of CD4 levels in Th2 cells to those characteristic of naive or Th1 cells corrected the Ca2+ response deficiency (Fig. 6B), just as this manipulation corrected the proximal phosphorylation defect. Expression of CD4-EGFP in Th1 cells using retroviral infection did not change the Ca2+ response of these cells (see supplementary information Fig. 2).
Figure 6.
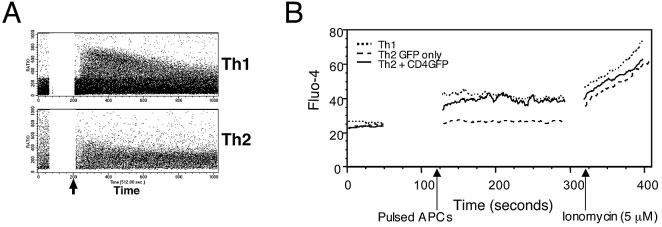
Th1-like pattern of TCR-induced intracellular Ca2+ elevation in Th2 cells with restored CD4 expression. (A) Polarized Th1 and Th2 5C.C7 T cells were loaded with the indicator dye Indo-1 and analyzed for changes in intracellular Ca2+ by FACS following stimulation with antigen-presenting cells pulsed with 10 μM MCC peptide (arrows). (B) Polarized Th1, Th2, and CD4-EGFP-positive Th2 5C.C7 T cells were loaded with the calcium indicator Fluo-4, and intracellular Ca2+ levels were analyzed by FACS. A 50-s baseline was recorded before addition of antigen-presenting cells pulsed with 100 μM PCC88–104 peptide. After 90 s of cell contact, the intracellular Ca2+ levels were measured again (first arrow) followed by exposure to ionomycin (5 μM; second arrow). Results are representative of five experiments.
Discussion
In comparison to naive T cells or Th1 cells, Th2 cells show a distinct pattern of TCR-dependent signaling, with lower and less complete ζ chain tyrosine phosphorylation, little or no ZAP-70 phosphorylation, and diminished or transient elevation of intracellular Ca2+ [6-11, 13]. These three aspects of TCR signal transduction all play important roles in downstream signaling for gene activation [21], which makes it surprising that they appear to be actively down-modulated in differentiated effector Th2 cells as compared to their naive counterparts or Th1 cells. At present there is no satisfying physiological rationale for this feature of Th2 biology nor for the capacity of these effector cells to respond (at least in vitro) with cytokine secretion when stimulated at ligand densities similar to those able to activate Th1 cells showing more prototypic TCR signaling capacity.
Given its paradoxical nature, it is surprising that only a few studies have sought to define the molecular basis of this altered signaling in Th2 effector cells as one step towards gaining a better understanding of this aspect of T cell biology. One report suggested that diminished signaling in Th2 cells arose from a change in the behavior of CD4, with its exclusion from membrane rafts and the immunological synapse following TCR ligation [11]. These findings implied a qualitative change in the molecular properties of the TCR-coreceptor axis in Th2 cells as compared to naive or Th1 cells.
Here we show that a modest quantitative change in CD4 levels is sufficient to account for much of what appears to be qualitatively altered signaling in Th2 cells. Polarized Th2 cells were found to have approximately 50% of the surface level of CD4 present on Th1 cells derived from the same TCR-transgenic, RAG−/− T cell population, with no differences in TCR, CD3, or CD45 expression. Retroviral transduction to increase CD4 expression to that seen on naive or Th1 cells allowed the polarized Th2 cells to respond to TCR ligation with a ratio of phosphorylated ζ chains (p21/p23) similar to that seen in Th1 cells, together with detectable tyrosine-phosphorylated ZAP-70, and with elevated Ca2+ responses. Pre-sorting of naive cells into CD4hi and CD4lo subpopulations did not influence the generation of Th1 or Th2 cells under standard polarizing culture conditions (Y.I., unpublished observations), indicating that the low level of CD4 expression among Th2 cells is a consequence of differentiation, not a reflection of preexisting microheterogeneity among precursors that can be discerned based on CD4 expression.
These findings agree with a model of CD4 function we have proposed previously [14], in which the surface density of CD4 plays a major role in dictating the ligand discrimination properties of the TCR. Low CD4 density on a cell would demand exposure to very avid TCR ligands for full agonist signaling to occur. For a given ligand-TCR pair, this means that T cells with low CD4 levels would be expected to show only a partial agonisttype signaling response to a peptide-MHC molecule combination that could act as a full agonist for cells with high CD4 expression, precisely as reported here. Whether the previously reported loss of lipid domain association of CD4 in Th2 cells might thus represent a secondary effect of this quantitative change and the alteration in signaling leads to this, will require additional investigation. Our description of very early acting SHP-1-dependent negative feedback in controlling the TCR signal quality and duration [19], along with the evidence from this and other laboratories that the early phosphorylation of TCR-associated components precedes synapse formation [22], is consistent with the view that these previously reported aspects of CD4 behavior may be a downstream consequence rather than a primary cause of the Th2 pattern of signaling.
The present data do not by themselves provide a physiological rationale for why Th2 cells decrease CD4 expression and limit the robustness of their signaling response, nor do they explain the molecular basis of the reduction in CD4 levels (less transcription, limited translation, more degradation, differential distribution between surface membrane and intracellular compartments). This latter issue also bears on the failure of gene array studies comparing Th1 and Th2 cells to note a difference in CD4 mRNA levels in these two cell types that would account for the data reported here. The surface difference we see is only twofold, which many array analyses use as a cut-off [23, 24]. Some have suggested that IL-4 is a “dangerous” cytokine and that the pseudo-monoallelic nature of IL-4 gene expression is a mechanism evolved to limit production of this potentially harmful molecule [25]. The attenuation of TCR signaling in Th2 effectors can be seen as reinforcing the same behavior. The paradox is that weak stimulation favors Th2 differentiation among naive cells before the development of this differentiated phenotype, so it is not obvious why cells that already are at the limit of signal input during their initial triggering would need further attenuation of signal transduction to constrain later responses [26].
The low expression of CD4 and the resulting diminished TCR-induced signaling in Th2 cells could make a contribution to preventing responses to low-avidity ligands like self-antigens [27]. A possible role for CD4 in regulating autoimmune T cell function is suggested by a report that pathogenic T cells express a higher level of CD4 than non-pathogenic T cells in NOD mice [28]. It is intriguing that Th1 cells have been reported to regulate their response to low-affinity ligands with partial activation followed by clonal anergy that can affect both proliferation and IFN-γ responses [29, 30], while in contrast, IL-4 production is not impaired in anergic Th2 cells [31, 32]. Therefore, mechanisms other than anergy may be required to prevent a Th2 effector response to low-avidity ligands. Down-regulation of CD4 may be one of these mechanisms, suggesting that a better understanding of the molecular basis for the change in CD4 expression in Th2 cells may help to provide a better understanding of autoimmune susceptibility.
Materials and methods
Preparation of Th1/Th2 cell lines
Th1 and Th2 cell lines were established from TCR-transgenic 5C.C7 mice [33] lacking RAG-2 expression [34] (Taconic Farms, Germantown, NY). Freshly isolated spleen and lymph node cells (2×106/well) were cultured with irradiated B10.A spleen cells (2×106/well) and the agonist peptide PCC88–104 (KERADLIAYLKQATAK; 1 μM) in 24-well plates. IL-12 (10 ng/mL) plus anti-IL-4 (clone 11B11; 10 μg/mL) were added to cultures to prepare Th1 cells, and IL-4 (30 ng/mL) plus anti-IL-12 (polyclonal goat antibody, 10 μg/mL; R&D Systems, Minneapolis, MN) were added to cultures to prepare Th2 cells. Cells were maintained in RPMI 1640 supplemented with 10% FCS, 50 μM 2-ME, 2 mM l-glutamine, 10 mM Hepes, and 1% nonessential amino acids. Two days after initial stimulation, recombinant IL-2 was added to maintain cell division and promote differentiation (1 ng/mL; Takeda Chemical Industries, Ltd., Osaka, Japan). Five to seven days after IL-2 addition, the cells were re-stimulated using similar culture conditions. All cells studied here for signaling or functional responses were used not earlier than 6 days after this single re-stimulation.
Measurement of TCR down-modulation and cytokine production by flow cytometry
One million P13.9 L-cell transfectants expressing I-Ek, CD54, and CD80 [35] were pre-incubated with PCC88–104 or in some cases the more potent MCC88–103 (ANRADLIAYLKQATK) peptide overnight as indicated in the Fig. legends, then washed with PBS before adding T cells. Polarized 5C.C7 T cells (5×105) were mixed with P13.9, spun for 5 min in 5-mL polypropylene tubes, and cultured at 37°C for 5 h. Monensin was added in the beginning of the re-stimulation culture for analysis of cytokine production. Cells were washed with PBS containing 0.5 mM EDTA before staining. Surface TCR was stained with anti-Vβ3 [15]. For intracellular cytokine analysis, cells were fixed with 4% paraformaldehyde in PBS and stained with PE-conjugated anti-IL-4 and/or FITC-conjugated anti-IFN-γ (BD PharMingen, San Diego, CA) in the presence of saponin [36].
Protein phosphorylation and immunoblotting analyses
Polarized 5C.C7 cells (3×106) were incubated with P13.9 (3×106) previously pulsed with peptide as specified in the Fig. legends. In some experiments, anti-CD4 (clone H129.19) was added to the cell mixture. After incubation, cells were lysed with 1% Nonidet-P40, 140 mM NaCl, 10 mM Tris-HCl (pH 7.2), 2 mM EDTA, 5 mM iodoacetamide, 1 mM Na3VO4, and proteinase inhibitor mixture (Boehringer Mannheim, Indianapolis, IN) on ice for 30 min. After centrifugation, the supernatant was incubated with anti-TCR (clone H57-597), biotinylated anti-CD3ε (clone 145-2C11), or antiserum to ZAP-70 ([17], kindly provided by Dr. J. B. Bolen) for 4 h and precipitated with protein A-Sepharose (Amersham Pharmacia Biotech, Sweden) or ImmunoPure immobilized streptavidin (Pierce, Rockford, IL) for biotinylated antibody. Precipitated proteins were separated using SDS-PAGE and transferred to Hybond-P membrane (Amersham Pharmacia Biotech, Little Chalfont, UK).
Tyrosine-phosphorylated proteins were detected with 4G10 (Upstate Biotechnology, Lake Placid, NY) and horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad Laboratories, Richmond, CA). Horseradish peroxidase activity was detected with ECL (Amersham Pharmacia Biotech). Previous studies have shown that these solubilization and precipitation conditions are appropriate for the detection of phosphoproteins associated with the proximal events in TCR-dependent signaling [14, 15, 17-19]. Controls for sample loading consisted of re-probing of blots for total ZAP-70. For quantitative analysis of protein expression, antiserum to ZAP-70 and anti-Lck mAb (clone 3A5; Upstate Biotechnology) were used for blotting followed by staining with horseradish peroxidase-conjugated goat anti-rabbit Ig and anti-mouse IgG (Bio-Rad Laboratories), respectively.
Retroviral gene transduction
For retroviral enhancement of CD4 expression on Th2 cells, the pIB-CD4-EGFP(C4) vector (a kind gift of M. Davis, HHMI Stanford University School of Medicine [37]) was transfected into Phoenix-Eco packaging cells. Viral supernatant collected from these transduced cells was added to previously generated 5C.C7 Th2 cells that were re-stimulated with splenic antigen-presenting cells and anti-CD3ε (clone 145-2C11; 1 μg/mL) in 24-well plates for 24 h. Cells were spun down at 2200 rpm for 90 min at 32°C in the presence of polybrene (8 μg/mL). Infection was repeated three times at 12-h intervals and the resulting cell population electronically sorted on the basis of EGFP expression. EGFP-negative cells from the same cultures were used as a control for signaling and functional experiments. Surface CD4 expression was measured by staining with PE-conjugated anti-CD4 (BD PharMingen) followed by flow cytometric analysis. Cells transduced with virus encoding only EGFP were used as controls.
Ca2+ measurements
Method 1
Resting Th1 and Th2 cell lines were prepared as described above. T cells (2×107) were suspended in 1 mL Hank's balanced salt solution (HBSS) containing 10 mM Hepes, 1 % FCS, 0.03% pleuronic, and 5 μM Indo-1 (Molecular Probes, Inc., Eugene, OR). After gently shaking for 45 min at 30°C, cells were washed twice in HBSS. MHC class I molecules on T cells were stained with FITC-conjugated anti-Dd for 15 min at room temperature. Cells were washed twice more before data acquisition. Indo-1-labeled T cells were spun down to produce conjugates with P13.9 previously pulsed with MCC88–103 (10 μM). After gating based on high FSC (P13.9) and expression of Dd (5C.C7 T cells) to identify conjugates, calcium ratios (bound/unbound) were measured using a FACSVantage (BD Biosciences; NIAID Flow Cytometry Unit).
Method 2
EGFP-negative and EGFP-positive cells Th2 cells were sorted from Th1- or Th2-polarized 5C.C7 T cell populations exposed to the CD4-GFP-encoding retrovirus, washed twice in PBS, and 2×106 cells for each sample were suspended in 500 μl of PBS containing 1 μM Fluo-4 (Molecular Probes, Inc.). After 20 min incubation at 37°C, cells were washed twice in warm RPMI medium and incubated at 37°C for 1 h before analysis. Fluo-4-labeled T cells were co-centrifuged with P13.9 cells previously pulsed with PCC88–104 (10 μM) and stained with SNARF (Molecular Probes, Inc.). After gating based on low red fluorescence and high green fluorescence to distinguish Fluo-4-labeled T cells from SNARF-labeled P13.9 cells and unlabeled T cells, calcium flux was analyzed using a FACSCaliber flow cytometer (BD Biosciences). The response to 5 μM ionomycin exposure was used as a control.
Acknowledgements
We thank Dr. Irena Stefanova for help with the phosphorylation experiments, Drs. Kevin L. Holms, Hai Qi, and Ruth Swofford for assistance with Ca2+ flux experiments, Dr. Keiko Hodohara for use of the cell sorter, and Drs. Mark Davis, J. B. Bolen, Toshio Kitamura, and Shoji Yamaoka for generously providing materials. This work was supported in part by the Intramural Research Program of the NIH, NIAID, as well as the Uehara Memorial Foundation, the Liver Disease Medical Research Foundation, and the Grants-in-Aid of the Japan Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- EGFP
enhanced green fluorescent protein
- ZAP-70
zeta-associated protein 70
References
- 1.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 3.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-α β-transgenic model. J. Exp. Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leitenberg D, Bottomly K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Semin. Immunol. 1999;11:283–292. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signaling pathways by Th1 and Th2 clones. J. Immunol. 1990;144:4110–4120. [PubMed] [Google Scholar]
- 7.Tamura T, Yanagida T, Nariuchi H. Difference in signal transduction pathway for IL-2 and IL-4 production in T helper 1 and T helper 2 cell clones in response to anti-CD3. J. Immunol. 1993;151:6051–6061. [PubMed] [Google Scholar]
- 8.Sloan-Lancaster J, Steinberg TH, Allen PM. Selective loss of the calcium ion signaling pathway in T cells maturing toward a T helper 2 phenotype. J. Immunol. 1997;159:1160–1168. [PubMed] [Google Scholar]
- 9.Tamura T, Nakano H, Nagase H, Morokata T, Igarashi O, Oshimi Y, Miyazaki S, Nariuchi H. Early activation signal transduction pathways of Th1 and Th2 cell clones stimulated with anti-CD3. Roles of protein tyrosine kinases in the signal for IL-2 and IL-4 production. J. Immunol. 1995;155:4692–4701. [PubMed] [Google Scholar]
- 10.Hannier S, Bitegye C, Demotz S. Early events of TCR signaling are distinct in human Th1 and Th2 cells. J. Immunol. 2002;169:1904–1911. doi: 10.4049/jimmunol.169.4.1904. [DOI] [PubMed] [Google Scholar]
- 11.Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and Th2 cells. Immunity. 2001;15:729–738. doi: 10.1016/s1074-7613(01)00223-0. [DOI] [PubMed] [Google Scholar]
- 12.Murphy KM, Ouyang W, Farrar JD, Yang J, Ranganath S, Asnagli H, Afkarian M, Murphy TL. Signaling and transcription in T helper development. Annu. Rev. Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 13.al-Ramadi BK, Nakamura T, Leitenberg D, Bothwell AL. Deficient expression of p56(lck) in Th2 cells leads to partial TCR signaling and a dysregulation in lymphokine mRNA levels. J. Immunol. 1996;157:4751–4761. [PubMed] [Google Scholar]
- 14.Madrenas J, Chau LA, Smith J, Bluestone JA, Germain RN. The efficiency of CD4 recruitment to ligand-engaged TCR controls the agonist/partial agonist properties of peptide-MHC molecule ligands. J. Exp. Med. 1997;185:219–230. doi: 10.1084/jem.185.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: Relationship to the quality of individual TCR signaling events. J. Immunol. 1999;162:2073–2080. [PubMed] [Google Scholar]
- 16.Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J. Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- 17.Madrenas J, Wange RL, Wang JL, Isakov N, Samelson LE, Germain RN. ζ-phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 18.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-ζ and lack of ZAP-70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 19.Stefanova II, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat. Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 20.Rooney JW, Hodge MR, McCaffrey PG, Rao A, Glimcher LH. A common factor regulates both Th1- and Th2-specific cytokine gene expression. EMBO J. 1994;13:625–633. doi: 10.1002/j.1460-2075.1994.tb06300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu. Rev. Immunol. 1994;12:555–592. doi: 10.1146/annurev.iy.12.040194.003011. [DOI] [PubMed] [Google Scholar]
- 22.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 23.Rogge L, Bianchi E, Biffi M, Bono E, Chang SY, Alexander H, Santini C, et al. Transcript imaging of the development of human T helper cells using oligonucleotide arrays. Nat. Genet. 2000;25:96–101. doi: 10.1038/75671. [DOI] [PubMed] [Google Scholar]
- 24.Chtanova T, Kemp RA, Sutherland AP, Ronchese F, Mackay CR. Gene microarrays reveal extensive differential gene expression in both CD4(+) and CD8(+) type 1 and type 2 T cells. J. Immunol. 2001;167:3057–3063. doi: 10.4049/jimmunol.167.6.3057. [DOI] [PubMed] [Google Scholar]
- 25.Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, Assenmacher M, et al. Regulation of expression of IL-4 alleles: Analysis using a chimeric GFP/IL-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- 26.Boyton RJ, Altmann DM. Is selection for TCR affinity a factor in cytokine polarization? Trends Immunol. 2002;23:526–529. doi: 10.1016/s1471-4906(02)02319-0. [DOI] [PubMed] [Google Scholar]
- 27.Viola A, Salio M, Tuosto L, Linkert S, Acuto O, Lanzavecchia A. Quantitative contribution of CD4 and CD8 to T cell antigen receptor serial triggering. J. Exp. Med. 1997;186:1775–1779. doi: 10.1084/jem.186.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lejon K, Fathman CG. Isolation of self antigen-reactive cells from inflamed islets of nonobese diabetic mice using CD4high expression as a marker. J. Immunol. 1999;163:5708–5714. [PubMed] [Google Scholar]
- 29.Madrenas J, Schwartz RH, Germain RN. Interleukin 2 production, not the pattern of early T-cell antigen receptor-dependent tyrosine phosphorylation, controls anergy induction by both agonists and partial agonists. Proc. Natl. Acad. Sci. USA. 1996;93:9736–9741. doi: 10.1073/pnas.93.18.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 31.Sloan-Lancaster J, Evavold BD, Allen PM. Th2 cell clonal anergy as a consequence of partial activation. J. Exp. Med. 1994;180:1195–1205. doi: 10.1084/jem.180.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 33.Seder RA, Paul WE, Davis MM, Fazekas de St., Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J. Exp. Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 35.Ding L, Linsley PS, Huang L-Y, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J. Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 36.Itoh Y, Germain RN. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J. Exp. Med. 1997;186:757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krummel MF, Sjaastad MD, Wulfing C, Davis MM. Differential clustering of CD4 and CD3 ζ during T cell recognition. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]


