Abstract
Purpose
This study assessed the relationship between CNS treatment and psychologic mood using the Profile of Moods State (POMS), a standardized measure of affect, among a large sample of young adult survivors of childhood acute lymphoblastic leukemia (ALL; N = 555).
Patients and Methods
Survivors of childhood ALL (ages 18 to 33 years at study entry) participated in a structured telephone interview eliciting demographic, health, and behavioral data and the POMS. Treatment data included total dose of CNS irradiation (CRT) and intrathecal methotrexate (MTX) obtained from medical records.
Results
Mood disturbance was reported by 24% of survivors. High-dose CRT and MTX predicted disturbance rates modestly and primarily in combination with education variables. Interactions between educational achievement, a history of attendance in special education classes, and sex were better predictors than treatment type or dose. Non-white males, those younger than 12.5 years of age at diagnosis, and those with negative perceptions of current health and cancer’s impact on employment were also at greater risk for mood disturbance (P < .01 to .001).
Conclusion
Although most survivors are doing well psychologically, a subset of long-term survivors show potentially serious mood disturbance. Mood disturbance seems to be a function of interactions between preexisting individual difference variables (eg, sex, race/ethnicity), treatment factors, and posttreatment educational experiences. Prevention strategies aimed at childhood cancer survivors at greatest risk for mood disturbance may be improved by focus on posttreatment psychosocial and educational supports.
RESEARCH ON survivors of childhood acute lymphoblastic leukemia (ALL) suggests that as expected survival time after treatment for ALL increases, the risk of neuropsychologic and psychosocial impairments in functioning may increase.1,2 Although many survivors of childhood ALL develop into well-adjusted adults, a subset of survivors experience difficulties with mood, school or work adjustment, and cognitive functioning.3–8 CNS treatment for ALL may serve as one risk factor that helps identify this subset of survivors who are at risk for long-term adjustment difficulties.
Compared with other treatments, high-dose cranial radiation treatment (CRT) has been linked to lower intelligence quotient levels,9 poor academic performance,10 an increase in learning problems in school,11 and a reduced likelihood of attending college as survivors become young adults.12 Even low-dose CRT has been associated with mild delayed intelligence quotient decline, increased learning disabilities, and academic failure.13–15 As concerns about the excessive toxicity of CNS radiation emerged, treatment protocols were shifted to include increasing doses of intrathecal (IT) methotrexate (MTX) chemotherapy and less CRT. These less toxic treatment protocols have sometimes8,15 but not always resulted in reduced negative cognitive or psychologic outcomes.16–18 Variation in dose of MTX and combinations of CNS radiation with MTX may be important factors in the equivocal data across studies.
The relative benefit of MTX compared with CRT may also depend on demographic variables, such as age and sex. Treatment with CNS radiation at a young age may place survivors at greatest risk for neuropsychologic impairment.2,13,14 However, poorer psychologic adjustment in patients who are older at diagnosis have also been reported.3,19 Sex effects are also equivocal. Some studies suggest that female patients are at greater risk for cognitive deficits after treatment than male patients,1,20,21 but the reverse has also been shown.15
The present study sought to use multivariate analyses to explore the relative role of CRT and MTX dosage and demographic variables on mood state in a large sample of young adult survivors of childhood ALL. High cumulative dose (CRT or MTX) was predicted to be associated with greater disturbance in mood. Age at diagnosis and sex were hypothesized to further impact psychologic outcomes.
PATIENTS AND METHODS
Childhood ALL survivors were recruited from 23 institutions participating in the Children’s Cancer Group (CCG) project. Of 731 survivors, 593 completed the study interview and met all eligibility criteria: a diagnosis of ALL between 1970 and 1986, treatment on a CCG protocol before the age of 20 years, age at least 18 years at interview, survival for at least 2 years after diagnosis, in remission, and not receiving antileukemia treatment at study entry. Missing data resulted in 555 survivors available for the current analyses. A sibling control group was also assessed, and data on that cohort are provided elsewhere.22
Each participant was mailed a consent form approved by each institution’s Human Subjects Protection Committee. After receipt of signed consents, a telephone interview was arranged with each patient. The interview followed a structured format and was conducted by trained interviewers. Questions were asked about education, marital status, employment status, health, fertility, offspring, and risk behaviors. There were two questions on the interview that asked about survivors’ perceptions of the impact of treatment on their employment (ability to get a job) and their education. These responses were analyzed to determine the effects of survivors’ own perceptions of the impact of their leukemia treatment on current emotional state. At the end of the interview, the Profile of Mood States (POMS) and a self-esteem measure were administered by telephone. Patients’ age at the time of the interview was also noted. The age at which subjects were diagnosed with ALL, the type and amount of treatment received (CRT, MTX, other), and other pertinent medical data were obtained from survivors’ medical records.
POMS
The POMS23 is a 65-item self-report questionnaire designed to measure six identifiable mood states (tension/anxiety, depression, anger, confusion, vigor, fatigue) with demonstrated reliability and validity. High scores on the vigor subscale indicate persons with high energy, whereas high scores on the fatigue subscale suggest persons with low energy. Patients were asked to describe the extent to which the adjectives describe the way they had been feeling during the past week, on a scale ranging from 0 (not at all) to 4 (extremely). A total score is derived by summing each of the six subscales, with vigor weighted negatively. The possible range of scores is −40 through 192. Higher scores indicate greater mood disturbance. Although the POMS is a self-report measure of affect at one point in time (ie, state v trait), it has been shown to be useful for assessing psychiatric outpatients and for documenting patient responses to psychotherapy.23 The POMS has also been used among a variety of cancer patient samples and their families.24,25
Defining Treatment Groups
Survivors were grouped by the type of treatment received. Treatment type/dose followed CCG protocols in place at the time of diagnosis. Evaluation of the IT chemotherapy received by the survivors demonstrated that the majority of survivors had received MTX (n = 490; 85%). Only 50 survivors received IT cytarabine. Only 50 survivors received intravenous MTX. Thus MTX was defined as the unit of analysis for CNS chemotherapy effects on outcome. We defined Low MTX as a total cumulative dose of less than 83 mg (based on a median split) and High MTX as a total cumulative dose of ≥ 83 mg. This same median split categorization was used in a previous analysis of impact of treatment on educational outcomes in the same survivor sample.12
Total CNS irradiation dose ranged from 2 to 40 Gy. However, the doses were not evenly distributed, because the CCG ALL treatment protocols included either a relatively low dose (18 Gy) or a high dose (24 Gy) or no irradiation. To maximize statistical power by collapsing groups and thereby increase the number of patients in each group, survivors who received less than 21 Gy were categorized as receiving Low CNS irradiation, whereas those who received ≥ 21 Gy were categorized as receiving High CNS irradiation. Although the dosage range seemed wide, most patients who received CNS irradiation did receive at or close to 18 or 24 Gy. Those who received no CNS irradiation were categorized as No (none).
Because there was an inverse relationship between total dose of CNS irradiation and total dose of MTX, treatment groups were next categorized in relation to both variables to examine the effects of different combinations of treatment. For the final analyses, survivors were categorized into four treatment groups: (1) No or Low MTX (< 83 mg) and CRT (< 21 Gy), (2) High MTX (> 83 mg) and No or Low CRT, (3) High CRT (> 21 Gy) and No or Low MTX, and (4) High CRT and MTX.
Analyses
As recommended,23 a total mood disturbance score was used in analyses to reflect overall affective state. Unreported analyses showed similar results across all subscales. Previous analyses22 indicated no difficulties with skewness, outliers, and influential observations, and the total POMS score is well described by a normal distribution.
A subset of survivors at risk for mood disturbance was identified based on a review of distributions in previous cancer studies using the POMS and suggestions by Van’t Spijker et al.26 The survivor sample was split at a total POMS score of 33. Those above 33 (approximately 24% of the total sample) were labeled as mood-disturbed and those below were labeled as healthy. This score is nearly two times higher than the mean of the total POMS score reported for other cancer survivors.24,25 Scores above 33 are therefore out of the normal range for cancer survivors and likely to be clinically significant.
Backward elimination logistic regression techniques were used to identify the survivor characteristics associated with greater likelihood of mood disturbance (total POMS score > 33). The following predictors were tested: treatment group, age at diagnosis, age at interview, evidence of relapse (yes/no), sex, race/ethnicity, marital status, education level, employment status, perceptions of current health (excellent/good/poor), and the yes/no response to two questions about whether the cancer experience had impacted abilities at school or at work. Exploratory classification tree analysis methods27 were used to select a sample split cutoff for age at diagnosis. Analyses revealed the greatest predictive value for 12.5 years of age at diagnosis (young = < 12.5 years; older = > 12.5 years). All two-way interactions were tested during the step-wise variable selection. For the final multivariate model, nonsignificant variables were dropped except those retained as covariates: mother’s highest education (a proxy for socioeconomic status), relapse history (a proxy for disease severity), and age at interview (a potential predictor and proxy for historical changes in treatment over time).
RESULTS
Historical Changes in Treatment
Review of CCG studies conducted during the 16-year time span indicated that the major change was a shift from higher total doses of CNS irradiation (24 Gy) during the earlier years toward lower total doses (18 Gy or none), with the addition of higher doses of MTX (> 83 mg) during the later years. Timing of treatment was considered early if it took place during the first 8 years (1970 to 1977; n = 322), whereas later treatment took place from 1978 to 1986 (n = 213; Table 1). These data reflect the overall changes in treatment to less or no CNS irradiation and, to compensate, higher amounts of MTX.
Table 1.
Changes in Treatment Over Time
| % of Patients
|
||
|---|---|---|
| 1970–1977 (n = 322) | 1978–1986 (n = 213) | |
| CNS irradiation | ||
| None | 6.0 | 12.6 |
| 18 Gy | 18.8 | 82.7 |
| 24 Gy | 75.2 | 4.7 |
| IT-MTX | ||
| None | 27.5 | 0.0 |
| < 83 mg | 50 | 29.4 |
| ≥ 83 mg | 22.5 | 70.6 |
NOTE. The percentage of survivors receiving high-dose CNS irradiation decreased, whereas the percentage receiving high-dose methotrexate increased.
Abbreviation: IT-MTX, intrathecal methotrexate.
Univariate Analyses
Treatment variables.
Descriptive statistics for the POMS total score for each treatment group (examined across groups using analysis of variance) indicate groups were not significantly different. When percentage of mood disturbed was examined across all treatment groups using χ2 methods, there were also no significant effects. Nonetheless, a two-group comparison provided evidence of a greater percentage of mood disturbance among survivors given High CRT (28.5%) compared with High MTX (20.3%; χ2[366] = 3.32; P = .05; Table 2).
Table 2.
Descriptive Statistics for the POMS Total Score and Percentage Mood Disturbed As a Function of Treatment Group
| No or Low CRT and MTX | High MTX | High CRT | High CRT and MTX | Total | |
|---|---|---|---|---|---|
| Total No. of patients in treatment group | 131 | 187 | 179 | 58 | 555 |
| Distressed | |||||
| No. of patients | 32 | 38 | 51 | 12 | 133 |
| % | 24.1 | 20.3 | 28.5 | 20.7 | 24.0 |
| Total POMS Score | |||||
| Mean | 16.9 | 15.7 | 20.0 | 16.2 | 17.4 |
| SD | 32.3 | 24.7 | 27.6 | 29.0 | 28.0 |
Abbreviations: POMS, Profile of Moods State; CRT, CNS irradiation; MTX, methotrexate; SD, standard deviation.
There was a significantly greater proportion of mood-disturbed survivors diagnosed when younger than 12.5 years of age (29.5%) than those diagnosed at an older age (15.7%; P < .001). Only 25 patients (4.4%) had evidence in medical records of prior relapse at the time of the interview, and this variable was not associated with mood disturbance.
Demographic predictors.
Racial and ethnic minorities showed more mood disturbance (34.3%) than white patients (22.5%; P < .03). Those with a history of special education were more likely to be mood disturbed (42.9%) than those without such a history (22.1%; P < .001).
Subjective perceptions.
Only 16.8% of survivors reporting excellent health were mood disturbed, compared with 25.4% of those reporting good health and 51.1% reporting fair or poor health (P < .001). Also, disturbance rates were 40.4% among survivors who said cancer limited their ability to work, compared with 20.6% among those who said it had not (P < .001).
Multivariate Analyses
Variables in the final model are listed in Table 3. Nonsignificant main effect terms accompanied by a significant interaction (eg, sex) were retained in the model but are not shown in Table 4 for simplicity of viewing.
Table 3.
Logistic Regression Model for Prediction of Mood Disturbance
| Raw Proportion
|
||||||
|---|---|---|---|---|---|---|
| Treatment Variable | No. of Patients | % | Model Risk (%) | Model Relative Odds | 95% CI | P |
| Age at diagnosis, years | ||||||
| Older than 12.5 | 35/223 | 15.7 | 18.3 | 1.0 | ||
| Younger than 12.5 | 98/332 | 29.5 | 45.2 | 3.7 | 2.0 to 6.6 | < .001 |
| Treatment | ||||||
| No-low | 32/131 | 24.1 | 36.3 | 1.0 | ||
| High MTX | 38/187 | 20.3 | 19.6 | 0.4 | 0.2 to 1.2 | .11 |
| High CRT | 51/179 | 28.5 | 20.1 | 0.4 | 0.2 to 1.2 | .10 |
| High both | 12/58 | 20.7 | 49.4 | 1.7 | 0.4 to 6.8 | .45 |
| Treatment X, highest education | ||||||
| Some college | ||||||
| No-low | 22/73 | 30.1 | 28.9 | 1.0 | ||
| High MTX | 21/105 | 20.0 | 20.7 | 0.6 | 0.3 to 1.4 | .26 |
| High CRT | 14/70 | 20.0 | 13.2 | 0.4 | 0.2 to 0.9 | .02 |
| High both | 6/28 | 21.4 | 21.1 | 0.7 | 0.2 to 2.0 | .46 |
| High school graduate | ||||||
| No-low | 7/50 | 14.0 | 18.4 | 1.0 | ||
| High MTX | 15/68 | 22.0 | 37.3 | 2.6 | 0.9 to 7.9 | .09 |
| High CRT | 34/98 | 34.7 | 35.7 | 2.5 | 0.9 to 6.5 | .07 |
| High both | 5/28 | 17.9 | 29.2 | 1.8 | 0.5 to 7.2 | .39 |
| Dropout | ||||||
| No-low | 3/8 | 37.5 | 67.0 | 1.0 | ||
| High MTX | 2/14 | 14.3 | 8.5 | 0.1 | 0.0 to 0.8 | .03 |
| High CRT | 3/11 | 27.3 | 15.7 | 0.1 | 0.0 to 1.3 | .07 |
| High both | 1/2 | 50.0 | 89.3 | 4.1 | 0.1 to 171 | .47 |
| Demographics | ||||||
| Highest education | ||||||
| Some college | 63/276 | 22.8 | 20.4 | 1.0 | ||
| High school graduate | 61/244 | 25.0 | 29.5 | 1.63 | 0.6 to 4.8 | .37 |
| Dropout | 9/35 | 25.7 | 42.4 | 2.87 | 0.6 to 13.1 | .17 |
| Ethnicity | ||||||
| White | 110/448 | 24.6 | 24.8 | 1.00 | ||
| Minority | 23/67 | 34.3 | 35.8 | 1.7 | 0.1 to 3.2 | .01 |
| Sex X, ethnicity | ||||||
| White | ||||||
| Male | 45/244 | 18.4 | 15.9 | 1.00 | ||
| Female | 65/244 | 26.6 | 36.7 | 3.1 | 1.4 to 6.8 | .006 |
| Nonwhite | ||||||
| Male | 13/34 | 38.2 | 41.6 | 3.8 | 1.6 to 9.2 | .004 |
| Female | 10/33 | 30.3 | 30.3 | 2.3 | 0.7 to 7.2 | .15 |
| Sex X, special education | ||||||
| Male | ||||||
| No special education | 51/253 | 20.2 | 26.3 | 1.00 | ||
| Yes special education | 7/25 | 28.0 | 27.4 | 1.1 | 0.3 to 4.1 | .94 |
| Female | ||||||
| No special education | 61/253 | 24.1 | 17.0 | 1.00 | ||
| Yes special education | 14/24 | 58.3 | 55.2 | 6.0 | 1.8 to 20.0 | .003 |
| Special education X, highest education | ||||||
| Some college | ||||||
| No special education | 61/266 | 22.9 | 35.8 | 1.0 | ||
| Yes special education | 2/10 | 20.0 | 10.5 | 0.2 | 0.0 to 1.4 | .11 |
| High school graduate | ||||||
| No special education | 48/216 | 22.2 | 25.1 | 1.00 | ||
| Yes special education | 13/28 | 46.4 | 34.4 | 1.6 | 0.6 to 4.0 | .35 |
| Dropout | ||||||
| No special education | 3/24 | 12.5 | 9.6 | 1.00 | ||
| Yes special education | 6/11 | 54.5 | 83.6 | 48.2 | 5.1 to 457 | < .001 |
| Perceptions | ||||||
| Has cancer limited your ability to work? | ||||||
| No | 96/466 | 20.6 | 19.2 | 1.0 | ||
| Yes | 37/89 | 40.4 | 43.6 | 3.3 | 1.8 to 5.8 | < .001 |
| Self-reported health | ||||||
| Excellent | 38/226 | 16.8 | 17.7 | 1.00 | ||
| Good | 72/284 | 25.4 | 26.4 | 1.71 | 0 to 2.7 | .04 |
| Poor | 23/45 | 51.1 | 50.6 | 4.7 | 2.2 to 10.5 | < .001 |
NOTE. Model retains nonsignificant covariates: age at interview, relapse (yes or no), and mother’s highest level of education.
Abbreviations: MTX, methotrexate; CRT, CNS irradiation.
Treatment variables.
Treatment alone was not a significant predictor of adult mood disturbance, but interacted with highest education to increase risk. As shown in Figure 1, college-educated survivors who had received High CRT were at less risk for mood disturbance (13.2%) than those treated with No-Low doses (28.9%; P = .02). Similarly, dropouts treated with either High MTX (risk estimate, 8.5%) or High CRT (risk estimate, 15.7%) were protected from risk of mood disturbance (P = .03 and P = .07, respectively) as compared with survivors treated with No-Low (risk estimate, 67%) or High Both (MTX and CRT; 89.3%). Among survivors with only a high school education, treatment effects were not significant.
Fig 1.
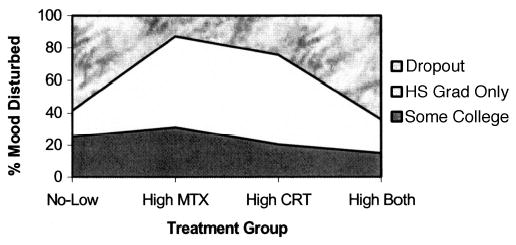
Risk of mood disturbance as a function of treatment and highest education achieved. MTX, methotrexate; CRT, cranial radiation treatment; hs, high school, grad, graduate.
Being a preadolescent at diagnosis increased odds of adult mood disturbance by 3.7 compared with postadolescence, even after controlling for other predictors and covariates in the final model (P < .001).
Demographic predictors.
Highest education, although not a significant predictor of mood disturbance alone, interacted with treatment and with a history of special education, as seen in Table 3 and illustrated in Fig 2. Whereas special education history increased risk among dropouts (risk estimate with [83.6%] v without special education [9.6%]; P < .001]), there was a trend for special education history to reduce risk of mood disturbance among college attendees (P = .11). Among survivors with only a high school education, a special education history did not influence risk of mood disturbance. The impact of special education history was also modulated by sex. Among males, having attended special education classes did not increase mood disturbance rates compared with males without that history. In contrast, females with a history of special education were six times more likely to be mood disturbed than females without such a history (P = .003).
Fig 2.
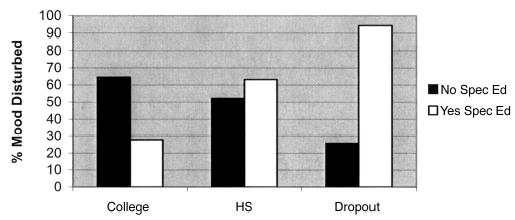
Risk of mood disturbance as a function of special education history and highest education achieved. HS, high school; spec ed, special education.
Sex also interacted with race and ethnicity. Female mood disturbance rates were not influenced by race/ethnicity; both white and nonwhite female patients showed greater risk than male patients. In contrast, male minorities had an almost fourfold increase in risk compared with white male patients (P = .004). Age at interview, relapse (yes or no), and mother’s highest level of education were not significantly predictive in the multivariate model but were retained as covariates. Marital and employment status were not significantly associated with mood disturbance and were dropped.
Subjective perceptions.
Survivors who reported fair or poor health remained at greater risk for mood disturbance in the multivariate model compared with those reporting good or excellent health (P = .04 and P < .001, respectively). Also, survivors who said cancer had impacted their ability to work were at increased risk for mood disturbance compared with those who said cancer had not influenced work (P < .001).
Model Fit
The model accounts for modest variance in mood disturbance rates (R2 = 24.2%), but correctly classifies 71% of mood-disturbed and 71% of healthy patients in the sample and fits the data well (mean deviance of 1 and a Hosmer and Lemeshow Goodness of Fit P value > .30). Cross-validation of randomly split halves shows the model has generalizability; outcomes remain similar for independent samples of data. Results were unaffected by the order of regressor elimination.
DISCUSSION
This, the largest study of adult survivors of childhood ALL, found that nearly one quarter of patients (24%) reported high levels of mood disturbance (a POMS score of nearly twice the mean of POMS scores from other cancer survivor studies). Survivors treated with High CRT had a greater percentage of mood disturbance than survivors treated with High MTX, No-Low MTX, or No-Low CRT. However, multivariate analyses indicated treatment group was not a direct mediator of adult mood status. Instead, treatment acted to increase or decrease risk rates depending on the survivor’s educational achievement. In contrast, being a preadolescent at diagnosis was a strong, direct predictor of mood disturbance. Female patients, minority (non-white) male patients, female patients with a special education history, and high-school dropouts with a special education history were also at significantly greater risk.
Being a preadolescent at diagnosis increased risk for adult mood disturbance almost four-fold, and this effect was not modulated by educational achievement or treatment dose. These data are in keeping with studies indicating that a young age at diagnosis is predictive of lower school achievement8 and more negative life outlook28 but stand in contrast to others showing no effect of age at diagnosis on coping and social functioning5,29 or a reverse effect (general psychologic functioning worse for survivors who were older at diagnosis).19 Equivocal findings may be due to the cross-study variations in survivor age at assessment. The impact of age at diagnosis on psychologic problems may change as survivors move from late adolescence to adulthood and face new life challenges. Developmental issues are further implicated by the mood disturbance rate here (24%), which replicates the extent of psychologic problems found in recent studies of young adult survivors of childhood cancer29–31 but stand in contrast to those combining teens and young adults.8,28,32
Survivors with past attendance in special education classes were more than twice as likely to show mood disturbance, and this risk increased six-fold among female patients. Assuming special education history is a proxy for neurocognitive deficits, these data suggest that female patients are not only at greater risk for cognitive deficits after pediatric cancer treatment,9,20,21 but such deficits may also increase their risk for mood disturbance. Dropouts with a special education history were also at high risk for mood disturbance.
A heightened risk for mood disturbance among No or Low-dose treatment groups led to surprising protective effects of high-dose treatment in interaction with education variables. For example, high-dose treatment and a special education history among college attendees significantly reduced risk relative to No-Low doses. Also, survivors who attended college or who were high school dropouts were less likely to have mood disturbance if treated with High CRT than if treated with No-Low doses. High MTX likewise reduced risk for mood disturbance among high school dropouts compared with No-Low doses. This protective effect of high-dose treatment did not occur among high school graduates; both High CRT and High MTX were risk factors for mood disturbance compared with No-Low doses. These surprising findings may be data anomalies related to small sample size in subgroups. Or, perhaps survivors who overcome the disadvantage of high-dose treatment or learning problems and go to college despite these handicaps may be particularly resilient psychologically. In support, survivor scores on a measure of self-esteem were highly correlated with total POMS scores (r = −0.54; P = .01) and were higher among survivors who attended college than for those who did not attend college (P = .01).33
Although being female or nonwhite increased risk for mood disturbance, nonwhite male patients were at comparatively greatest risk. This sex by race or ethnicity effect is unique to the present study because of the statistical power achieved here by the large sample. The associations between mood disturbance and negative perceptions of current health and cancer’s impact on ability to work are in keeping with previous evidence that subjective perceptions of the cancer experience and its impact play a significant role in psychologic late effects.8,28,30 However, without prospective data, it is not possible to determine whether such perceptions are underlying mediators of treatment and education interactions, direct causal factors in mood disturbance, or a consequence of current mood state.
The present study has numerous advantages over previous research. The sample is homogeneous for cancer type (ALL) and developmental stage (young adults), representative of many communities (multisite), and of sufficient sample size to stratify/statistically control for multiple variables. Given these strengths, the conclusion about the relative value of treatment severity in predicting mood disturbance is unequivocal. Objective measures of treatment severity offer some prognostic value for identifying at-risk groups but are overshadowed by other person and situation variables. Just as trauma severity is limited in predicting those at risk for posttraumatic stress disorder,30,34–37 treatment severity is limited as a predictor of mood disturbance.
Other conclusions are less definitive as a result of several methodologic limitations. First, POMS elevations are not synonymous with the presence of a mental disorder. Also, the cutoff score used here was chosen to represent a level of mood disturbance that is likely to be functionally significant, but further research on the validity of the POMS for predicting functional health is needed. Second, establishing that a relationship exists between long-term mood disturbance and treatment, demographic, and perceptual variables does not elucidate the mechanisms of such associations. For example, increased mood disturbance in those younger at diagnosis may simply reflect longer exposure to parental anxiety generated by the cancer; high rates of posttraumatic stress disorder have been found among mothers of children who have survived cancer.34,31,38 Third, recent studies of coping, social relationships, and self-image8,28,29,32 confirm that there are multiple aspects to psychologic health after cancer. Future studies of psychologic well-being in pediatric cancer survivors may be greatly enhanced by data reflecting multiple time points, informants (eg, parents, spouses/partners, doctors, teachers, and friends), and levels of analyses (eg, psychophysiologic and behavioral).
In summary, one quarter of this large sample of young adult survivors of childhood ALL reported mood disturbance, but CRT and MTX dose were not direct risk factors alone. With the exception of nonwhite male ethnicity/sex and preadolescent age at diagnosis, treatment and demographic risk factors occurred only in conjunction with postdiagnosis educational experiences that are potentially amenable to intervention. These findings hold out hope that greater educational support provided during and after pediatric cancer treatment may offset the potentially negative sequelae of high-dose regimens.
Appendix
Acknowledgments
Dorie A. Glover, PhD, thanks the University of California at Los Angeles Jonsson Comprehensive Cancer Center and the American Cancer Society for postdoctoral support during the writing of this manuscript and Jonathan Bergman for assistance in literature reviews.
The acknowledgment and appendix are included in the full-text version of this article, available on-line at www.jco.org. They are not included in the PDF (via Adobe® Acrobat Reader®) version.
Footnotes
Supported by grants from the Division of Cancer Treatment, National Cancer Institute, and the National Institute of Child Health and Development, National Institutes of Health, Department of Health and Human Services, Bethesda, MD. Contributing Children’s Cancer Group investigators, institutions, and grant numbers are given in the Appendix.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The authors indicated no potential conflicts of interest.
References
- 1.Brown RT, Madan-Swain A, Walco GA, et al. Cognitive and academic late effects among children previously treated for acute lymphocytic leukemia receiving chemotherapy as CNS prophylaxis. J Pediatr Psychol. 1998;23:333–340. doi: 10.1093/jpepsy/23.5.333. [DOI] [PubMed] [Google Scholar]
- 2.Maidan-Swain A, Brown RT. Cognitive and psychosocial sequelae for children with acute lymphocytic leukemia and their families. Clin Psychol Rev. 1991;11:267–294. [Google Scholar]
- 3.Koocher GP, O’Malley JE, Gogan JL, et al. Psychological adjustment among pediatric cancer survivors. J Child Psychol Psychiatry. 1980;21:163–173. doi: 10.1111/j.1469-7610.1980.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 4.Koocher GP, O’Malley JE: The Damocles Syndrome. New York, NY, McGraw-Hill, 1981
- 5.Mackie E, Hill J, Kondryn H, et al. Adult psychosocial outcomes in long-term survivors of acute lymphoblastic leukaemia and Wilms’ tumour: A controlled study. Lancet. 2000;355:1310–1314. doi: 10.1016/S0140-6736(00)02112-7. [DOI] [PubMed] [Google Scholar]
- 6.Gray RE, Doan BD, Shermer P, et al. Psychologic adaptation of survivors of childhood cancer. Cancer. 1992;70:2713–2721. doi: 10.1002/1097-0142(19921201)70:11<2713::aid-cncr2820701124>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Bauld C, Anderson V, Arnold J. Psychosocial aspects of adolescent cancer survival. J Paediatr Child Health. 1998;34:120–126. doi: 10.1046/j.1440-1754.1998.00185.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill JM, Kornblith AB, Jones D, et al. A comparative study of the long term psychosocial functioning of childhood acute lymphoblastic leukemia survivors treated by intrathecal methotrexate with or without cranial radiation. Cancer. 1998;82:208–218. [PubMed] [Google Scholar]
- 9.Meadows AT, Gordon J, Massari DJ, et al. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukemia treated with cranial irradiation. Lancet. 1981;2:1015–1018. doi: 10.1016/s0140-6736(81)91216-2. [DOI] [PubMed] [Google Scholar]
- 10.Peckham VC, Meadows AT, Bartel N, et al. Educational late effects in long-term survivors of childhood acute lymphocytic leukemia. Pediatrics. 1988;81:127–133. [PubMed] [Google Scholar]
- 11.Van der Does-Van den Berg A, de Vaan GAM, Van Weerden JF, et al. Late effects among long-term survivors of childhood acute leukemia in the Netherlands: A Dutch Childhood Leukemia Study Group report. Pediatr Res. 1995;38:802–807. doi: 10.1203/00006450-199511000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Haupt R, Fears TR, Robison LL, et al. Educational attainment in long-term survivors of childhood acute lymphoblastic leukemia. JAMA. 1994;272:1427–1432. [PubMed] [Google Scholar]
- 13.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 14.Mulhern RK: Neuropsychological late effects, in Bearison DJ, Mulhern RK (eds): Pediatric Psychooncology. New York, NY, Oxford University Press, 1994, pp 99–121
- 15.Langer T, Martus P, Ottensmeier H, et al. CNS late-effects after ALL therapy in childhood: Part III. Neuropsychological performance in long-term survivors of childhood ALL—Impairments of concentration, attention, and memory. Med Pediatr Oncol. 2002;38:320–328. doi: 10.1002/mpo.10055. [DOI] [PubMed] [Google Scholar]
- 16.Mulhern RK, Wasserman AL, Friedman AG, et al. Social competence and behavioral adjustment of children who are long-term survivors of cancer. Pediatrics. 1989;83:18–25. [PubMed] [Google Scholar]
- 17.Mulhern RK, Fairclough D, Ochs J. A prospective comparison of neuropsychologic performance of children surviving leukemia who received 18-Gy, 24-Gy, or no cranial irradiation. J Clin Oncol. 1991;9:1348–1356. doi: 10.1200/JCO.1991.9.8.1348. [DOI] [PubMed] [Google Scholar]
- 18.Copeland DR, Donell RE, Fletcher JM, et al. Neuropsychological test performance of pediatric cancer patients at diagnosis and one year later. J Pediatr Psychol. 1985;13:183–196. doi: 10.1093/jpepsy/13.2.183. [DOI] [PubMed] [Google Scholar]
- 19.Elkin TD, Phipps S, Mulhern RK, et al. Psychological functioning of adolescent and young adult survivors of pediatric malignancy. Med Pediatr Oncol. 1997;29:582–588. doi: 10.1002/(sici)1096-911x(199712)29:6<582::aid-mpo13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: Cranial radiation requires an accomplice. J Clin Oncol. 1995;13:2490–2496. doi: 10.1200/JCO.1995.13.10.2490. [DOI] [PubMed] [Google Scholar]
- 21.Waber DP, Urion DK, Tarbell NJ, et al. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent. Dev Med Child Neurol. 1990;32:238–248. doi: 10.1111/j.1469-8749.1990.tb16930.x. [DOI] [PubMed] [Google Scholar]
- 22.Zeltzer LK, Chen E, Weiss R, et al. A comparison of psychological outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: A cooperative Children’s Cancer Group and National Institutes of Health Study. J Clin Oncol. 1997;15:547–556. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]
- 23.McNair DM, Lorr M, Droppleman LF: Profile of Mood States. San Diego, CA, Educational and Industrial Testing Service, 1971
- 24.Cassileth BR, Lusk EJ, Brown LL, et al. Psychosocial status of cancer patients and next of kin: Normative data from the Profile of Mood States. J Psychsocial Oncol. 1986;3:99–105. [Google Scholar]
- 25.Ganz PA, Coscarelli A, Fred C, et al. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 26.Van’t Spijker A, Trijsburg RW, Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: A meta-analytic review of 58 studies after 1980. Psychosom Med. 1997;59:280–293. doi: 10.1097/00006842-199705000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Breiman L, Friedman JH, Olshen RA, et al: Classification and Regression Trees. Belmont, CA, Wadsworth International Group, 1984
- 28.Zebrack BJ, Chesler M. Health-related worries, self-image and life outlooks of long-term survivors of childhood cancer. Health Soc Work. 2001;26:245–256. doi: 10.1093/hsw/26.4.245. [DOI] [PubMed] [Google Scholar]
- 29.Boman K, Bodegard G. Long-term coping in childhood cancer survivors: Influence of illness, treatment and demographic background factors. Acta Paediatr. 2000;89:105–111. doi: 10.1080/080352500750029167. [DOI] [PubMed] [Google Scholar]
- 30.Hobbie WL, Stuber M, Meeske K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J Clin Oncol. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- 31.Kazak AE. Posttraumatic distress in childhood cancer survivors and their parents. Med Pediatr Oncol. 1998;60–68(suppl) doi: 10.1002/(sici)1096-911x(1998)30:1+<60::aid-mpo9>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Evans SE, Radford M. Current life-style of young adults treated for cancer in childhood. Arch Dis Child. 1995;72:423–426. doi: 10.1136/adc.72.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitzman RL, Glover DA, Meadows AT, et al: Self-concept in adult survivors of childhood acute lymphoblastic leukemia. Med Pediatr Oncol (in press) [DOI] [PubMed]
- 34.Stuber ML, Kazak AE, Meeske K, et al. Predictors of posttraumatic stress symptoms in childhood cancer survivors. Pediatrics. 1997;100:958–964. doi: 10.1542/peds.100.6.958. [DOI] [PubMed] [Google Scholar]
- 35.Ehlers A, Mayou RA, Bryant B. Cognitive predictors of posttraumatic stress disorder in children: Results of a prospective longitudinal study. Behav Res Ther. 2003;41:1–10. doi: 10.1016/s0005-7967(01)00126-7. [DOI] [PubMed] [Google Scholar]
- 36.Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: Relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev Psychopathol. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- 37.Breslau N. The epidemiology of posttraumatic stress disorder: What is the extent of the problem? J Clin Psychiatry. 2001;62(suppl):16–22. [PubMed] [Google Scholar]
- 38.Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27:805–819. doi: 10.1016/s0306-4530(01)00081-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


