Summary
In humans and experimental animals, damage to the hippocampus or related medial temporal lobe structures severely impairs the formation of new memory but typically spares very remote memory. Questions remain about the importance of these structures for the storage and retrieval of remote autobiographical memory. We carried out a detailed volumetric analysis of structural brain images from eight memory-impaired patients. Five of the patients had damage limited mainly to the medial temporal lobe. These patients performed normally on tests of remote autobiographical memory. Three patients had medial temporal lobe damage plus significant additional damage to neocortex, and these patients were severely impaired. These findings account for previously reported differences in the recollective ability of memory-impaired patients and demonstrate that the ability to recollect remote autobiographical events depends not on the medial temporal lobe but on widely distributed neocortical areas, especially the frontal, lateral temporal, and occipital lobes.
Introduction
In both humans and experimental animals, damage to the hippocampus or related medial temporal lobe structures typically impairs recent memory but spares remote memory (Squire et al., 2004). This pattern of memory loss, termed temporally graded retrograde amnesia, has usually been interpreted to mean that medial temporal lobe structures become less important for memory storage and retrieval as time passes after learning.
Within this framework, there is disagreement about the status of autobiographical memory. Autobiographical memory refers to memory for unique personal experiences that are specific to a particular time and place. According to one view, autobiographical memories, like other kinds of memory, gradually become independent of the medial temporal lobe as time passes (McClelland et al., 1995; Squire and Alvarez, 1995). A different view states that autobiographical memory depends on specific contextual information and always requires the hippocampus and related structures. Accordingly, autobiographical memory remains dependent on the medial temporal lobe for as long as the memory persists (Fujii et al., 2000; Rosenbaum et al., 2001).
Strong evidence for the first view has been provided by a recent study (Bayley et al., 2003) in which six patients with damage limited primarily to the hippocampal region and two other patients with more extensive damage to the medial temporal lobe successfully recollected remote autobiographical memories. The memories of the patients were indistinguishable from the memories of 25 controls with respect to the number of details recalled, the number of prompts needed to begin a narrative, and the duration of the narratives. These results were also in agreement with earlier studies, using less sensitive methods, that had found remote autobiographical memory to be intact after damage limited to the medial temporal lobe (Zola-Morgan et al., 1986; MacKinnon and Squire, 1989; Rempel-Clower et al., 1996; Reed and Squire, 1998; Kapur and Brooks, 1999). Evidence for the second view comes from patients who are deficient at recalling autobiographical episodes even from early life and whose impairments have been attributed to medial temporal lobe damage (Hirano and Noguchi, 1998; Nadel et al., 2000; Cipolotti et al., 2001).
Two important issues merit further consideration. The first concerns the locus and extent of neuropathology in the patients under study. What is the nature of the damage in patients who can successfully recollect autobiographical memories, compared to patients who cannot recollect autobiographical memories? It has been difficult to make this comparison, because findings are frequently reported from single cases, and often only limited anatomical information is available. The second issue concerns the quality of autobiographical recollections that are produced by the patients being studied. Even if the recollections of patients and controls appear similar according to certain quantitative measures, the recollections might differ in other ways. For example, one patient (Y.K.) was reported to have some knowledge of remote incidents in his life but was unable to “remember” them (Hirano and Noguchi, 1998; Hirano et al., 2002). Using the Remember and Know procedure (Tulving, 1985), Y.K. assigned K responses to all of his remote recollections, indicating that he had knowledge of the events as facts but could not actually place himself mentally at the scenes where the events occurred. In contrast, the recollections produced by controls were mostly assigned R responses. That is, controls indicated that they had a sense of being able to reexperience the events that they recalled. This report highlights the need for systematic study of the quality of autobiographical recollections.
We addressed these issues in two experiments. In experiment 1, a detailed volumetric analysis was performed on the magnetic resonance images from memory-impaired patients and controls. Five of the patients could recollect remote autobiographical memories as successfully as their controls, but three other patients were strikingly impaired. Volume estimates were obtained for the hippocampal region (hippocampus proper, dentate gyrus, and subicular complex), parahippocampal gyrus, fusiform gyrus, insular cortex, and each of the major lobes of the brain. We identified significant brain damage in the neocortex of patients who were impaired that was not present in the patients who were unimpaired.
Experiment 2 examined the subjective experiences that accompany autobiographical recall, focusing on the five patients who could successfully recollect remote autobiographical memories. First, participants were asked to classify their autobiographical memories using the same Remember/Know procedure that was used to test patient Y.K. (Hirano et al., 2002). Second, participants were asked to rate the vividness of the visual imagery in their autobiographical recollections. Visual imagery is central to autobiographical memory, and damage to the visual cortex can severely impair autobiographical recall (Ogden, 1993; Rubin and Greenberg, 1998). Third, participants were asked to state the viewpoint from which the visual imagery in their recollections was seen (i.e., first-person or third-person viewpoint). Images occurring during autobiographical remembering are usually viewed from the first-person perspective (Heaps and Nash, 2001). With respect to these three aspects of the recollective experience, the question of interest was whether the recollections of memory-impaired patients were similar to or different from the recollections of healthy individuals.
Results
Experiment 1
Volumetric Data: MTL Group
Magnetic resonance images (MRI) for the two patients in the medial temporal lobe (MTL) group who have the largest lesions (E.P. and G.P.) are shown in Figure 1. Figure 2 shows the volumes of the major lobes of the five amnesic patients in the MTL group. The volumes of medial temporal lobe structures are presented in Table 1, and the volumes of the fusiform gyrus and insular cortex are presented in Table 2. When measurements of brain regions were undertaken by two independent scorers, the results were consistently within 13% of each other (also see Gold and Squire, 2005). Three of the patients (R.S., G.W., J.R.W.) have a substantial volume reduction within the hippocampal region but, with one exception, no reduction in the parahippocampal gyrus, the fusiform gyrus, the insular cortex, or the major lobes of the brain. The one exception is R.S., whose parietal lobes are unusually small (Figure 2). However, this finding likely reflects natural variation in parietal lobe volume rather than damage to this region, because (1) no evidence of parietal lobe damage is apparent in his MRI scan; and (2) he obtained normal scores on tests sensitive to parietal lobe function, including a score of 28 out of 36 for his copy of the Rey-Osterrieth figure (Osterrieth, 1944), a score of 99 on the Attention subscale of the Wechsler Memory Scale-Revised (Wechsler, 1987) (Table 3), and a scaled score of 11 on the Block Design subtest of the Weschler Adult Intelligence Scale-Revised (Wechsler, 1981). Note that volumes of some brain regions have been reported previously for R.S, G.W., and J.R.W. (Gold and Squire, 2005), for E.P. and G.P. (Levy et al., 2004), and for H.C. (Stark and Squire, 2003). The differences between volumes reported earlier and the volumes reported here are due to the different numbers of controls in the earlier studies and to differences in normalization procedures. Here, normalization to intracranial volume has been carried out uniformly for medial temporal lobe structures, fusiform gyrus, and insular cortex.
Figure 1.
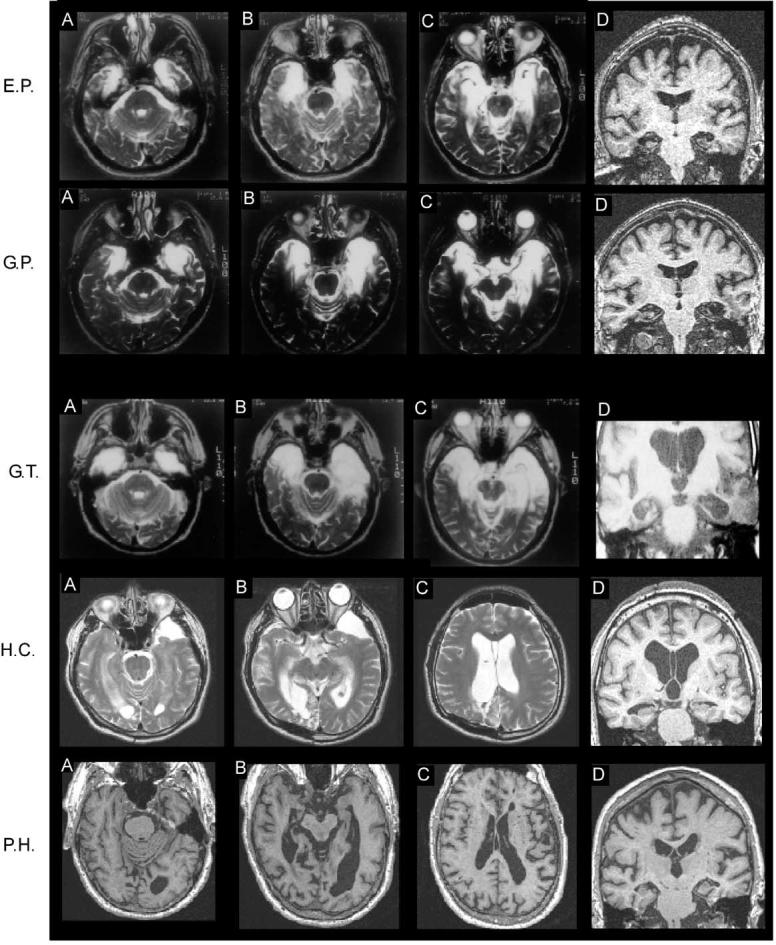
Magnetic Resonance Images Showing the Extent of Brain Damage for Five of the Eight Patients
(A), (B), and (C) are T2-weighted axial images (T1-weighted axial images for P.H.) arranged from ventral (A) to dorsal (C). Damaged tissue is indicated by areas of bright signal (but by dark signal for P.H.). (D) in each row is a coronal, T1-weighted image taken at the level of the hippocampus. For all images, the left side of the brain is on the right side of the image. See text for detailed description of the lesions.
Figure 2.
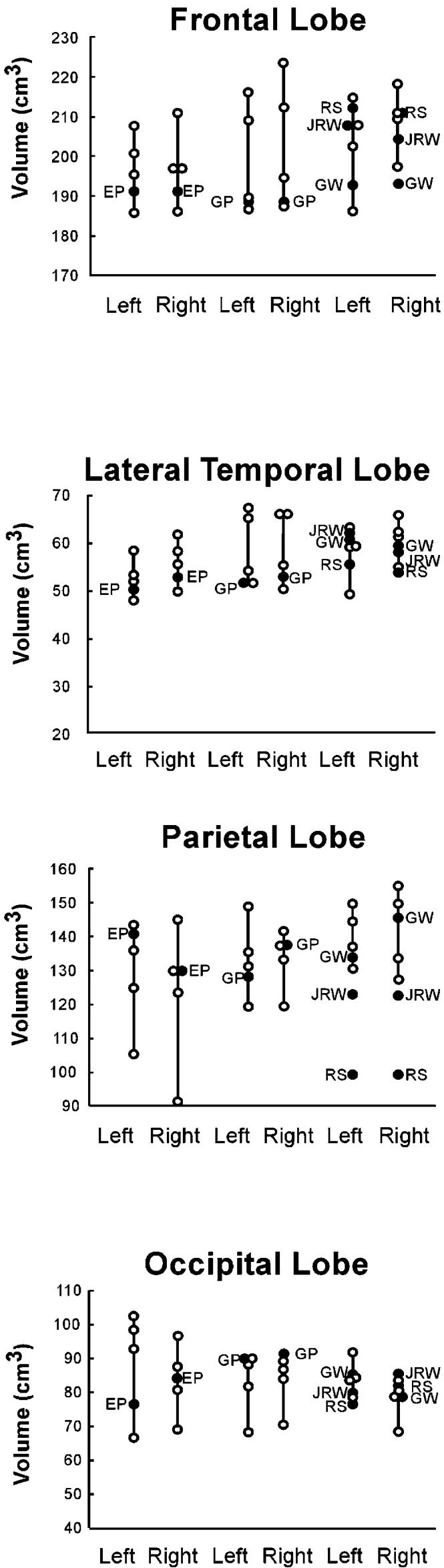
Volumes of Major Brain Regions for the Five Amnesic
Patients with Lesions Limited Primarily to the Medial Temporal Lobe Patients are represented by filled circles. Separate control groups (n = 4; unfilled circles) were matched to patient E.P, patient G.P., and patients R.S., G.W., and J.R.W.
Table 1.
Percent Reduction in the Volume of Medial Temporal Lobe Regions for the Patients Relative to Four Controls for Each Patient
| Hippocampal Region | Parahippocampal Gyrus | |||
|---|---|---|---|---|
| Patient | Left | Right | Left | Right |
| R.S. | 24% | 35%* | 2% | 0% |
| G.W. | 49%* | 42%* | 16% | 9% |
| J.R.W. | 43%* | 40%* | −5% | 18% |
| E.P. | 98%* | 97%* | 92%* | 94%* |
| G.P |
100%* |
93%* |
96%* |
87%* |
| H.C. | 7% | 12% | 40%* | 15% |
| P.H. | 54%* | 34%* | 35%* | 26%* |
| G.T. | 89%* | 67%* | 98%* | 94%* |
Volumes were corrected for differences in brain size by dividing by intracranial volume. An asterisk denotes a reduction in volume >2 standard deviations from the control mean. The hippocampal region includes the CA fields, dentate gyrus, and subicular complex. The parahippocampal gyrus includes the perirhinal, entorhinal, and parahippocampal cortices.
Table 2.
Percent Reduction in the Volume of the Fusiform Gyrus and the Insular Cortex for the Patients Relative to Four Controls for Each Patient
| Fusiform Gyrus | Insular Cortex | |||
|---|---|---|---|---|
| Patient | Left | Right | Left | Right |
| R.S. | −22% | 8% | −13% | −32% |
| G.W. | 14% | 8% | 1% | 0% |
| J.R.W. | 6% | 18% | 1% | −2% |
| E.P. | 39% | 68%* | 32% | 30% |
| G.P. |
41%* |
56%* |
80%* |
49% |
| H.C. | 0% | −6% | −14% | −23% |
| P.H. | 31% | 10% | 19% | 14% |
| G.T. | 53%* | 64%* | 66%* | 29% |
Volumes were corrected for differences in brain size by dividing by intracranial volume. An asterisk denotes a reduction in volume >2 standard deviations from the control mean.
Table 3.
Characteristics of Patients
| Patient |
Group |
Year of Birth |
Education (Years) |
WAIS-III IQ |
WMS-R |
||||
|---|---|---|---|---|---|---|---|---|---|
| Attention | Verbal | Visual | General | Delay | |||||
| R.S. | MTL | 1956 | 12 | 99 | 99 | 85 | 81 | 82 | <50 |
| G.W. | MTL | 1959 | 12 | 108 | 105 | 67 | 86 | 70 | <50 |
| J.R.W. | MTL | 1963 | 12 | 90 | 87 | 65 | 95 | 70 | <50 |
| E.P. | MTL | 1922 | 12 | 98 | 94 | 57 | 82 | 61 | 56 |
| G.P. | MTL | 1946 | 16 | 98 | 102 | 79 | 62 | 66 | <50 |
| H.C. | MTL+ | 1961 | 22 | 98 | 96 | 83 | 53 | 68 | 51 |
| P.H. | MTL+ | 1922 | 19 | 105 | 117 | 67 | 83 | 70 | 57 |
| G.T. | MTL+ | 1936 | 12 | 84 | 120 | 57 | 50 | 50 | <50 |
The Wechsler Adult Intelligence Scale-III (WAIS-III) (Wechsler, 1997) and the Wechsler Memory Scale-Revised (WMS-R) (Wechsler, 1987) yield mean scores of 100 in the normal population with a standard deviation of 15. The WMS-R does not provide numerical scores for individuals who score below 50. IQ scores for R.S. and J.R.W. are from the Wechsler Adult Intelligence Scale-Revised (Wechsler, 1981). The first three patients in the medial temporal lobe (MTL) group have damage thought to be limited to the hippocampal region. E.P. and G.P. have hippocampal damage as well as damage to adjacent medial temporal cortex. The MTL+ group has medial temporal lobe damage as well as additional damage to other regions of neocortex.
Both patients E.P. and G.P. have more extensive medial temporal lobe damage than the other patients in the MTL group. Specifically, for both these patients the hippocampal region and parahippocampal gyrus are markedly reduced in volume bilaterally (Table 1). The damage also extends laterally to include the fusiform gyrus bilaterally (Table 2), although volume reduction in the left fusiform gyrus in E.P. falls just short of significance (defined as >2 standard deviations from the control mean). The insular cortex is moderately reduced in volume in E.P. In G.P., the insular cortex is significantly reduced in volume on the left (Table 2). The major lobes of patients E.P. and G.P. appear to be of normal volume (Figure 2).
Volumetric Data: MTL+ Group
The three patients in this group have reduced volumes of medial temporal lobe structures (Table 1) and additional reductions in the volumes of one or more of the major lobes (Figure 3). Specifically, H.C. has reduced volumes of the frontal, parietal, and occipital lobes (Figures 1 and 3). These reduced volumes are bilateral, although the volume reduction in his frontal lobe reaches significance only in the right hemisphere. The left parahippocampal gyrus is also reduced in volume (Table 1). Patient P.H. has reduced volume of the left frontal lobe (Figure 3). P.H. also has reduced volumes of medial temporal lobe structures, including the hippocampal region and the parahippocampal gyrus bilaterally (Figure 1; Table 1). Patient G.T. has reduced volumes of the lateral temporal lobes bilaterally (Figures 1 and 3). The hippocampal region and the parahippocampal gyrus are also reduced in volume bilaterally (Table 1), as is the fusiform gyrus (Table 2). The insular cortex is reduced in volume on the left.
Figure 3.
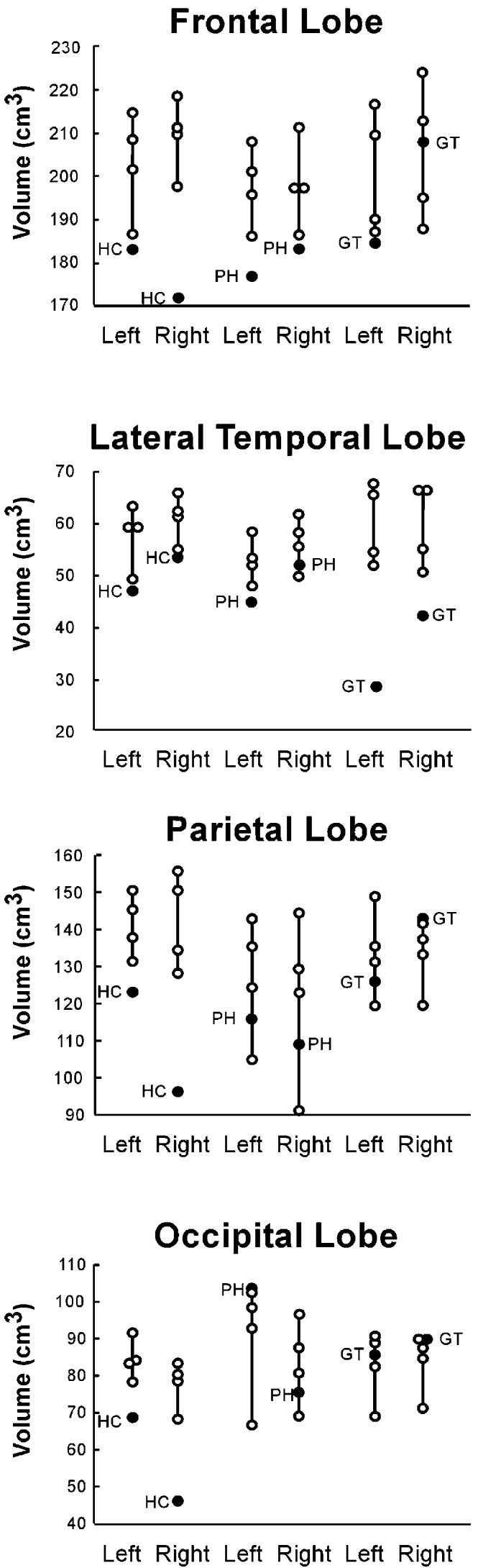
Volumes of Major Brain Regions for Three Amnesic Patients with Large Medial Temporal Lobe Lesions and Additional Damage to Neocortex
Patients are represented by filled circles. Separate control groups (n = 4; unfilled circles) were matched to patient H.C., patient P.H., and patient G.T.
It should be noted that this summary of the volumetric data is conservative. The considerable between-subject variation in the size of brain regions as well as the modest number of controls available for each patient work against finding significant volume reductions. If, instead, one evaluates reductions for the patients with respect to all 12 controls (instead of four for each patient), one finds, in addition to what is reported above, a significant reduction in E.P.'s left fusiform gyrus and G.P.'s right insula, and a nearly significant reduction (p = 0.07) in P.H.'s right frontal cortex.
Autobiographical Memory
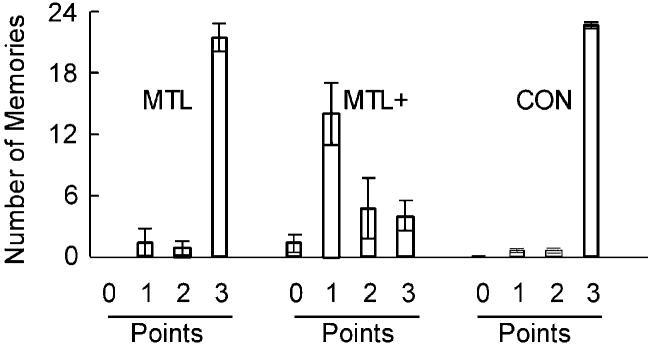
The patients in the MTL group and their controls were able to provide unique autobiographical memories (scoring the maximum of 3 points) in response to most of the 24 cue words (Figure 4; MTL patients, 21.6 memories; controls, 22.9 memories; for individual data, see Table 4). In contrast, the three patients in the MTL+ group were severely impaired at recalling autobiographical memories and provided an average of only 4.0 unique (3 point) memories in response to the 24 cue words. Most of the memories provided by the MTL+ group were awarded just one point (Figure 4), suggesting that these patients were able to recall some general information but had difficulty providing memories that were specific to a particular time and place. For each participant, a mean score (0 to 3) was calculated across all 24 words. The MTL+ group had a lower score (1.47) than either the MTL group (2.84) [t(6) = 7.3; p < 0.01] or the control group (2.93) [t(27) = 19.0; p < 0.01]. The control and MTL groups performed similarly [t(29) = 1.52; p > 0.10].
Figure 4.
Performance on the Test of Remote Autobiographical Memory
Participants were given 24 cue words (e.g., river, bottle, nail) and asked to recollect a specific event that involved the word. Patients were asked to recall events from the first third of their life before the onset of amnesia, and controls were asked for events from the same portion of their lives. Tape-recorded narratives were scored (0 to 3) for how well they described an event (0 = no response or a generic response, 1 = vague reference to a memory without any reference to time or place, 2 = memory that had some specificity but was not specific to one time and place, 3 = memory that was specific to one time and place). The bars show the mean number of narratives given each score, and the brackets show SEM. MTL, five patients with medial temporal lobe lesions; MTL+, three patients with medial temporal lobe lesions and additional lesions to neocortex; CON, 26 controls.
Table 4.
Performance on the Test of Remote Autobiographical Memory
| Patient | Group | 0 Point | 1 Point | 2 Point | 3 Point |
|---|---|---|---|---|---|
| R.S. | MTL | 0 | 0 | 0 | 24 |
| G.W. | MTL | 0 | 0 | 2 | 22 |
| J.R.W. | MTL | 0 | 1 | 3 | 20 |
| E.P. | MTL | 0 | 6 | 0 | 18 |
| G.P. | MTL | 0 | 0 | 0 | 24 |
| H.C. | MTL+ | 1 | 8 | 10 | 5 |
| P.H. | MTL+ | 3 | 16 | 4 | 1 |
| G.T. | MTL+ | 0 | 18 | 0 | 6 |
| CON | 0.1 | 0.5 | 0.4 | 22.9 |
Twenty-four tape-recorded narratives from each participant were scored on a 0 to 3 point scale (see Figure 4). For each patient, the number of narratives given each score is shown. For the controls, the mean number of narratives given each score is shown. MTL, five patients with medial temporal lobe lesions; MTL+, three patients with medial temporal lobe lesions and additional lesions to neocortex; CON, 26 controls.
To determine the reliability of the 0–3 point scoring method, narratives from 24 of the participants (all eight patients and 16 of the 26 controls) were scored by a second rater who was blind to the identity of the participants. Eight narratives were selected randomly for each participant, giving a total of 192 narratives. The scores of the blind rater and the original rater were highly correlated (r = 0.92; p < 0.001).
Autobiographical Memory Interview
The patients in the MTL+ group performed poorly compared to the patients in the MTL group and controls (all ps < 0.05). The mean scores for autobiographical incidents from childhood are shown in Figure 5A, and the mean scores for personal semantic memory are shown in Figure 5B.
Figure 5.
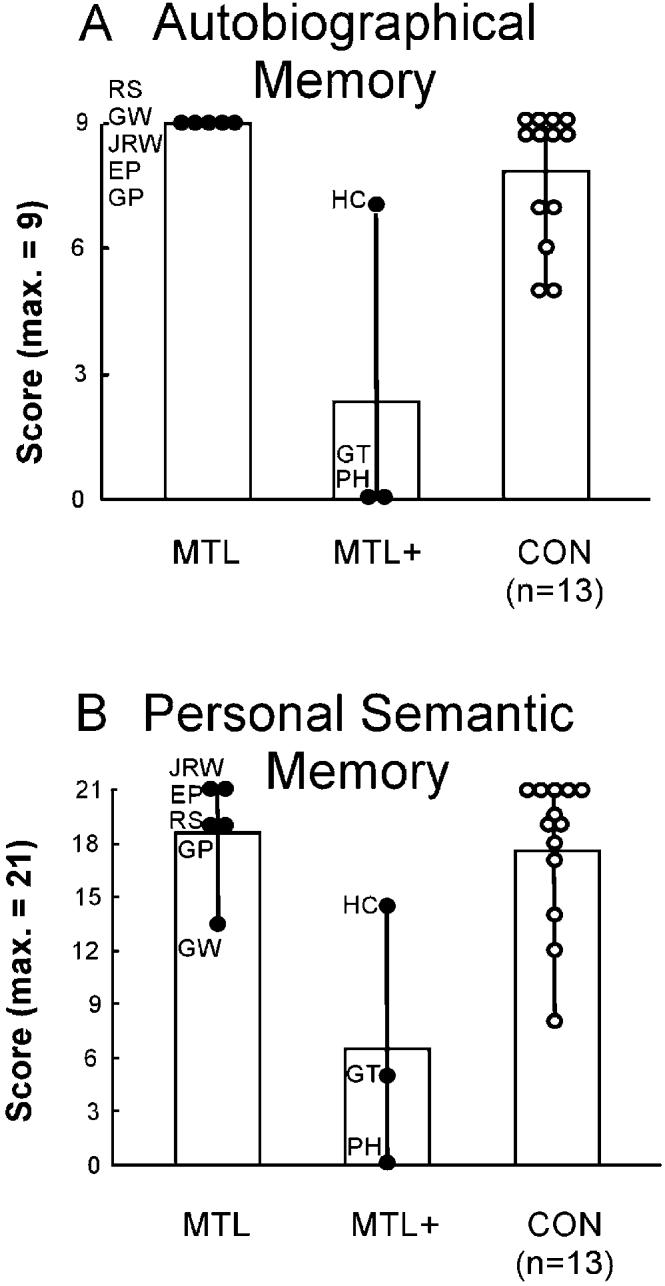
Performance on the Childhood Portion of the Autobiographical Memory Interview
(A) Scores on items that assessed memory for autobiographical events (maximum score = 9).
(B) Scores on items that assessed personal semantic knowledge (maximum score = 21). MTL, five patients with medial temporal lobe lesions; MTL+, three patients with medial temporal lobe lesions and additional lesions to neocortex; CON, 13 controls.
Experiment 2
Remember and Know
The patients in the MTL group and the controls performed similarly overall, rating most of their memories as Remember (MTL group, 87.1% Remember versus 10.7% Know; control group, 80.3% Remember versus 18.6% Know). Patients failed to recall 2.2% of their memories, and controls failed to recall 1.1% of their memories (Figure 6A).
Figure 6.
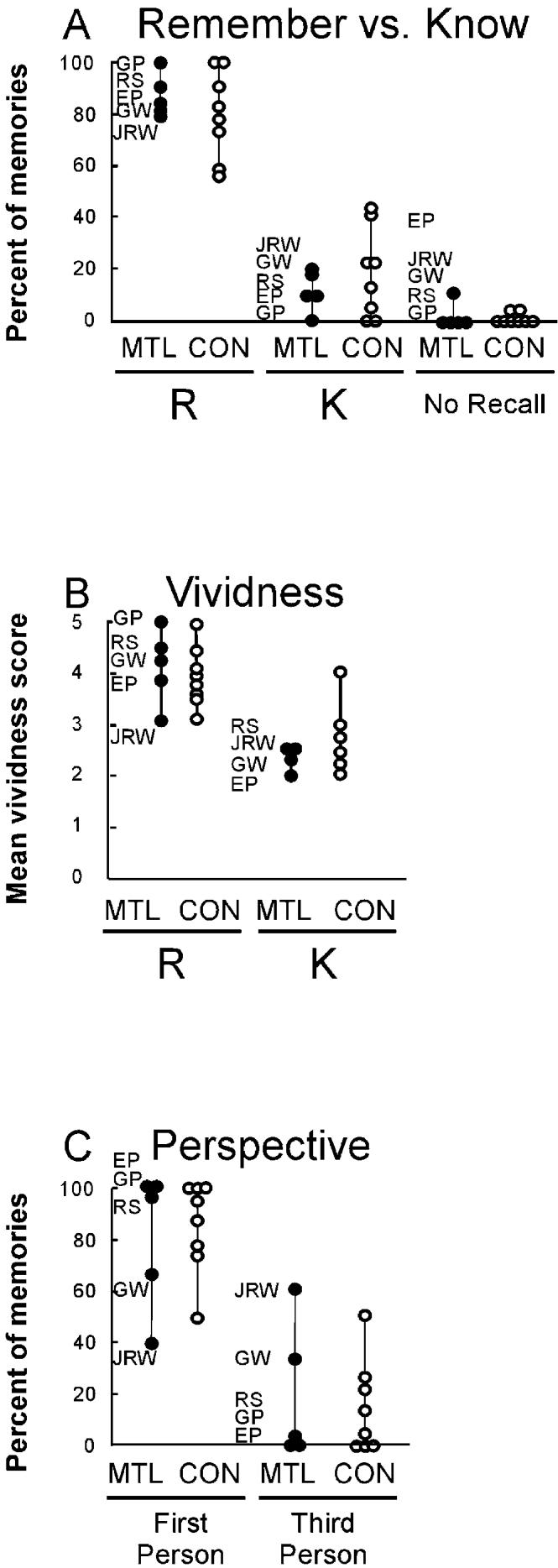
Characteristics of the Remote Autobiographical Memories
(A) The percentage of Remember (R) and Know (K) responses for each autobiographical memory. A Remember response was scored if participants reported that a recollection was associated with the subjective feeling of mentally traveling back in time to the place that the event occurred. A Know response was scored if participants reported that the event had occurred but could not directly reexperience it.
(B) Vividness of the visual imagery during autobiographical recall. Vividness scores are shown separately for autobiographical memories classified as Remember (R) and Know (K), as described in (A). Note that two of the eight controls did not report any Know memories, so that only six controls are shown in this condition. Participants were asked to rate the vividness of their mental imagery using a 5 point scale, 1 (“No image at all”) to 5 (“Perfectly clear and vivid as normal vision”).
(C) The perspective from which visual imagery was viewed during autobiographical recall. Participants were asked to judge whether their recollections were viewed from the first-person perspective (from the participant's own viewpoint) or from the third-person perspective (from a third-person viewpoint). MTL, five patients with medial temporal lobe lesions; CON, 8 controls.
Patient H.C. was asked to recollect a total of five autobiographical recollections (this is the number of recollections for which he received the maximum 3 point score). He rated three of these memories as Know, and two as Remember. Thus, unlike the other participants, H.C. rated the majority of his memories as Know. The other two patients in the MTL+ group could not be evaluated in experiment 2. Thus, P.H. produced only one recollection that received a maximum 3 point score. G.T. was not available.
Vividness
Figure 6B shows the vividness scores that participants gave for their autobiographical memories. Patients in the MTL group and controls rated memories that they had classified as Remember as being more vivid than those they had classified as Know (patients, Remember = 4.1, Know = 2.3; controls, Remember = 3.9, Know = 2.7). An ANOVA confirmed that, overall, memories classified as Remember were more vivid than memories classified as Know [F(1, 8) = 79.3; p < 0.01]. There was no effect of group [F(1, 8) = 0.50] and no group × rating interaction [F(1, 8) = 0.99].
Patient H.C. also rated the two memories he classified as Remember as being more vivid than the three memories he classified as Know (Remember = 5.0, Know = 3.3).
Perspective
The patients and the controls performed similarly, reporting that most of their recollections were experienced from the first-person perspective (MTL group, 80.3% first-person versus 19.7% third-person; control group, 85.6% first-person versus 14.4% third-person) (Figure 6C). Patient H.C. stated that all five of his autobiographical memories were seen from the first-person perspective.
Discussion
The ability to recall remote autobiographical memories was assessed in detail in a group of memory-impaired patients for whom quantitative volumetric data were obtained to describe the locus and extent of brain damage. Five of the patients had damage limited mainly to the medial temporal lobe, and three had medial temporal lobe damage plus significant additional damage to neocortex. There were two major findings. First, the patients with damage restricted mainly to the medial temporal lobe performed normally on tests of remote autobiographical memory, whereas the patients with significant damage to the neocortex were severely impaired. Second, by three measures (Remembering versus Knowing, the vividness of visual imagery, and the perspective from which visual imagery was seen), the subjective experience of remote autobiographical recollection was normal in the five patients with damage restricted mainly to the medial temporal lobe. Of the three patients with more extensive damage, only H.C. could be evaluated. He performed abnormally by the first measure and normally by the other two measures.
The three patients who had difficulty recollecting remote autobiographical memories all had damage to one or more areas of the neocortex, including the frontal, lateral temporal, and occipital lobes. Damage to the lateral temporal cortex is known to impair remote autobiographical memory (Graham and Hodges, 1997). In our study, lateral temporal lobe damage might explain the poor performance of patient G.T. Damage to the frontal lobe impairs a variety of “executive” functions that are important for the strategic aspects of recall as well as for active or effortful reconstructive processes (Kopelman, 2002), and frontal lobe damage is associated with impaired autobiographical memory (Kopelman et al., 2003). In our study, frontal lobe damage might explain the poor performance of patients H.C. and P.H. Finally, damage to the occipital lobe can also impair autobiographical recollection, perhaps because recollecting a past event depends importantly on the successful retrieval of visual images (Rubin and Greenberg, 1998). In our study, occipital lobe damage might contribute to the poor performance of patient H.C.
The five patients with damage limited mainly to the medial temporal lobe not only produced detailed, well-formed remote autobiographical memories that resembled the recollections of the control group (also see Bayley et al., 2003), they also produced recollections that were qualitatively normal by three different measures. First, autobiographical memories were classified using the Remember/Know procedure according to those that included a feeling of being able to reexperience the original event (Remembering) and those that did not include this feeling (Knowing). Both patients and controls labeled most of their remote autobiographical memories as Remember, and the two groups had similar proportions of Remember and Know responses. Second, the rated vividness of autobiographical memories was similar for patients and controls. Third, the patients and controls experienced the imagery in most of their recollections from the same first-person perspective. These findings suggest that the recollective experience of patients with damage limited mainly to the medial temporal lobe was qualitatively normal.
In view of these findings, the earlier report that a memory-impaired patient assigned Know responses to all of his remote autobiographical recollections (patient Y.K.; Hirano et al., 2002) raises the possibility that this patient has damage to structures outside the medial temporal lobe. It is noteworthy that Y.K. was impaired on neuropsychological measures of frontal lobe function, and the authors suggested that Y.K.'s performance might be the result of frontal lobe damage (Hirano et al., 2002). This suggestion is supported by the observation that our patient H.C., who has significant frontal lobe damage, also classified the majority of his memories as Know.
The present findings can be contrasted to reports in which memory-impaired patients had difficulty recalling remote autobiographical memories. In one study (Moscovitch et al., 2000), five patients with this impairment had various etiologies: closed head injury (patient K.C.), viral encephalitis, diencephalic damage following astrocytoma, basal forebrain damage following anterior communicating artery aneurysm, and Alzheimer's disease. Except for K.C., the brain damage has not been described, and the relationship between impaired remembering and the integrity of specific brain structures is difficult to determine. Certainly, one cannot suppose that the deficits in autobiographical memory reported for these patients are due specifically to medial temporal lobe damage. In the case of K.C., whose damage has been carefully documented, the damage includes the medial temporal lobe bilaterally but also involves left frontal, left parietal, left retrosplenial, and left occipital cortex, and there is a small lesion in the right parietal cortex (Tulving et al., 1991; Rosenbaum et al., 2004). K.C. can recall few, if any, autobiographical episodes from his life before his injury (Tulving et al., 1988; Hayman et al., 1993; Westmacott et al., 2001). Some neuropsychological data have been taken to suggest that K.C.'s poor remote autobiographical memory is related to his medial temporal lobe damage and not to damage in the posterior neocortex or the frontal lobes (Rosenbaum et al., 2004). However, this view is difficult to sustain. K.C. has less medial temporal lobe damage than patients E.P. and G.P. (compare the status of the parahippocampal gyrus of E.P. and G.P. in our Table 1 with the description of K.C. by Rosenbaum et al. [2000]). Yet E.P. and G.P. can recollect autobiographical memories better than K.C. can. Accordingly, K.C.'s severe impairment in autobiographical recollection is unlikely to be due to his medial temporal lobe damage.
Other memory-impaired patients with significant neocortical damage have also been reported to do poorly at recollecting autobiographical memory. Kapur (1999) identified 20 published cases where this impairment was especially prominent. Although some of the cases had damage to the medial temporal lobe, damage was not limited to this region in any of the cases. For example, the encephalitic patient L.D. had severe retrograde amnesia for remote personal events and damage to the left medial temporal lobe, the right medial and lateral temporal lobe, the basal forebrain bilaterally, and the right parietal lobe (O'Connor et al., 1992). A similar retrograde memory impairment was documented for the encephalitic patient S.S. (Cermak and O'Connor, 1983), who has extensive bilateral damage to the medial temporal lobe, as well as the insular cortex, septal region, and lateral temporal lobe bilaterally (Verfaellie et al., 2000). Similar findings were also reported by Viskontas et al. (2000), who studied 25 patients with temporal lobe epilepsy, 12 of whom had undergone unilateral resective surgery. Although the impairment in recollecting remote autobiographical events was attributed to medial temporal lobe damage, the status of medial and lateral temporal lobe tissue was not described.
An additional patient (V.C.) also exhibited impaired remote autobiographical memory following repeated episodes of cerebral ischemia accompanied by seizures (Kartsounis et al., 1995; Cipolotti et al., 2001). A volumetric analysis of the temporal lobe revealed severe hippocampal atrophy and additional damage to the left parahippocampal gyrus, which was reduced in volume by 2.9 standard deviations (Cipolotti et al., 2001). Like patient K.C., who was discussed above, V.C. has less medial temporal lobe damage than either patient E.P. or G.P., but E.P. and G.P. succeeded at autobiographical recollection. It is therefore difficult to attribute V.C.'s remote memory impairment to medial temporal lobe damage.
Lastly, the well-studied patient H.M. underwent a bilateral medial temporal lobe resection at the age of 27 years (Corkin et al., 1997) and has long been described as having good access to autobiographical memories from before the age of 17 years (Corkin, 1984; Sagar et al., 1985). Recently, H.M. was described as deficient on a new test of autobiographical memory that collected one memory from each of five time periods covering most of his life span (Steinvorth et al., 2005). Importantly, he achieved a normal score (by their criteria) for one of the two remote time periods tested, providing a detailed autobiographical memory from age 15 years. Nevertheless, H.M. was judged to be deficient and provided only one or two additional autobiographical memories. It is worth noting two factors that may have contributed to his performance. First, H.M. became amnesic at a relatively young age, which limited the number of premorbid, remote memories that he could be expected to have. Second, memory formation may have been disrupted by his epilepsy, which developed beginning at age 10. Further studies of these issues will be useful. A second patient (W.R.) in the same study was marginally impaired in three premorbid time periods and entirely normal in another (early adulthood). She also had bilateral atrophy in the parietal lobe and lesions of the right superior temporal gyrus and right thalamus, which make it difficult to interpret her performance.
There appear to be two ways to reconcile the discrepancy in findings for memory-impaired patients who do poorly on tests of remote autobiographical memory and patients who succeed on these tests, as in the present study. From the anatomical evidence considered above, one explanation is that the two kinds of patients differ importantly in the locus and extent of their damage. Specifically, most of the patients who perform poorly are known to have significant damage outside the medial temporal lobe, whereas those patients who perform well have damage limited to the medial temporal lobe. An alternative possibility is that the differences between patients reflects important differences in test procedures. For example, it has been proposed that the autobiographical narratives of patients with medial temporal lobe lesions lack the richness of detail that appears in the narratives of controls and that this deficiency can be detected only using sensitive tests (Nadel et al., 2000). Similarly, Rosenbaum et al. (2004) pointed out that, until comparable techniques are used across laboratories, differences in how remote memory is assessed might explain any differences in findings.
The state of affairs is not as challenging as these last comments suggest, because comparable techniques have been used across laboratories. Whereas methods that collect and score detailed narratives will always be difficult to standardize across research settings, published data are available for many of the patients under study, including our own patients, from a simple, standardized test of autobiographical memory (the Autobiographical Memory Interview [AMI]; Kopelman et al., 1989). This test includes three items that ask about autobiographical incidents that occurred during childhood (maximum score = 9 points). The critical finding is that patients reported to have impaired remote autobiographical memory, including those whose impairment has been attributed to medial temporal lobe damage, performed poorly on the Childhood portion of the autobiographical incidents schedule from the AMI (patient Y.K. = 4/9 points; Hirano and Noguchi, 1998; patient V.C. = 1/9; Cipolotti et al., 2001; patient K.C. = 2/9; Rosenbaum et al., 2004; patient R.S. = 0/9; Kitchener et al., 1998; see Figure 5A for our patients H.C., P.H., and G.T.). In contrast, in the present study, patients E.P. and G.P. both obtained the maximum score of nine points on the same test (Figure 5A). Further, each of the three patients who had damage restricted primarily to the hippocampal region also obtained the maximum score of nine points. Scores for 13 controls on this test ranged from 5 to 9 (mean = 7.9). Scores in this same range have also been reported for other memory-impaired patients thought to have damage restricted to the medial temporal lobe (patient M.R. = 6/9; Eslinger, 1998; patient P.D. = 7/9; Eslinger, 1998; patient B.E., whose score was reported as “normal”; Kapur and Brooks, 1999). These results show that, even when a simple, standardized test is used, considerable differences remain across patients in the ability to recall remote autobiographical memories. It follows that the origin of this difference cannot lie in differences in the test procedures used to assess remote memory. Further, detecting this difference between patients does not require the use of especially sensitive testing methods or the detailed analysis of narrative content.
If testing method does not account for who is impaired and who is not, the most likely alternative is that important differences exist among patients with respect to the locus and extent of brain damage. In our study, five patients with damage limited mainly to the medial temporal lobe, including patients E.P. and G.P., recalled remote autobiographical memories as well as controls. Three other patients did poorly at recalling remote autobiographical memories, and these patients had significant neocortical damage outside the medial temporal lobe. Other patients with identified damage outside the medial temporal lobe have also been reported to do poorly at remote autobiographical remembering (e.g., patients K.C., L.D., R.S., and S.S., as cited above). We are unaware of any memory-impaired patients with damage limited to the medial temporal lobe (and with quantitative MRI data from the entire brain to support this anatomical description) who are incapable of recollecting remote autobiographical memories. These considerations fit with the view that medial temporal lobe structures are needed for the formation of new memories and for the retrieval of older memories, especially recently formed ones. However, the ability to retrieve remote memories depends on neocortical regions, especially within the frontal, lateral temporal, and occipital lobes. Studies of experimental animals have documented the increasing importance of neocortex as memories grow older (Frankland et al., 2004; Maviel et al., 2004; Wiltgen et al., 2004; Frankland and Bontempi, 2005), and more than a dozen lesion studies of experimental animals have demonstrated the temporary role of the hippocampus and related structures for memory storage and retrieval (Squire et al., 2004). The present study suggests that remote autobiographical memory similarly depends on the neocortex and is independent of the medial temporal lobe.
Experimental Procedures
Experiment 1
Participants
Eight memory-impaired patients participated (Tables 1, 2, and 3; Figures 1, 2, and 3). Of these, three patients (R.S., G.W., J.R.W.) have damage thought to be limited primarily to the hippocampal region, and two (E.P. and G.P.) have damage to the hippocampal region as well as adjacent medial temporal lobe cortex (Figure 1). Patient J.R.W. became amnesic in 1990, following an anoxic episode associated with cardiac arrest. Patients R.S. and G.W became amnesic in 1998 and 2001, respectively, following a drug overdose and respiratory failure. E.P. and G.P. became amnesic in 1992 and 1987, respectively, after contracting viral encephalitis. In the present report, all five of these patients are designated as belonging to the MTL group.
Three other patients (MTL+ group; H.C., P.H., G.T.) have medial temporal lobe lesions and additional damage to other regions of neocortex that are intact in the MTL group (Figure 1). Patient H.C. became amnesic in 1997 when he underwent a right parietal craniotomy to evacuate a right occipital and parietal hematoma after a ruptured arteriovenous malformation. His memory impairment is thought to have resulted from ischemia associated with this rupture.
Patient P.H. had a 6 year history of 1–2 min “attacks” (with a possible epileptic basis) that were associated with gastric symptoms and transient memory impairment. In 1989, he suffered from a series of brief episodes that resulted in marked and persisting memory loss. Beginning after 1997, and after exhibiting stable and circumscribed memory impairment for about 8 years, his condition began to worsen. For example, his score on the Initiation/Perseveration subscale of the Dementia Rating Scale (DRS; Mattis, 1976) declined from 34 out of 37 points in 1997, to 30 in 2000, to 15 in 2002 (his MRI was obtained in 2001). Performance on this subscale is sensitive to frontal lobe damage (Janowsky et al., 1989). Similarly, his confrontational naming ability, as measured by the Boston Naming Test (Kaplan et al., 1983), declined from 56 out of 60 in 1997 (a normal score; Squire et al., 1990) to 49 in 1999 to 30 in 2002. Performance on naming tests is sensitive to lateral temporal lobe damage (Hermann et al., 1999).
Patient G.T. became severely amnesic in 1990 after contracting viral encephalitis. As a group, the three patients in the MTL+ group performed more poorly on both the Initiation/Perseveration subscale of the DRS and on the Boston Naming Test than the five patients in the MTL group (Initiation/Perseveration, 23.0 versus 35.3; data available for three MTL patients; Boston Naming, 35.3 versus 47.0; data available for four MTL patients). Thus, as expected from the fact that the MTL+ group had lesions in neocortex, this group had neuropsychological deficits beyond memory functions that were not observed in the MTL group.
Acquisition of Volumetric Data
Volumetric data were obtained for the brains of all eight patients. Volumetric data were also obtained for 12 male controls. Four of the controls were matched to patients E.P. and P.H. (mean age of controls = 78 years; range = 73–82 years), another four were matched to patients G.P. and G.T. (mean age of controls = 60 years; range = 56–65 years), and the remaining four were matched to patients H.C., R.S., G.W., and J.R.W (mean age of controls = 41 years; range = 35–47 years). The patients and controls were scanned in 1.5 T clinical scanners.
Medial temporal lobe structures were defined using criteria based on histological analysis of healthy brains (Amaral and Insausti, 1990; Insausti et al., 1998). Two regions were defined for each hemisphere: the hippocampal region (hippocampus proper, dentate gyrus, and subicular complex) and the parahippocampal gyrus (perirhinal, entorhinal, and parahippocampal cortices). See Gold and Squire (2005) for a detailed description of the volumetric methods. Volumes calculated for each region were normalized by dividing the volume of each structure by the intracranial volume.
To obtain volumetric data for the neocortex, twelve regions of interest were defined, including the frontal lobes, lateral temporal lobes, parietal lobes, occipital lobes, insular cortex, and fusiform gyrus (both left and right sides) (Duvernoy, 1991; Stefanacci et al., 2000). Magnetic resonance images were reconstructed using the Analysis of Functional NeuroImages (AFNI) software program (Cox, 1996) so that the images could be viewed in all three planes (except for patient G.T. [see below]). Before volumes were calculated, brains were aligned along a plane running through the anterior and posterior commissures (i.e., the AC-PC axis), ensuring that images of all brains were oriented to a uniformly and anatomically defined axis. Voxels were then resampled to 1 × 1 × 1 mm. Brain regions were next drawn by hand on contiguous 1 mm thick coronal brain slices using the Draw Dataset plug-in from AFNI. For each participant, the volume of a region was calculated as follows. First, all the voxels encompassed within a region were labeled on each section. Then, a segmentation program (Gyrus Finder, AFNI) was used to create an overlay for each brain that discriminated voxels representing gray and white matter from voxels representing cerebrospinal fluid, sinuses, and bone. Voxels not representing gray or white matter were then removed. Finally, the volume (mm3) of gray and white matter within each region was calculated as the sum of the voxels remaining in the region.
Volumes of the insular cortex and fusiform gyrus were normalized by dividing the volume of each structure by the intracranial volume. The volumes of the frontal, lateral temporal, parietal, and occipital lobes were not normalized, because the major lobes make up a significant percentage of the total intracranial volume.
Brain images for patient G.T. were available only on film. (G.T. died before higher-resolution scans could be obtained and before he could participate in experiment 2). Sagittal (7.5 mm thick) and coronal (5.0 mm thick) MRI sections were electronically scanned and imported into the Canvas software program, where regions were outlined using the polygon tool. The volume of each region was then calculated by multiplying the area of each region by the thickness of the images through the region.
Test of Autobiographical Memory
The patients described above, together with 26 healthy control subjects (22 males) participated in the study of autobiographical memory. Controls were matched to the patients with respect to age (controls = 59.6 years; range = 38–80 years; patients = 54.5 years, range = 39–76 years) and education (controls = 14.0 years, range = 12–20 years; patients = 15.2 years, range = 12–22 years).
Autobiographical memories were collected using a modified version of the Crovitz test of autobiographical memory (Crovitz and Schiffman, 1974; Bayley et al., 2003). Patients were asked to recollect autobiographical memories from the first third of life before the onset of their amnesia. Controls were asked to recollect events from the same time periods. Specifically, a list of 24 high-frequency nouns (e.g., river, bottle, nail) were presented one at a time with the instruction to recollect a unique event that involved the stimulus word and that was specific to time and place. Narratives were tape recorded for later scoring.
Specific instructions were as follows: “I am going to give you a word and I would like you to tell me something that is connected with that word that happened to you one time during the time period zero to __ years old (each participant was given a specific age). The memory can be anything, as long as it happened to you, not something that you heard about from someone else.” If the participant was unable to provide a memory that was specific in time and place, then prompts were given as follows.
Prompts before the Narrative Recollection Was Begun
Prompts were given as needed before the participant began to describe a specific event. For example, to help the participant remember an event involving the cue word “lake,” the interviewer might ask “Perhaps you remember one day when you went swimming in a lake?”
Prompts during the Narrative Recollection
Once the participant began to describe an event, prompts were given as needed in order to elicit more details. For example, the interviewer might ask “You said that you graduated from high school. Can you tell me more about the day you graduated?” or “What did you do after the graduation ceremony?”
More specific prompts were also given to try to elicit as much detail as possible (e.g., “What was the name of the racehorse who won the race you were watching?”). Prompts continued until the interviewer judged that the participant had recalled as many details as possible. Similar methods for eliciting autobiographical memories have been described in other studies (Moscovitch et al., 2000; Levine et al., 2002).
Scoring
All narratives were scored on a 0 to 3 scale (Zola-Morgan et al., 1983). Three points were awarded for an episodic memory that was specific to time and place (e.g., a description of the events on the day the participant passed the driving test). Two points were awarded for a memory that had some specificity, but was not specific to one time and place and was therefore not recalled as a unique event (e.g., “I used to stay at my grandma's house on weekends”). One point was awarded for a vague reference to a memory but without any time or place reference (e.g., “I read a lot of books”). Zero points were given for no response or for a generic response (e.g., “You can open and close a door”). Additional detailed analysis of the narratives from the five patients in the MTL group was also carried out to determine the number of details in the recollections that were produced for each cue word. These data were presented previously and showed that the recollections of the patients contained the same number of details (±5%) as the recollections of the controls (Bayley et al., 2003).
AMI
In order to permit comparison between our patients and patients tested in other settings, the three patients in the MTL+ group were also assessed with the AMI (Kopelman et al., 1989). Data for the MTL group have been published previously (Bayley et al., 2003), and they are included here for reference. This standardized test quantifies the recall of autobiographical incidents and personal facts from childhood (until age 18) and two later time periods. Following published procedures, participants were asked to recall three unique events from childhood (autobiographical memory) as well as 12 facts about their childhood (personal semantic memory). Results were compared to findings for 13 controls (nine male; age = 65.6 years; education = 14.6 years).
Experiment 2
Participants
The five patients in the MTL group from experiment 1 and one patient from the MTL+ group (H.C.) participated in experiment 2. Patient G.T. was unavailable, and P.H. was not tested because only one of his autobiographical memories received the maximum score of three points. Eight controls from experiment 1 also participated, and they were matched to the patients with respect to gender (all male), mean age at the time of testing (controls = 54.5 ± 6.3 years; patients = 53.5 ± 7.4 years), and years of education (controls = 12.8 ± 0.5; patients = 12.8 ± 0.8).
Procedure
Testing occurred on average 1.7 years (range = 0.7–4.8 years) after experiment 1. Participants were asked to describe each recollection that had been given a maximum score of three points (Figure 4) in experiment 1 (mean = 21.6 memories for the five patients in the MTL group; 5 memories for the MTL+ patient H.C.; mean = 23.1 memories for the eight controls). For each recollection, participants were given the cue word that had been given previously, together with a maximum of six details that they had originally provided in response to the cue word (see below). Specific instructions were as follows: “During a previous test session, you told us about an incident that happened to you that was connected with [the cue word] that happened to you before the age of [each participant was given a specific age; see Experimental Procedures]. The incident involved [subjects were initially provided with two details from their original narrative]. I would like you to tell me about this incident again.” Participants were encouraged to provide the entire narrative in as much detail as possible. If a participant was unable to recollect a narrative, he was prompted with additional details from his original narrative. Additional details were given one at a time, and the inquiry was terminated if the participant remained unable to recollect the narrative after four additional details had been given.
Participants were able to recollect most of the incidents they had described earlier. The R and K procedure was then applied as follows: “I want you to decide whether you ‘Remember’ the incident or only ‘Know’ that it happened. Say ‘Remember’ if you can actually remember the event as if you were there and you can mentally travel back in time to the place that the incident occurred and imagine that you are there. You should say ‘Know’ if you know that it happened to you, but you cannot travel back in time to the place that the incident occurred, and you cannot imagine yourself there. Say ‘Know’ if it sounds familiar, and that you know it happened to you, but you cannot really imagine yourself there. For example, you would say ‘Know’ if you feel that the information you have about the incident is just occurring automatically and that you have no actual feeling of it.” A card was placed in front of the participants during the entire test session that summarized the distinction as “Remember: if you can remember the incident and can imagine yourself there or Know: if you know the incident took place but you cannot imagine yourself there.”
After rating their narratives using the R and K procedure, participants next rated the visual imagery in their recollections on a 5 point scale. Participants were asked: “How clear is your visual image for this incident?” The scale was explained on the card that was placed in front of them: 5 = “Perfectly clear and vivid as normal vision,” 4 = “Clear and reasonably vivid,” 3 = “Moderately clear and vivid,” 2 = “Vague and dim,” 1 = “No image at all.” Finally, participants were asked to state the viewpoint from which their imagery was experienced. They were asked: “Is your image seen from your own perspective or as an observer?” To assist the participants, the card in front of the participants was marked “Your own perspective—As seen through your own eyes” and “An observer perspective—You see yourself in the image.”
Acknowledgments
Supported by the Medical Research Service of the Department of Veterans Affairs, NIMH Grant 24600, and the Metropolitan Life Foundation. We thank Jennifer Frascino and Leah Swalley for assistance, and Dr. T. Jernigan for providing magnetic resonance images of control brains.
References
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The Human Nervous System. Academic Press; San Diego, CA: 1990. pp. 711–755. [Google Scholar]
- Bayley PJ, Hopkins RO, Squire LR. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;37:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Cermak LS, O'Connor M. The anterograde and retrograde retrieval ability of a patient with amnesia due to encephalitis. Neuropsychologia. 1983;19:213–224. doi: 10.1016/0028-3932(83)90039-8. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Shallice T, Chan D, Fox N, Scahill R, Harrison G, Stevens J, Rudge P. Long-term retrograde amnesia … the crucial role of the hippocampus. Neuropsychologia. 2001;39:151–172. doi: 10.1016/s0028-3932(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Semin. Neurol. 1984;4:249–259. [Google Scholar]
- Corkin S, Amaral DG, Gonzalez RG, Johnson KA, Hyman BT. H.M.'s medial temporal lobe lesion: findings from magnetic resonance imaging. J. Neurosci. 1997;17:3964–3979. doi: 10.1523/JNEUROSCI.17-10-03964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bull. Psychon. Soc. 1974;4:517–518. [Google Scholar]
- Duvernoy HM. The Human Brain. Springer-Verlag; New York: 1991. [Google Scholar]
- Eslinger PJ. Autobiographical memory after temporal lobe lesions. Neurocase. 1998;4:481–495. [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat. Rev. Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Fujii T, Moscovitch M, Nadel L. Memory consolidation, retrograde amnesia, and the temporal lobe. In: Cermak L, editor. Handbook of Neuropsychology. Elsevier; Amsterdam: 2000. pp. 223–250. [Google Scholar]
- Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Hodges JR. Differentiating the roles of the hippocampal complex and the neocortex in long-term memory storage: evidence from the study of semantic dementia and Alzheimer's disease. Neuropsychology. 1997;11:77–89. doi: 10.1037//0894-4105.11.1.77. [DOI] [PubMed] [Google Scholar]
- Hayman CA, MacDonald CA, Tulving E. The role of repetition and associative interference in new semantic learning in amnesia: a case experiment. J. Cogn. Neurosci. 1993;5:375–389. doi: 10.1162/jocn.1993.5.4.375. [DOI] [PubMed] [Google Scholar]
- Heaps CM, Nash M. Comparing recollective experience in true and false autobiographical memories. J. Exp. Psychol. 2001;27:920–930. doi: 10.1037//0278-7393.27.4.920. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Perrine K, Chelune GJ, Barr WB, Loring W, Strauss E, Trenerry MR, Westerveld M. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Hirano M, Noguchi K. Dissociation between specific personal episodes and other aspects of remote memory in a patient with hippocampal amnesia. Percept. Mot. Skills. 1998;87:99–107. doi: 10.2466/pms.1998.87.1.99. [DOI] [PubMed] [Google Scholar]
- Hirano M, Noguchi K, Hosokawa T, Takayama T. I cannot remember, but I know my past events: remembering and knowing in a patient with amnesic syndrome. J. Clin. Exp. Neuropsychol. 2002;24:548–555. doi: 10.1076/jcen.24.4.548.1041. [DOI] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela M, Salinas A, Martinez-Penuela J. Human medial temporal lobe in aging: anatomical basis of memory preservation. Microsc. Res. Tech. 1998;43:8–15. doi: 10.1002/(SICI)1097-0029(19981001)43:1<8::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav. Neurosci. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea Febiger; Philadelphia: 1983. [Google Scholar]
- Kapur N. Syndromes of retrograde amnesia: a conceptual and empirical synthesis. Psychol. Bull. 1999;125:800–825. doi: 10.1037/0033-2909.125.6.800. [DOI] [PubMed] [Google Scholar]
- Kapur N, Brooks DJ. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. 1999;9:247–254. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Kartsounis LD, Rudge P, Stevens JM. Bilateral lesions of CA1 and CA2 fields of the hippocampus are sufficient to cause a severe amnesic syndrome in humans. J. Neurol. Neurosurg. Psychiatry. 1995;59:95–98. doi: 10.1136/jnnp.59.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener EG, Hodges JR, McCarthy R. Acquisition of post-morbid vocabulary and semantic facts in the absence of episodic memory. Brain. 1998;121:1313–1327. doi: 10.1093/brain/121.7.1313. [DOI] [PubMed] [Google Scholar]
- Kopelman MD. Disorders of memory. Brain. 2002;125:2152–2190. doi: 10.1093/brain/awf229. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 1989;5:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Lasserson D, Kingsley DR, Bello F, Rush C, Stanhope N, Stevens TG, Goodman G, Buckman JR, Heilpern G, et al. Retrograde amnesia and the volume of critical brain structures. Hippocampus. 2003;13:879–891. doi: 10.1002/hipo.10140. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol. Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: Medial vs. lateral temporal lobe. Proc. Natl. Acad. Sci. USA. 2004;101:6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon D, Squire LR. Autobiographical memory in amnesia. Psychobiology. 1989;17:247–256. [Google Scholar]
- Mattis S. Dementia Rating Scale. In: Bellack R, Keraso B, editors. Geriatric Psychiatry. Grune and Stratton; New York: 1976. pp. 77–121. [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;3:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Yaschyshyn T, Ziegler M, Nadel L. Remote episodic memory and retrograde amnesia: was Endel Tulving right all along. In: Tulving E, editor. Memory, Consciousness and the Brain: The Tallinn Conference. Psychology Press/Taylor & Francis; Philadelphia: 2000. pp. 331–345. [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10:352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- O'Connor M, Butters N, Miliotis P, Eslinger P, Cermak LS. The dissociation of anterograde and retrograde amnesia in a patient with herpes encephalitis. J. Clin. Exp. Neuropsychol. 1992;14:159–178. doi: 10.1080/01688639208402821. [DOI] [PubMed] [Google Scholar]
- Ogden JA. Visual object agnosia, prosopagnosia, achromatopsia, loss of visual imagery, and autobiographical amnesia following recovery from cortical blindness: Case M.H. Neuropsychologia. 1993;31:571–589. doi: 10.1016/0028-3932(93)90053-3. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Arch. Psychol. (Frankf.) 1944;30:206–356. [Google Scholar]
- Reed JM, Squire LR. Retrograde amnesia for facts and events: Findings from four new cases. J. Neurosci. 1998;18:3943–3954. doi: 10.1523/JNEUROSCI.18-10-03943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel-Clower N, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment following bilateral damage limited to the hippocampal formation. J. Neurosci. 1996;16:5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DL, Priselac S, Kohler S, Black S, Gao F, Nadel L, Moscovitch M. Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nat. Neurosci. 2000;3:1044–1048. doi: 10.1038/79867. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Winocur G, Moscovitch M. New views on old memories: re-evaluating the role of the hippocampal complex. Behav. Brain Res. 2001;127:183–197. doi: 10.1016/s0166-4328(01)00363-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, McKinnon MC, Levine B, Moscovitch M. Visual imagery deficits, impaired strategic retrieval, or memory loss: disentangling the nature of an amnesic person's autobiographical memory deficit. Neuropsychologia. 2004;42:1619–1635. doi: 10.1016/j.neuropsychologia.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Greenberg DL. Visual memory-deficit amnesia: a distinct amnesic presentation and etiology. Proc. Natl. Acad. Sci. USA. 1998;95:5413–5416. doi: 10.1073/pnas.95.9.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar HJ, Cohen NJ, Corkin S, Growdon JH. Dissociations among processes in remote memory. Ann. N Y Acad. Sci. 1985;444:533–535. doi: 10.1111/j.1749-6632.1985.tb37637.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Amaral DG, Press GA. Magnetic resonance measurements of hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. J. Neurosci. 1990;10:3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Clark RE, Bayley PJ. Medial temporal lobe function and memory. In: Gazzaniga M, editor. The Cognitive Neurosciences III. The MIT Press; Cambridge, MA: 2004. pp. 691–708. [Google Scholar]
- Stark CEL, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13:281–292. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Buffalo EA, Schmolck H, Squire LR. Profound amnesia following damage to the medial temporal lobe: a neuroanatomical and neuropsychological profile of patient E.P. J. Neurosci. 2000;20:7024–7036. doi: 10.1523/JNEUROSCI.20-18-07024.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: evidence from H.M. and W.R. Neuropsychologia. 2005;43:479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Can. Psychol. 1985;26:1–12. [Google Scholar]
- Tulving E, Schacter DL, McLachland D, Moscovitch M. Priming of semantic autobiographical knowledge: a case study of retrograde amnesia. Brain Cogn. 1988;8:3–20. doi: 10.1016/0278-2626(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Tulving E, Hayman CAG, MacDonald CA. Long-term perceptual priming and semantic learning in amnesia: a case experiment. J. Exp. Psychol. Learn. Mem. Cogn. 1991;17:595–617. doi: 10.1037//0278-7393.17.4.595. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Keane MM, Johnson G. Preserved priming in auditory perceptual identification in Alzheimer's disease. Neuropsychologia. 2000;38:1581–1592. doi: 10.1016/s0028-3932(00)00073-7. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Remote episodic memory deficits in patients with unilateral temporal lobe epilepsy and excisions. J. Neurosci. 2000;20:5853–5857. doi: 10.1523/JNEUROSCI.20-15-05853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. The Psychological Corp; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. The Psychological Corp; New York: 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Third The Psychological Corp; San Antonio, TX: 1997. [Google Scholar]
- Westmacott R, Leach L, Freedman M, Moscovitch M. Different patterns of autobiographical memory loss in semantic dementia and medial temporal lobe amnesia: a challenge to consolidation theory. Neuroimage. 2001;7:37–55. doi: 10.1093/neucas/7.1.37. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004;44:101–108. doi: 10.1016/j.neuron.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Cohen NJ, Squire LR. Recall of remote episodic memory in amnesia. Neuropsychologia. 1983;21:487–500. doi: 10.1016/0028-3932(83)90005-2. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]