Abstract
Killikaike blakei is a new genus and species of anthropoid from the late Early Miocene of southeastern Argentina based on the most pristine fossil platyrrhine skull and dentition known so far. It is part of the New World platyrrhine clade (Family Cebidae; Subfamily Cebinae) including modern squirrel (Saimiri) and capuchin monkeys (Cebus) and their fossil relatives known from Early to Middle Miocene and subrecent periods. Living cebines are relatively large-brained, adroit predatory foragers and live within complex social groups, and wild capuchins exhibit a wide range of behaviors associated with enhanced intelligence. We show that K. blakei lacks diagnostic derived characteristics of the lower face and premolar dentition that are shared by modern cebines, but its strongly vaulted frontal bone and capacious anterior cranial fossa indicate the early evolution of an enlarged forebrain.
Keywords: Miocene, Paleoprimatology, Patagonia, Platyrrhini, Cebinae
Intact craniodental remains of pre-Pleistocene fossil New World anthropoids are exceedingly rare (1–3). Of the five Miocene genera that are known from crania (Dolichocebus, Tremacebus, Homunculus, Chilecebus, and Lagonimico), the first three are essentially edentulous and all but one are broken and/or deformed. Chilecebus carracoensis from a site in the western Andes of Chile (4) is an exception. It preserves an unspoiled skull with teeth in situ, albeit heavily worn. Here we report a fossil of a platyrrhine that preserves the entire face and also an unworn, little damaged dentition that is the best anatomical evidence of the maxillary teeth of any Santacrucian fossil monkey. It also provides relatively complete and undistorted evidence of the anterior braincase of a fossil platyrrhine. The two specimens of this species come from the classic Santacrucian sediments of Patagonian southeastern Argentina (5, 6), which has produced a substantial primate fauna (1, 7, 8, ††). They are the southernmost fossil platyrrhine primates ever described.
Systematic Paleontology.
The classification of Killikaike blakei is as follows: Order Primates (Linnaeus, 1758); Suborder Anthropoidea (Mivart, 1864); Superfamily Ateloidea (Gray, 1825); Family Cebidae (Bonaparte, 1831); Subfamily Cebinae (Bonaparte, 1831; Mivart, 1865); K. blakei, gen. et sp. nov.
Holotype.
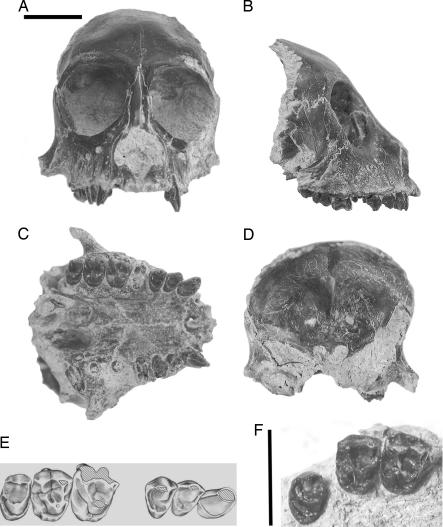
MPM-PV 5000 (Museo Regional Provincial “Padre Manuel Jesús Molina” Padre Molina, Vertebrate Paleontology Collection) is an intact, undistorted face of an adult individual that preserves the forehead, orbits, snout, dental arcade, and roots of the pterygoid processes. The dentition includes right C, P2–3, root of P4, crowns of M1–3; left C, broken crowns of P2–3, and roots of M1–3 (Fig. 1A–E).
Fig. 1.
Holotype cranium and referred upper molars of K. blakei, a new genus of platyrrhine primate from the Miocene of Argentina. Frontal (A), right lateral (B), palatal (C), and posterior (D) views of MPM-PV 5000, and an illustration of the cheek teeth (E). (F) MPM-PV 1607 in occlusal view. (Scale bar, 1 cm.)
Hypodigm.
Holotype and MPM-PV 1607, a right M1–3 associated in a piece of maxilla (Fig. 1F).
Locality and age.
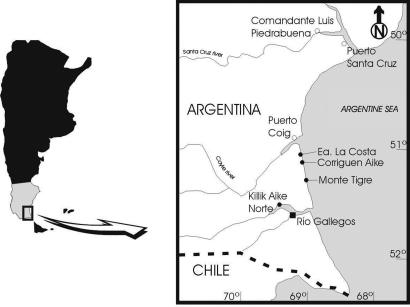
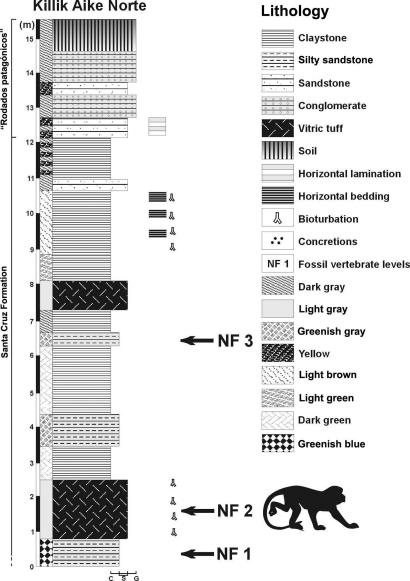
MPM-PV 5000 was collected >25 years ago at the locality Killik Aike Norte (formerly known as Estancia Felton) (5, 6, 9), ≈50 km northwest of the city of Río Gallegos, Santa Cruz Province, Argentina (Fig. 2). MPM-PV 1607 was collected in January of 2005 at the same locality. Killik Aike Norte is part of the Santa Cruz Formation (10) exposed in the northern cliffs of the estuary of the Rio Gallegos river. The stratigraphy is divided into two members (6): Estancia La Costa (120 m thick with 18 fossiliferous levels) and Estancia Angelina (103 m thick with four fossiliferous levels). There are two types of volcanic ash at the locality. The exact provenience of the MPM-PV 5000 specimen is not known, but the mineralogy and chemistry of the rock that encased it are consistent with an origin in the lower volcanic tuff, which is part of the Estancia La Costa member (Fig. 3). MPM-PV 1607 was also collected from the lower tuff. The level 6 tuff of the Estancia La Costa member at the locality Estancia La Costa, northeast of Killik Aike Norte, previously yielded a primate skull attributed to Homunculus patagonicus (7). Correlation of the level 6 at La Costa with the tuff at Killik Aike Norte is not confirmed yet.
Fig. 2.
Location map of Killik Aike Norte.
Fig. 3.
Stratigraphic section and lithology of Killik Aike Norte showing the fossiliferous levels (NF) and the two tuffs mentioned in the text.
We have determined the age of the MPM-PV 5000 through 40Ar/39Ar laser incremental-heating analyses of plagioclase separated from the tuff level where the skull was found at Killik Aike Norte. Three separate incremental-heating experiments yielded reproducible plateau ages of 16.45 ± 0.14, 16.5 ± 0.2, and 16.5 ± 0.3 mega-annum (Ma) (see Fig. 4, which is published as supporting information on the PNAS web site).
The Ca/K ratio, derived from the 37Ar/39Ar ratio obtained during the 40Ar/39Ar dating of the plagioclase, can be used to delimit possible correlative Santa Cruz tuffs previously dated at the coastal sites of Monte Observación and Monte León, which are ≈100 km north of Killik Aike Norte. These sites contain key fossil mammal localities of the Santa Cruz Formation and have yielded 40Ar/39Ar ages similar to that reported here for Killik Aike Norte. The low Ca/K ratios of the Killik Aike Norte plagioclase compare best with Monte Observación MO2, although the variation in the Ca/K measurements does not preclude possible correlation with MO64 or ML18 tuffs, and further geochemical study is needed (11, ‡‡)
Our 40Ar/39Ar age for Killik Aike Norte is appreciably younger than the older age limit of 17.7 Ma for the Santa Cruz Formation based on a K-Ar date from the same site (12). The older age may be attributed to contaminant (detrital grains incorporated into the bulk K-Ar sample). The younger age for Killik Aike Norte reported here corroborates the assessment of Fleagle et al. (10, 11) that the base of the Santacrucian at the classic coastal localities between Río Gallegos and Río Coyle is not older than ≈16.5 Ma. The older age limit for the Santacrucian based on the 17.7 Ma K-Ar date may be in error. The main terrestrial fossiliferous units of the Santacrucian Land Mammal Age appear to have been laid down over a relatively short time period of ≈400,000 years, approximately between 16.1 and 16.5 Ma.
Etymology.
Killikaike, after Killik Aike Norte, the name of the ranch and fossil site; blakei, in honor of the Blake family, who donated the fossil to the Padre Molina museum.
Diagnosis.
Killikaike is unique among platyrrhines in the following combination of craniodental characters: relatively short face that is not wide anteriorly, narrow interorbital region, deep recessed orbits that lack an imperforate medial orbital wall, high arched frontal bone conforming to an enlarged anterior cranial fossa, and nonbunodont cheek teeth with subtriangular premolars and hypocone-bearing quadrate molars. It is a platyrrhine similar in size to Saimiri, Callicebus, or Leontopithecus based on dental and cranial dimensions (Tables 1 and 2), with an upper tooth count of 2.1.3.3. Unlike Cebus and Saimiri, the face is relatively narrow and the snout tapers anteriorly, making it shorter than Aotus and Pithecia, for example, but without abbreviated premaxillae as in Callicebus. The interorbital region is relatively narrow, in contrast to callitrichines, pitheciines, atelines, and Chilecebus, but less so than in existing cebines and Dolichocebus. Orbits are not enlarged as in Aotus and Tremacebus, and the medial orbital walls are closely spaced and not fenestrated, in contrast to Dolichocebus and Saimiri. The orbits are recessed beneath the arched and uptilted frontal bone, as in modern cebines and unlike most other platyrrhines, where the frontal trigon is flattened in the midline. A relatively large lateral orbital foramen is partially preserved, unlike most other platyrrhines. Postcanine crowns are moderately crested, resembling Saimiri and unlike the bunodont Cebus. However, in contrast to Saimiri and Cebus, where P2–3 are transversely wide and lingually expanded and sometimes have a lingual cingulum, in Killikaike these teeth are relatively narrower and more triangular, and they lack lingual cingulum. M1–2 are quadrate with high cusps and well developed, offset hypocones, strong lingual cingula, and well developed crests, distinguishing the genus from all pitheciines, atelines, callitrichines, and Szalatavus. The molars are relatively narrower than in Branisella and Chilecebus. M1 is considerably wider than M2, and M3 is reduced but otherwise an unremarkable two-cusped crown.
Table 1.
Dental measurements (means in millimeters) of fossils and selected living species
| C |
P2 |
P3 |
M1 |
M2 |
M3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | MD | BL | MD | BL | MD | BL | MD | BL | MD | BL | MD | |
| MPM-PV 5000 | 3.4 | 2.8 | 3.6 | 2.8 | 4 | 3 | — | 4.1 | 4.9 | 3.7 | 4.5 | 2.9 |
| MPM-PV 1607 | — | — | — | — | — | — | 5.7 | 4.1 | 5.5 | 4 | 4.9 | 3.4 |
| Saimiri sciureus (3m, 3f) | 2.9 | 3.1 | 3.6 | 2.4 | 3.9 | 2.2 | 4 | 3 | 3.6 | 2.6 | 2.7 | 1.7 |
| Saimiri oerstedi (3m, 3f) | 3 | 3.3 | 3.2 | 2.3 | 3.5 | 2 | 3.6 | 2.6 | 3.4 | 2.4 | 2.4 | 1.6 |
| Cebus apella (4m, 4f) | 6 | 6.7 | 6.5 | 3.9 | 3.5 | 3.5 | 6 | 4.4 | 5.4 | 3.9 | 4.4 | 3 |
| Callicebus moloch (4m, 4f) | 3.1 | 2.6 | 3.2 | 2.1 | 3.6 | 2.2 | 4.4 | 3.2 | 4.3 | 3 | 3.3 | 2.3 |
| Aotus vociferans (3m, 3f) | 2.5 | 2.8 | 2.9 | 1.9 | 3.2 | 2 | 3.9 | 3.1 | 3.7 | 2.9 | 3.2 | 2.1 |
| Leontopithecus rosalia (2m, 1f, 3?) | 3 | 3.3 | 3.2 | 2.6 | 3.8 | 2.3 | 4.1 | 3.2 | 3.3 | 2.4 | na | na |
Sexes and sample sizes are in parentheses. Data on body weight and cranial capacity and the Index of Cranial Capacity are from Martin (17) and are not based on the same individuals. BL, buccolingual breadth; MD, mesiodistal length; m, males; f, females; na, not applicable.
Table 2.
Cranial measurements (means in millimeters) of fossils and selected living species
| Interorbital breadth | Biorbital breadth | Orbital height | Orbital width | Postorbital constriction | Bicanine breadth | Bimolar width | Body weight, g | Cranial capacity, cm3 | Index of cranial capacity | |
|---|---|---|---|---|---|---|---|---|---|---|
| MPM-PV 5000 | 4.4 | 30.5 | 13.9 | 13.1 | 27.3 | 14.4 | 23.9 | — | — | — |
| Saimiri sciureus (3m, 3f) | 3.2 | 33 | 15.3 | 13.9 | 29.8 | 17.3 | 20.4 | 914 | 23.6 | 7 |
| Saimiri oerstedi (3m, 3f) | 3 | 32.5 | 14.7 | 13.9 | 29.7 | 15.9 | 19.6 | — | — | — |
| Cebus apella (4m, 4f) | 5.2 | 51.2 | 21.3 | 20.2 | 38.6 | 28 | 28.2 | 2537 | 76.2 | 11.7 |
| Callicebus moloch (4m, 4f) | 6.7 | 36 | 15.4 | 14.1 | 31.4 | 14.3 | 21.3 | 1078 | 18.3 | 4.9 |
| Aotus vociferans (3m, 3f) | 4.9 | 42 | 19.3 | 19.1 | 31 | 15.4 | 20.4 | 985 | 16.9 | 4.8 |
| Leontopithecus rosalia (2m, 1f, 3?) | 6.4 | 28.6 | 11.7 | 11.3 | 23.2 | 15.9 | 20.7 | — | — | — |
Sexes and sample sizes are in parentheses. Data on body weight and cranial capacity and the Index of Cranial Capacity are from Martin (17) and are not based on the same individuals. m, males; f, females.
Results and Discussion
MPM-PV 5000 is the forehead, face, and palate of a young adult, probably female. It evidently broke away from the neurocranium, which has not been recovered, behind the craniofacial junction. The snout and orbits are remarkably well preserved, bilaterally undamaged except for a slight crack at the left lateral corner of the orbit near the zygomaticofrontal junction as well as some abrasion of its lateral margin and the face beneath the socket. Foramina and sutures are well preserved. Anterior temporal lines are faint. The root of the zygomatic process is a simple bar that sweeps backward and slightly inferiorly, conforming to the lightly built pattern of cebids as opposed to the more robust pattern of atelids (13). Postorbital closure was complete; there is only a small gap evident as a lateral orbital fissure separating the enclosing zygomatic from the maxillary tuberosity in the temporal fossa. The morphology in all respects resembles that of living cebines.
The frontal bone, which is essentially complete, also preserves detailed impressions of the cerebral surface endocranially. The roof of the anterior cranial fossa is raised high above convex promontories that are the impressions of the orbits. Between them is the olfactory recess leading to the cribiform plate. It is long and tear-drop in shape, resembling the slit-like condition seen in Saimiri. The widest part of the anterior cranial fossa conforms to the postorbital constriction. It gives no indication of further widening toward the rear, which would be indicative of a much broader biparietal dimension of the middle cranial fossa, as see in Saimiri, for example.
The size of the anterior cranial fossa can be gauged by measuring the space anterior to and above the optic foramina. The volume of this space is 1.6 ml and was measured as the space of the anterior fossa demarcated by a coronal plane passing through the posterior border of the anterior cranial fossa at the midline. It measures a sizeable portion of the frontal lobe, which is very highly correlated with brain volume in primates and other mammals (14). In adult samples of two platyrrhines with differently shaped braincases and forebrains, Saimiri and Callicebus, each weighing ≈1 kg (15), the space averages 1.8 ml (range, 1.6–2.0; n = 4) and 1.0 ml (range, 0.9–1.2; n = 4), respectively. Based on Martin’s figures (15), this measurement represents ≈5% of the total brain size in Callicebus and 7% of brain size in Saimiri. Overall brain size in Saimiri is 29% larger than that of Callicebus. Thus it is evident that the highly vaulted frontal bone of the fossil does indeed reflect an absolutely and relatively enlarged forebrain compared with non-cebine platyrrhines.
There are four incisor alveoli, and they show no evidence of procumbency as seen in living and fossil pitheciins, for example. The morphology of the canine closely resembles female Saimiri. It is moderately projecting and bilaterally compressed, with a lingual cingulum and a modest lingual torus, which demarcates a short mesial sulcus. P2–3 are unexceptional crowns, each with a single occlusal fovea. Little can be said of the broken crown of P4, but it was probably wider than P3. The buccal cusps of M1 are broken away in MPM-PV 5000 but are intact and unworn in MPM-PV 1607. Molar morphology is described in the diagnosis. It provides empirical confirmation of the ancestral cebine morphotype, originally reconstructed based on comparisons from the living forms, as being generally Saimiri-like in crown morphology (16, 17), with a moderately crested relief pattern, a low hypocone, strong pre- and postparacristae, and strong lingual cingulum.
Killikaike is the seventh pre-Pleistocene cranium of a fossil platyrrhine. The youngest, Lagonimico conclucatus, is badly crushed (18). The oldest, C. carrascoensis (4), is well preserved but not fully described yet, and its teeth are well worn. One of the most complete is Tremacebus harringtoni, but its braincase is somewhat deformed and its orbits are somewhat misshapen and damaged (3, 19, 20). Teeth are unknown, except for the very fragmentary left M1–3. The face, orbits, and palate of the edentulous Dolichocebus gaimanensis (3, 13) are damaged and distorted, and its braincase is artificially compressed bilaterally. H. patagonicus (3, 8, 21) is known by two partial crania preserving the left part of the face and part of the braincase; three others have recently been reported in an abstract.§§ Teeth in the first two specimens are broken or badly worn, revealing little or no occlusal morphology. Debate regarding the systematics of these forms, which represent nearly one-quarter of the pre-Pleistocene fossil record of platyrrhines, has been fueled by the poor quality of the fossils (1–3, 21, 22, §§). K. blakei, as the best-preserved cranium of a Tertiary New World monkey, thus has important bearing on the evolution of the platyrrhine radiation.
The narrow interorbital region, elongate descending process of the frontals, rounded, recessed orbits, and vaulted frontal bones are a combination of features found only among extant platyrrhines in Saimiri and Cebus and in the fossil Dolichocebus. These features together provide strong evidence that K. blakei is a member of the cebine clade, whose monophyly is well corroborated by morphology and molecules (22–24). The origins of this clade may have occurred >22 million years ago (24). Absent in Killikaike are derived rostral and dental features shared by these sister taxa, such as the U-shaped dental arcade, large transversely broadened and rectangular upper premolars, a foreshortened face, and enlarged sexually dimorphic canines (16, 25).
On the other hand, the domed frontal bone and measured estimates of anterior cranial fossa size given above leave little doubt that the forebrain of K. blakei was shaped and proportioned like Saimiri. Saimiri and Cebus are two highly encephalized anthropoids (15, 25). Martin (15) calculated an Index of Cranial Capacity for primates that measures the amount of encephalization in a species above what is expected for its body size, based on a “basal” insectivore regression model (Table 2; also see Fig. 5, which is published as supporting information on the PNAS web site). The three highest index values were as follows: Homo sapiens, 23; Australopithecus africanus, 12.4; and Cebus sp., 11.7. Capuchins ranked well above chimpanzees (8.2) and orangutans (7.7). The Saimiri value was 7.0, well within the range of apes and Old World monkeys. Thus, as with Cebus and Saimiri, expansion of the forebrain in K. blakei is a likely indication that overall brain size was also large relative to body size.
At ≈16.4 million years, K. blakei presents a primitive cebine facial and dental structure but an advanced brain, whereas contemporaneous apes had brain sizes that were unexceptional and probably smaller than the modern norms (26). The importance of facial foreshortening and broadening of the snout and premolars in modern cebines remains to be determined, but these features are probably connected with processing tough insects and ripping or tearing fibrous woody materials to harvest embedded food. The absence of these features in Killikaike suggests a brain-first/teeth-second adaptive sequence in the mosaic evolution of their extractive foraging–feeding specialization, with problem-solving skills and manual dexterity preceding oral-harvesting and food-processing adaptations.
Materials and Methods
We have compared the fossil platyrrhines described here with the material of living and fossil platyrrhines deposited in the collections of the Museo Argentino de Ciencias Naturales (Buenos Aires) and the American Museum of Natural History (New York).
During the process of determining the age of the tuff sample, before measurement, the plagioclases were irradiated at the Oregon State University Triga Research Reactor facility for 6 h. Interference corrections used were 36Ar/37ArCa = 2.72 ± 0.01(× 10−4), 39Ar/37ArCa = 7.11 ± 0.024 (× 10−4), and 40Ar/39ArK = 7 ± 3 (× 10−4). Mass discrimination was monitored during the analyses from replicate aliquots delivered from an online air pipette system averaging 1.0011 ± 0.0005 per atomic mass unit. J, the irradiation parameter, was calculated from replicate analyses of the coirradiated Fish Canyon sanidine (FC-2, 28.02 Ma) at 1.579 ± 0.041 (× 10−4). The plagioclases were heated in stepwise increments for 60 s each, by using a 40 W CO2 laser directed through a 6 mm square integrator lens. The released argon isotopes were measured using a MAP215-50 mass spectrometer. General gas extraction, isotope correction, and calibration procedures follow those outlined in Renne et al. (27) and references therein. Measurement and age calculation were accomplished using the program mass spec written by A. Deino (Berkeley Geochronology Center, Berkeley, CA).
Supplementary Material
Acknowledgments
We thank John and Mónica Blake, owners of Killik Aike Norte farm, for donating the fossil skull and allowing us to work on their land. We also thank Sergio Vincon, Sonia Cardozo, and Laureno González for their collaboration in the field. Eugenio Aragón kindly helped with the chemical analysis of the tuff. Mrs. M. Woll enabled us to perform computed tomography scans on the skull while it was embedded in rock. We are grateful to have been supported by a research grant from the L. S. B. Leakey Foundation (to M.F.T.) and by a Tow Faculty Travel Fellowship from Brooklyn College and a Professional Staff Congress of the City University of New York Research Award Program (to A.L.R.).
Abbreviation
- Ma
mega-annum.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Fleagle, J. G., Buckley, G. A. & Schloeder, M. E. (1988) J. Vert. Paleontol. 8, 14a (abstr.).
Fleagle, J. G. Perkins, M., Bown, T. M., Tauber, A. A. & Dozo, M. T. (2004) J. Vert. Paleont. 24, Suppl. 3, 58A (abstr.).
Kay, R. F., Vizcano, S. F., Tauber, A. A., Bargo, M. S., Williams, B. A., Luna, C. & Colbert, M. W. (2005) Am. J. Phys. Anthropol. Suppl., 131 (abstr.).
References
- 1.Fleagle J. G., Tejedor M. F. In: The Primate Fossil Record. Hartwig W. C., editor. Cambridge, U.K.: Cambridge Univ. Press; 2002. pp. 161–174. [Google Scholar]
- 2.Hartwig W. C., Meldrum D. J. In: The Primate Fossil Record. Hartwig W. C., editor. Cambridge, U.K.: Cambridge Univ. Press; 2002. pp. 175–188. [Google Scholar]
- 3.Fleagle J. G., Rosenberger A. L. In: Morphologie Evolutive Morphogenese du Crane et Origine de L’Homme. Sakka M., editor. Paris: Centre National de la Recerche Scientifique; 1983. pp. 141–153. [Google Scholar]
- 4.Flynn J. J., Wyss A. R., Charrier R., Swisher C. C., III Nature. 1995;373:603–607. doi: 10.1038/373603a0. [DOI] [PubMed] [Google Scholar]
- 5.Tauber A. A. Ph.D. thesis. Córdoba, Argentina: Universidad Nacional de Córdoba; 1994. [Google Scholar]
- 6.Tauber A. A. Ameghiniana. 1997;34:413–426. [Google Scholar]
- 7.Tauber A. A. Acad. Nac. Cienc. Córdoba. 1991;82:1–32. [Google Scholar]
- 8.Tejedor M. F. Ph.D. thesis. Buenos Aires: Universidad Nacional de La Plata; 2000. [Google Scholar]
- 9.Hatcher J. B. Reports of the Princeton University Expeditions to Patagonia, 1896–1899. Volume I: Narrative of the Expeditions. Geography of Southern Patagonia. Princeton: Princeton Univ. Press; 1903. [Google Scholar]
- 10.Russo A., Flores M. A. In: Geología Regional Argentina. Leanza A. F., editor. Córdoba, Argentina: Academia Nacional de Ciencias de Córdoba; 1972. pp. 707–725. [Google Scholar]
- 11.Fleagle J. G., Bown T. M., Swisher C. C., III, Buckley G. Actas VI Congr. Argent. Paleontol. Bioestratigr. 1995:129–135. [Google Scholar]
- 12.Marshall L. G., Drake R. R., Curtis G. H., Butler R. F., Flanagan K. M., Naeser C. W. J. Geol. 1986;94:449–457. [Google Scholar]
- 13.Rosenberger A. L. Ph.D. Thesis. New York: City University of New York; 1979. [Google Scholar]
- 14.Jerison H. J. In: The Human Frontal Lobes, Second Edition: Functions and Disorders. Miller B. L., Cummings J. L., editors. New York: Guilford; 2006. in press. [Google Scholar]
- 15.Martin R. D. Primate Origins and Evolution: A Phylogenetic Reconstruction. Princeton: Princeton Univ. Press; 1990. [Google Scholar]
- 16.Rosenberger A. L. Am. J. Phys. Anthropol. 1992;88:525–562. doi: 10.1002/ajpa.1330880408. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberger A. L. J. Hum. Evol. 1977;6:541–561. [Google Scholar]
- 18.Kay R. F. Am. J. Phys. Anthropol. 1994;95:333–353. doi: 10.1002/ajpa.1330950305. [DOI] [PubMed] [Google Scholar]
- 19.Hershkovitz P. Folia Primatol. 1974;21:1–35. doi: 10.1159/000155594. [DOI] [PubMed] [Google Scholar]
- 20.Kay R. F., Campbell V. M., Rossie J. B., Colbert M. W., Rowe T. B. Anat. Rec. 2004;281A:1157–1172. doi: 10.1002/ar.a.20121. [DOI] [PubMed] [Google Scholar]
- 21.Ameghino F. Rev. Argent. Hist. Nat. (Buenos Aires) 1891;1:383–397. [Google Scholar]
- 22.Rosenberger A. L. In: Ecology and Behavior of Neotropical Primates. Coimbra-Filho A. F., Mittermeier R. A., editors. Rio de Janeiro, Brazil: Academia Brasilia de Ciencias; 1983. pp. 9–27. [Google Scholar]
- 23.Harada M. L., Schneider H., Schneider M. P., Sampaio I., Czelusniak J., Goodman M. Mol. Phyl. Evol. 1995;4:331–349. doi: 10.1006/mpev.1995.1029. [DOI] [PubMed] [Google Scholar]
- 24.Schneider H. An. Acad. Bras. Cienc. 2000;72:165–172. doi: 10.1590/s0001-37652000000200005. [DOI] [PubMed] [Google Scholar]
- 25.Fedigan L. M., Rosenberger A. L., Boinski S., Norconk M. A., Garber P. A. In: Adaptive Radiations of Neotropical Primates. Norconk M. A., Rosenberger A. L., Garber P. A., editors. New York: Plenum; 1996. pp. 219–228. [Google Scholar]
- 26.Begun R. In: The Primate Fossil Record. Hartwig W. C., editor. Cambridge, U.K.: Cambridge Univ. Press; 2002. pp. 339–368. [Google Scholar]
- 27.Renne P. R., Swisher C. C., III, Deino A. L., Karner D. B., Owens T. L., DePaolo D. J. Chem. Geol. 1998;145:117–152. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.