Abstract
Interactions between neoplastic and stromal cells contribute to tumor progression. Wnt genes, involved in cell migration and often deregulated in cancers, are attractive candidates to regulate these effects. We have recently shown that coculture of breast cancer cells with macrophages enhances invasiveness via matrix metalloproteases and TNF-α. Here we demonstrate that coculture of MCF-7 cells and macrophages leads to up-regulation of Wnt 5a in the latter. This was accompanied by activation of AP-1/c-Jun in MCF-7. Recombinant Wnt 5a mimicked the coculture effect. Wnt 5a was also detectable in tumor-associated macrophages in primary breast cancers. Experiments with agonists and antagonists of Wnt signaling revealed that a functional canonical pathway in the tumor cells was a necessary prerequisite; however, noncanonical signaling via Wnt 5a and the Jun-N-terminal kinase pathway was critical for invasiveness. It was also responsible for induction of matrix metalloprotease-7, known to release TNF-α. All these effects could be antagonized by dickkopf-1. Our results indicate that Wnt 5a is essential for macrophage-induced invasiveness, because it regulates tumor cell migration as well as proteolytic activity of the macrophages. The function of Wnt 5a as either a suppressor or promoter of malignant progression seems to be modulated by intercellular interactions. Wnt 5a detection in tumor-associated macrophages in breast cancer biopsies supports the assumption that similar events play a role in vivo.
Keywords: tumor microenvironment, TNF-α, matrix metalloproteases
Genes of the Wnt family play a critical role in cellular proliferation, migration, and tissue patterning during embryonic development (1). Wnts bind to G-protein-coupled receptors of the Frizzled (Fz) and low-density lipoprotein receptor-related protein (LRP) family and activate multiple signaling pathways. The canonical pathway leads via the disheveled proteins to β-catenin. In benign cells, most of the β-catenin molecules are transcriptionally inactive and localized at the plasma membrane where they link E-cadherin to the actin cytoskeleton via α-catenin. Additionally, β-catenin is inactivated by a multimolecular complex containing the adenomatous polyposis coli protein, axin, and glycogen synthase kinase-3β (GSK-3β). Serine/threonine phosphorylation of β-catenin by GSK-3β results in its ubiquitination and proteolysis. Canonical Wnt signaling inhibits β-catenin degradation by inactivation of GSK-3β. Hypophosphorylated β-catenin then translocates to the nucleus, where it binds to transcription factors of the T cell factor/lymphocyte enhancer factor (TCF/LEF) family and initiates transcription of target genes, such as c-myc, cyclin D1, and the matrix metalloprotease (MMP) matrilysin (mmp-7).
Physiological Wnt inhibitors are the secreted Fz-related proteins (sFRP), Wnt inhibitory factor, and the members of the dickkopf (DKK) family. DKK-1 binds to the coreceptors LRP 5/6, thereby preventing the Wnt-induced Fz-LRP5/6 complex formation and disrupting canonical signaling (2, 3). Recent evidence demonstrates that DKK-1 is also able to antagonize β-catenin-independent noncanonical signaling (4, 5).
The noncanonical Wnt/Ca2+ pathway is triggered by Wnt 4, Wnt 5a, and Wnt 11. It induces intracellular Ca release and activation of PKC and is involved in an antagonistic crosstalk with the canonical pathway (6, 7). Other noncanonical Wnt signals activate small Rho-GTPases and regulate cytoskeletal architecture and cellular polarity. Downstream events are formation of complexes between specific domains of disheveled and either Rho or Rac, the latter leading to activation of Jun-N-terminal kinase (JNK) (8, 9, 10).
Wnt signaling has been implicated in malignant transformation and tumor progression. Wnts are overexpressed in a variety of cancers, their expression correlating with the transition from normal tissue to progressive malignancy (11–13). Secretion of the antagonists DKK-1, Wnt inhibitory factor, and the sFRPs is often down-regulated (14–16). Many cancer cells show accumulation and nuclear localization of β-catenin (17) due to impaired degradation (18, 19). β-Catenin translocation was demonstrated especially at the invasive frontier between tumor and surrounding tissue, suggesting that malignant and stromal cells interact in some way to regulate β-catenin activation (20, 21).
In contrast to the highly transforming Wnts 1, 3a, and 7a (22), Wnt 5a has been considered mainly a tumor suppressor. It promotes GSK-3β-independent β-catenin degradation (23) and enhances cell–cell adhesion on collagen matrices (24). Metastatic neuroblasts in a xenograft model displayed lower Wnt 5a expression than the primary neuroblastoma cells (25). In a retrospective analysis of breast cancers, immunohistochemical Wnt 5a detection was inversely correlated with metastasis formation and survival (26). On the other hand, Wnt 5a was found to be overexpressed in a variety of cancers in comparison to the respective benign tissues (11, 27). Wnt 5a expression was one of the most robust markers for aggressive behavior of cutaneous melanomas (28), where it was present predominantly in actively invasive cells. Artificial overexpression resulted in increased invasiveness via PKC activation (29). These apparently contradictory findings suggest that the function of Wnt 5a is modified by additional regulators. Interestingly, overexpression of Wnt 5a has been demonstrated not only in tumor cells, but also in the tumor stroma, especially in tumor-associated macrophages (TAM) (13).
There is growing evidence that interactions between tumor cells and the stromal compartment are crucial for malignant progression. TAM play an ambiguous role in this context. Although macrophages (Mφ) in general are known for their tumoricidal capacity, many authors have demonstrated a tumor-promoting effect (30). High numbers of infiltrating TAM are correlated with poor clinical outcome in breast cancers (31). Metastatic disease occurs less frequently in breast-cancer-bearing mice with defective Mφ recruitment than in their intact counterparts (32). We have recently shown that coculture of weakly invasive breast cancer cells with Mφ significantly enhances invasiveness of the tumor cells in a TNF-α-dependent way due to up-regulation of MMP-2, -3, -7, and -9 in the Mφ (33). MMP-7 is known to release active TNF-α from its membrane-bound proform (34). It also represents one of the target genes of Wnt signaling.
Based on these findings, we hypothesized that Wnt genes might be involved in the regulation of Mφ-induced tumor cell invasion. To further clarify this question, the influence of the coculture on the expression of Wnt family genes as well as on the production of MMPs and TNF-α was investigated in breast cancer cell lines and Mφ. Using agonists and antagonists of both canonical and noncanonical Wnt signaling, we tried to identify the responsible signaling pathways. Immunohistochemistry of malignant breast lesions served to assess the biological significance of the obtained results.
Results
DKK-1 Inhibits Coculture-Induced Invasiveness Without Influencing Cellular Viability.
Recently, we have shown that coculture of weakly invasive breast cancer cells with Mφ leads to enhanced invasiveness of the malignant cells. To clarify whether Wnt signals are involved in Mφ-induced invasion, the effect of the Wnt inhibitor DKK-1 on the coculture was investigated.
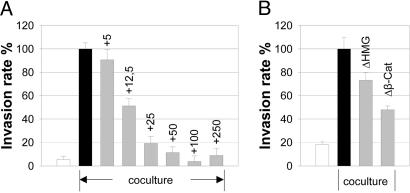
MCF-7 cells were cocultivated with Mφ in a modified Boyden chamber. Recombinant DKK-1 down-regulated the Mφ-induced invasiveness of MCF-7 in a dose-dependent manner (Fig. 1A). The inhibition was maximal at 50 ng/ml and could not be further increased by higher concentrations. A similar although weaker effect was found with the cell line SK-BR-3 (Table 2, which is published as supporting information on the PNAS web site). Because DKK-1 is able to antagonize proliferation and sensitize cells to proapoptotic stimuli, we studied the influence of DKK-1 on cellular viability through measurement of [3H]thymidine incorporation as well as 2,3-diphenyl-5-methyltetrazolium chloride conversion. DKK-1-mediated decreased invasion was not due to an inhibitory effect on either proliferation or mitochondrial metabolism (Table 3, which is published as supporting information on the PNAS web site).
Fig. 1.
DKK-1 inhibits coculture-induced enhanced invasiveness in a dose-dependent manner. (A) Microinvasion assay of MCF-7 cells (controls = white bar, + Mφ = black bar), cocultured with Mφ± recombinant DKK-1 at the indicated concentrations (nanograms per milliliter) (gray bars). Invasiveness is given as the rate of invasive tumor cells in percent of the coculture control (means ± SD, n = 5–6). (B) Inhibition of the canonical Wnt pathway downstream from β-catenin antagonizes coculture-induced enhanced invasiveness. Microinvasion assay of MCF-7 cells, cocultured with Mφ. Cells were transfected with either empty control vectors (white and black bars) or dominant negative LEF-1-ΔHMG and LEF-1-Δβcat (gray bars), respectively (means ± SD, n = 5–6).
Although Not Activated by Coculture, a Functional Canonical Pathway in the Tumor Cells Is a Prerequisite for Induced Invasiveness.
Because DKK-1 is known predominantly as an inhibitor of the canonical Wnt signaling pathway, we asked whether coculture-induced invasiveness requires TCF/LEF transcriptional activity in the tumor cells. Two dominant-negative LEF constructs as well as empty control vectors were transiently transfected into MCF-7 cells. Overexpression of LEF-1-ΔβCat reduced coculture-induced invasiveness by >50%. LEF-1-ΔHMG was also effective, although to a lesser extent (Fig. 1B).
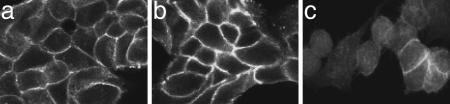
We then evaluated whether β-catenin-dependent signaling was activated by the coculture. In MCF-7, β-catenin was found predominantly in its signaling-inactive form at the plasma membrane with only weak cytoplasmic and nuclear staining. Coculture with Mφ did not alter its subcellular localization (Fig. 2a and b). There was no influence of coculture on the cellular β-catenin content regarding either the total amount or the levels of unphosphorylated β-catenin (Fig. 8, which is published as supporting information on the PNAS web site). Equally, DKK-1 treatment did not affect these levels. The content of cellular E-cadherin, known to be complexed with β-catenin at the plasma membrane, remained stable under both conditions.
Fig. 2.
Coculture does not influence the predominant localization of β-catenin at the cell membrane in contrast to LiCl. Immunofluorescence staining for total β-catenin in MCF-7 cells: (a) controls, (b) cocultivated with Mφ (20 h), and (c) +3 mM LiCl.
Wnt 5a Is Up-Regulated in Macrophages upon Coculture.
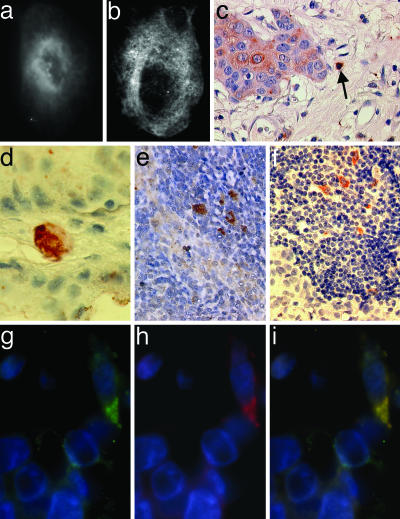
We then investigated the expression pattern of members of the Wnt family in MCF-7 as well as in Mφ under control and coculture conditions (Table 1; also see Table 4, which is published as supporting information on the PNAS web site). Both cell types expressed mRNAs of various Wnt ligands, receptors, downstream signaling molecules, and target genes. Although most of them were not influenced by coculture, mRNA of the noncanonical ligand Wnt 5a in Mφ was detectable only after cocultivation with MCF-7 (Fig. 9, which is published as supporting information on the PNAS web site). This could be confirmed also on the protein level (Fig. 3a and b). Wnt 5a protein staining in MCF-7 cells was only weak.
Table 1.
mRNA expression of Wnt family and associated genes in breast cancer cells and macrophages
| MAC | MAC coculture | MCF-7 | MCF-7 coculture | |
|---|---|---|---|---|
| Wnt 2* | − | − | − | − |
| Wnt 3* | − | − | + | + |
| Wnt 5a* | − | + | + | + |
| Wnt 7b* | − | − | + | + |
| Wnt 10b* | − | − | + | + |
| Wnt 13* | + | + | + | + |
| Fz 1** | − | − | + | + |
| Fz 2* | + | + | + | + |
| Fz 3** | − | − | + | + |
| Fz 4** | − | − | + | + |
| Fz 6* | − | − | + | + |
| Fz 7** | − | − | + | + |
| Fz 8** | − | − | + | + |
| LRP 5* | + | + | + | + |
| LRP 6* | − | − | + | + |
| DKK1* | − | − | + | + |
*RT-PCR.
**cDNA array.
Fig. 3.
Coculture induces up-regulation of Wnt 5a in Mφ. Wnt 5a-immunofluorescence staining of Mφ alone (a) and Mφ cocultivated with MCF-7 cells for 20 h (b). Wnt 5a is expressed in TAM in primary breast cancer tissues and lymph node metastases. (c and d) Wnt 5a-positive cell with Mφ-like morphology (arrow) in the stromal layer of a Wnt 5a-positive (c) and a Wnt 5a-negative ductal invasive breast adenocarcinoma (d) (magnification 10 × 63 and 10 × 100). (e) Wnt 5a-positive Mφ-like cells at the invasive front of a lymph node infiltrating tumor. (f) CD68 staining of an adjacent area of the same lymph node showing multiple TAM (×10 × 63). Immunofluorescence double staining of CD68 (g) and Wnt 5a (h) in a ductal invasive breast adenocarcinoma, demonstrating colocalization (i) of both signals (×10 × 63).
Wnt 5a Is Expressed in Tumor-Associated Macrophages in Breast Cancer Tissues and Lymph Node Metastases.
Next, we asked whether coculture-induced up-regulation of Wnt 5a in Mφ is restricted to the in vitro model or is reproducible also in malignant breast lesions. Thirty-two tissue samples (see Materials and Methods) were stained with antibodies against Wnt 5a and CD68 as a marker for TAM (Fig. 3 c–f). In 10 of 17 ductal invasive cancers, the tumor tissue was Wnt 5a-negative or weakly positive; seven were positive or strongly positive. In the 10 cases where primary tumors and matching lymph node metastases were available, Wnt 5a expression of the tumor cells themselves was comparable. Tumors and lymph nodes contained variable amounts of CD68-positive cells with Mφ-like morphology. Wnt 5a was detectable in some of these cells, their percentage varying between 5% and 15% in the different samples. Wnt 5a-positive Mφ could be detected especially at the invasive front of lymph node metastases. Immunofluorescence double staining confirmed the colocalization of Wnt 5a and CD68 signals (Fig. 3 g–i) and demonstrated that some but not all of the TAM expressed Wnt 5a. Only one of the five ductal carcinomas in situ contained CD68-positive cells as well as very few isolated Wnt 5a-positive Mφ. In the other four, no Mφ were detectable.
Noncanonical Signaling via Macrophage-Derived Wnt 5a Is Critical for Coculture-Induced Invasiveness.
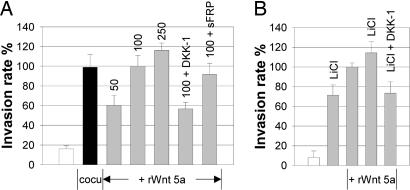
Because Wnt 5a was up-regulated in Mφ upon coculture with MCF-7 cells, we asked whether addition of Wnt 5a alone would be sufficient to elicit a similar increase in invasion. MCF-7 cells were subjected to microinvasion assays containing recombinant Wnt 5a (rWnt 5a) instead of Mφ. Invasiveness was enhanced by rWnt 5a in a dose-dependent manner (Fig. 4A and Fig. 10, which is published as supporting information on the PNAS web site). Addition of 100 ng/ml recombinant ligand increased invasiveness in a comparable way, as did the coculture. Wnt 5a-enhanced invasiveness was inhibited by DKK-1, whereas sFRP-1, which is known to block signaling of many Wnt ligands except Wnt 5a (35), had no effect. Similar results were achieved with the cell line SK-BR3 (Table 2).
Fig. 4.
Incubation of MCF-7 cells with rWnt 5a is sufficient to induce invasiveness. (A) Microinvasion assay of MCF-7 cells (controls = white bar, +Mφ = black bar) incubated with rWnt 5a (gray bars) at the indicated concentrations (nanograms per milliliter) ± the inhibitors DKK-1 (50 ng/ml) as well as sFRP-1 (400 ng/ml). (B) DKK-1 blocks Wnt 5a-induced invasion. Microinvasion assay of MCF-7 cells (controls = white bar) incubated with LiCl (3 mM), rWnt 5a (100 ng/ml), rWnt 5a + LiCl, rWnt 5a + LiCl, and DKK-1 (50 ng/ml). Invasiveness in both experiments is indicated as the rate of invasive tumor cells in percent of the 100 ng/ml Wnt 5a-control (means ± SD, n = 5–6).
To further specify the role of noncanonical Wnt 5a signaling, microinvasion assays with addition of the GSK-3β inhibitor lithium chloride LiCl were performed. LiCl effectively activated the canonical pathway, as demonstrated by translocation of β-catenin from the cell membrane to the cytoplasm and the nucleus (Fig. 2c). It also induced invasion when given alone and enhanced the function of Wnt 5a when applied together (Fig. 4B). The additive effect of LiCl and Wnt 5a on invasiveness was reduced to the levels induced by LiCl alone by addition of DKK-1. Acting upstream from GSK-3β, DKK-1 is unable to antagonize LiCl-triggered β-catenin signaling. This indicates, that DKK-1 inhibits only the Wnt 5a-part of induced invasion and functions as an inhibitor of noncanonical Wnt signaling.
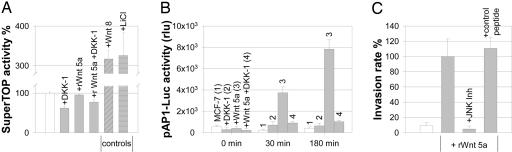
Wnt 5a has also been described to inhibit the canonical pathway. β-Catenin signaling was therefore measured via luciferase activity of the SuperTOP-vector, containing multiple TCF/LEF-binding sites. rWnt 5a neither activated nor inhibited canonical signaling, whereas the positive controls LiCl and Wnt 8 resulted in strong activation (Fig. 5A; for results of the negative control vector SuperFOP, see Table 5, which is published as supporting information on the PNAS web site).
Fig. 5.
Wnt 5a does not interfere with canonical signaling. (A) Luciferase activity in MCF-7 cells transfected with the SuperTOP-reporter construct (controls = white bar, means ± SE, n = 3–12, arbitrary units). For SuperFOP negative control, see Table 3. (B) Wnt 5a signals are mediated via AP-1. Luciferase activity in MCF-7 transfected with pAP1-Luc (means ± SD, n = 3, arbitrary units). (C) Inhibition of JNK abolishes Wnt 5a-induced invasion. Microinvasion assay of MCF-7 cells (white bar) incubated with 100 ng/ml rWnt 5a (gray bars) ± the JNK Inhibitor 1 or the respective control peptide, 1 μM each. Invasiveness is indicated as the rate of invasive tumor cells in percent of the Wnt 5a control (means ± SD, n = 5–6).
We then focused on the JNK/c-Jun pathway. As shown in Fig. 5B, AP1-promotor activity in MCF-7 cells increased dramatically after addition of rWnt 5a, which was again antagonized by DKK-1. Equally, c-Jun was rapidly phosphorylated at Ser-63 in response to rWnt 5a (Fig. 11, which is published as supporting information on the PNAS web site). Corresponding results were achieved in the coculture, where the low constitutive DNA binding activity of c-Jun in MCF-7 cells was up-regulated 5-fold by exposure to Mφ (Fig. 12, which is published as supporting information on the PNAS web site). Wnt 5 a-induced invasiveness was completely abrogated by addition of the JNK-inhibitor 1, whereas the control peptide had no effect (Fig. 5C).
Wnt 5a has been shown to enhance invasiveness via PKC activation. However, neither the amount of the total protein nor that of the phosphorylated form was influenced by addition of rWnt 5a (Fig. 13, which is published as supporting information on the PNAS web site).
Wnt 5a Triggers Production of MMP-7 and TNF-α by Macrophages.
As we have shown earlier, coculture-induced enhanced invasiveness relies on up-regulated proteolysis by the Mφ-derived gelatinases MMP-2 and -9 and the caseinases MMP-3 and -7. Induction of MMP-2 and –9, as well as invasiveness, is TNF-α-dependent.
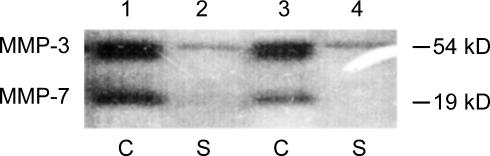
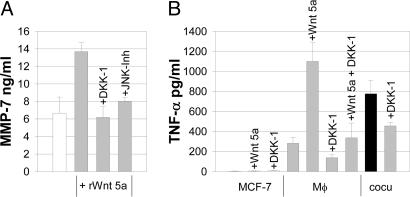
To determine whether inhibition with DKK-1 would interfere with the coculture-triggered MMP induction, we assessed MMP levels by gelatin and casein zymography. Although DKK-1 abolished invasiveness of the tumor cells almost completely, it had no influence on the coculture-induced increased production and secretion of either the gelatinases MMP-2 and -9 (Fig. 14, which is published as supporting information on the PNAS web site) or the caseinase MMP-3 by Mφ (Fig. 6). In contrast, production of MMP-7, known as a target of Wnt signaling, was down-regulated by DKK-1. Similar to the coculture, MMP-7 in Mφ was induced by addition of rWnt 5a (Fig. 7A). The effect was blocked by DKK-1 as well as by the JNK-inhibitor 1.
Fig. 6.
DKK-1 antagonizes coculture-induced MMP-7 production in Mφ. Casein zymography of cellular lysates (C) and supernatants (S) of Mφ cocultivated with MCF-7 for 96 h. Lanes 1 and 2, without DKK-1; lanes 3 and 4, with 50 ng/ml DKK-1.
Fig. 7.
Wnt 5a-induced up-regulation of MMP-7 is antagonized by DKK-1 and JNK inhibition. (A) MMP-7 ELISA with Mφ (white bar), incubated with 100 ng/ml rWnt 5a (gray bars) ± DKK-1 (50 ng/ml) and JNK inhibitor 1, 1 μM (means ± SD, n = 3). (B) DKK-1 inhibits coculture- as well as Wnt 5a-induced up-regulation of TNF-α. ELISA of TNF-α in supernatants of MCF-7 cells, Mφ, and MCF-7/Mφ cocultures ± 50 ng/ml DKK-1 (means ± SD, n = 3).
In parallel to the levels of the TNF-α-shedding protease MMP-7, the concentration of TNF-α in the supernatants was increased by coculture as well as by rWnt 5a. The effect was antagonized by addition of DKK-1 (Fig. 7B).
Discussion
Macrophages play an ambiguous role in cancers. Although generally known for their tumoricidal capacity, there is growing evidence that regulatory networks within tumor tissues redirect their function into a tumor-promoting activity. We have recently shown that cocultivation with Mφ increases invasiveness of weakly invasive breast cancer cells (33). The aim of this study was to investigate whether Wnt signaling is involved in this effect.
We found that the physiological Wnt inhibitor DKK-1 completely blocked Mφ-induced invasiveness. DKKs, especially DKK-1 and -3, have been implicated in tumorigenesis. Although most authors focused on down-regulation of DKK expression in various cancer models and tissues (5, 14, 36, 37), the role of DKKs for invasion has scarcely been investigated. Hoang et al. (38) described an inhibitory effect of DKK-3 on invasion and motility of osteosarcoma cells.
The obvious assumption that canonical Wnt signaling would be responsible for the coculture effect could not be confirmed. β-Catenin signaling in the investigated cell lines, which do not show constitutive β-catenin overexpression, was not activated by coculture. A functional canonical pathway was nevertheless necessary, because inhibition of TCF/LEF transcriptional activity via dominant negative LEF-1 antagonized induced invasion. Thus, canonical signaling in the tumor cells seems to play a permissive but not an active role for Mφ-mediated invasiveness.
The only Wnt gene clearly induced by coculture was the noncanonical ligand Wnt 5a. Demonstrating the functional impact of Wnt 5a expression in Mφ, incubation of the breast cancer cells with recombinant Wnt 5a alone was sufficient to mimic the coculture effect. As in the coculture experiments, Wnt 5a-induced invasiveness was antagonized by DKK-1. Secreted FRP-1, which neutralizes the major Wnt ligands except Wnt 5a (35), had no effect. This shows that Mφ-derived Wnt 5a is a critical signal for induced invasion and argues against involvement of other circulating Wnt ligands. It also demonstrates that DKK-1 is able to antagonize noncanonical signaling. That DKK-1 completely blocks the Wnt 5a-mediated part of the additive effect of LiCl and Wnt 5a on invasion underlines this finding.
Recent reports describe decreased migration of epithelial (cancer) cells on collagen upon exposure to relatively high concentrations of Wnt 5a and synthetic peptide derivatives (39). However, Wnt 5a can also act as an antagonist of canonical signaling (6). Although in our experiments there was no negative influence, higher concentrations of Wnt 5a could have interfered with the β-catenin pathway, thus inhibiting invasion.
As we have shown here and earlier (40), AP-1 promoter activity, c-Jun Ser 63 phosphorylation, and DNA-binding activity of c-Jun in the tumor cells were increased by coculture and rWnt 5a. Inhibition of JNK abolished coculture- and Wnt 5a-induced invasiveness, providing further evidence of β-catenin-independent signaling. Our observation that activation of the JNK/c-Jun pathway and a functional canonical pathway are both needed for these effects is compatible with recent findings of Nateri et al. (41). They described that signaling via the TCF/β-catenin and the JNK pathway cooperates via phosphorylation-dependent interaction between c-jun and TCF4.
Although Wnt 5a has been considered predominantly a tumor suppressor (25, 26, 42), there is evidence that it may also enhance malignant cell motility and foster tumor progression. Wnt 5a expression was associated with invasive behavior in cutaneous melanomas, being detectable predominantly in actually invasive cells (29). In contrast to our model, invasive signaling in melanoma cells was mediated via PKC activation.
The association of Wnt 5a with invasion in melanomas corresponds to our findings in human breast cancers. Some of the tumors stained positive and some negative for Wnt 5a, as described by others (26). However, in all of them a certain amount of the markedly varying number of TAM expressed Wnt 5a. Interestingly, Wnt 5a-positive TAMs could be found especially at the invasive front of lymph node metastases. It is tempting to speculate on why some of the TAM produce Wnt 5a and others do not. Wnt 5a expression may be involved in the still-unclear transition from tumoricidal to tumor-promoting TAM and reflect the activity of the regulatory network between tumor cells and the stromal compartment. Earlier studies did not focus on stromal cells. This may explain that Wnt 5a expression has been described to be associated with prolonged survival on the one hand and with invasion on the other.
Because Mφ-induced invasiveness depends on MMP up-regulation, we were interested in whether there would be any crosslinks between the proteolytic pathway and the migratory signaling through Wnts. TNF-α is part of both mechanisms and, as we have shown earlier, is indispensable for induced invasiveness. DKK-1 inhibits coculture- and Wnt 5a-mediated induction of MMP-7 as well as production of TNF-α in Mφ. This suggests that MMP-7, known as one of the shedding proteases of TNF-α, is not only a target of canonical Wnt signaling. Le Floch et al. (43) have recently shown that the mmp-7 promoter contains an AP-1-responsive element and is inducible also by noncanonical Wnt signals. Thus, it is attractive to hypothesize that coculture leads to up-regulation of Mφ-Wnt 5a, which, in turn, induces MMP-7. MMP-7 then releases TNF-α, which is followed by induction of MMP-2 and -9 and further activation of the proteolytic cascade. That MMP-2 and -9 production was not inhibited by DKK-1 does not contradict this hypothesis. The release of TNF-α was only partially antagonized by DKK-1, presumably leaving sufficient levels to induce these abundantly produced MMPs. Their up-regulation, however, was not able to trigger enhanced invasiveness, because the Wnt-governed migratory capacity of the tumor cells was impaired at the same time.
Taken together, both canonical and noncanonical Wnt signaling are required for Mφ-induced invasiveness. Canonical Wnt signaling does not play an active role in the coculture effect. However, it is a necessary prerequisite for the tumor cells to respond to the Mφ-derived Wnt 5a. Noncanonical Wnt 5a is the critical signal for coculture-induced invasiveness. Via release of TNF-α, Wnt 5a-induced MMP-7 seems to link the migration-regulating Wnt pathway with the proteolytic cascade, both mechanisms being equally indispensable for successful invasion. Our data suggest that the function of Wnt 5a either as a suppressor or as a promoter of malignant progression is determined not only by intracellular conditions but also by intercellular interactions among different cell types. The observation of Wnt 5a-expressing TAM in breast cancer tissues supports the assumption that similar events play a role in human breast cancer progression.
Materials and Methods
Cells and Tissues.
Human breast cancer cell lines (American Type Culture Collection) were grown on RPMI medium 1640 plus 10% FCS, depleted of gelatinase-type MMPs. Human Mφ were derived from peripheral blood mononuclear cells, as described (33). NIH 3T3 cells stably transfected with a plasmid containing murine wnt 5a as well as LacZ-negative controls were kindly provided by M. Kuehl (University of Ulm, Ulm, Germany). Tissues were obtained from 22 consecutive patients of the University Hospital of Göttingen with the following diagnoses: ductal invasive breast cancer (7 primary tumors, node negative; 10 primary tumors and 10 matching lymph node metastases; and 5 ductal carcinomas in situ).
Microinvasion Assay.
Invasion was measured in a modified Boyden chamber, where the tumor cells are cocultivated with Mφ without direct cell–cell contact. The detailed procedure has been described (33). The following Wnt agonists and antagonists were added: LiCl (3 mM) and rWnt-5a (R & D Systems), rDKK-1 (R & D Systems), JNK-inhibitor 1 and control peptide (Alexis, Grünberg, Germany; 1 μM), and sFRP-1 (R & D Systems; 400 ng/ml). Comparative experiments were performed with Wnt 5a-conditioned medium obtained from Wnt 5a-overexpressing NIH 3T3 fibroblasts (see above). They yielded the same results, thus demonstrating the functional activity of the recombinant protein (see Fig. 15, which is published as supporting information on the PNAS web site).
RNA Extraction, RT-PCR, and cDNA Arrays.
Total RNA was extracted with the RNeasy kit (Qiagen, Hilden, Germany). Reverse transcription was performed from 2 μg of total RNA by using oligo(dT) primers and M-MLV reverse transcriptase (Roche Diagnostics, Mannheim, Germany). Primers for Wnt 7b were: 5′-GTTACGGCATCGACTTCTCC, 3′-GTCCTCCTCGCAGTAGTTGG, primers for Wnt 2 (44), Wnt 3, Fz 6, LRP 6, DKK-1 (45), Wnt 5a, Wnt 10b, Wnt 13, Fz 2 (46), β-Catenin, Cyclin D1, c-myc, MMP 7, c-jun (47), and LRP-5 (48). Quantitative RT-PCR for Wnt 5a was performed on the Applied Biosystems 7900HT system using the TaqMan hWnt 5a and β2-microglobulin kits (Applied Biosystems).
For microarray studies, 7 μg of total RNA was labeled with the Superarray GEArray-RT-Labeling Kit (SuperArray Bioscience, Frederick, MD) according to the chemiluminescent detection protocol. Hybridization was carried out on the GEArray Q Series Human Wnt Signaling Pathway Microarray (HS-043; SuperArray Bioscience). Chemiluminescence detection on Hyperfilm (Amersham Pharmacia Biosciences) was performed with the Superarray Chemoluminescence Detection kit.
Constructs, Transfections, and Reporter Assays.
MCF-7 cells were seeded at a density of 105/ml into six-well dishes. After 12 h, the cells were transfected by using the CalPhos Mammalian transfection kit (BD Biosciences, Franklin Lakes, NJ) with 4 μg of the pBAT plasmid, containing either dominant negative LEF-1 [LEF-1-ΔβCat, lacking the β-catenin-binding site; LEF-1-ΔHMG, lacking the DNA-binding site (49)] or the empty control vector. To monitor β-catenin-dependent signaling and AP-1 activation, luciferase reporter assays were performed. Either 0.5 μg of the SuperTOP vector (50), containing multiple TCF/LEF-binding sites, or its negative control vector SuperFOP were cotransfected by using the MATra reagent (IBA, Göttingen, Germany) with pCMV-βgal to monitor for transfection efficiency. After 48 h, cells were harvested and luciferase activity was measured by using a luciferase assay kit (Promega) and normalized to β-galactosidase activity. The pAP1-Luc (BD Clontech) reporter assays were carried out as described (40). For all experiments, three independent transfections were performed.
ELISAs, Proliferation, and Viability Assays.
TNF-α and MMP-7 concentrations were determined by using commercial ELISA kits (R & D Systems). c-Jun phosphorylation was assessed with the Phospho-c-Jun (Ser-63) Sandwich ELISA (Cell Signaling Technology, Beverly, MA). Proliferation was analyzed by using [6-3H]thymidine incorporation (Amersham Pharmacia Biosciences), viability by measurement of 2,3-diphenyl-5-methyltetrazolium chloride conversion according to standard procedures.
c-Jun-Binding Assay.
Nuclear extracts from MCF-7 cells were prepared as described (40). After measurement of protein concentration (BCA assay; Pierce), the nuclear fractions were used to determine DNA binding of c-Jun with the Mercury TransFactor c-Jun kit (BD Biosciences).
Western Blot and Immunocytohistochemistry.
Cells were lysed and homogenized in sucrose buffer (250 mM sucrose/5 mM Hepes/0.5 mM EDTA, pH 7.5). Sixty micrograms of total protein (BCA assay, Pierce) was subjected to SDS/PAGE (10%) and transferred to a nitrocellulose membrane (Amersham Pharmacia). Protein detection was carried out with antibodies against total (Santa Cruz Biotechnology) and unphosphorylated β-catenin (Alexis), E-cadherin (Transduction Laboratories, Lexington, MA), total PKC (Santa Cruz Biotechnology), unphosphorylated PKC (Cell Signaling Technology), actin (Chemicon), horseradish peroxidase-coupled secondary antibodies (Santa Cruz Biotechnology), and the enhanced chemiluminescence detection system (Amersham Pharmacia).
For immunocytochemistry, cells were grown on 12-mm coverslips. Fixation (3.7% paraformaldehyde) and permeabilization (Triton X-100, 0.2%) was followed by incubation with the primary antibody (total β-catenin, 1:200; Wnt-5a, 1:100; R & D Systems) and the FITC/TRITC-coupled secondary antibodies (BD Biosciences). Control experiments to confirm the specificity of the antibody reaction were performed with Wnt 5a-overexpressing NIH 3T3 cells and the vector-containing LacZ-control cells (Figs. 15 and 16, which are published as supporting information on the PNAS web site), as well as by saturation of the primary antibody via addition of rWnt 5a.
Paraffin-embedded tissue sections were dewaxed, rehydrated, and boiled in Dako target retrieval buffer, pH 9 (DakoCytomation, Hamburg, Germany). The CD68 antibody KP-1(Dako; 1:250) and the above-mentioned antibodies were applied under the same conditions. For light microscopy, the Dako EnVision+ System horseradish peroxidase kit was used for signal detection.
Zymography.
Cell lysates and supernatants were separated on 10% SDS–polyacrylamide gels containing either gelatin (1 mg/ml) or β-casein (0.5 mg/ml). Gels were then incubated in renaturation buffer and stained with Coomassie brilliant blue. The detailed procedure has been described (33).
Supplementary Material
Acknowledgments
We are grateful to Professor Doris Wedlich (University of Karlsruhe, Karlsruhe, Germany), Dr. Hemmerlein (University of Göttingen, Göttingen, Germany), and Professor M. Kühl (University of Ulm, Ulm, Germany) for helpful discussions. This work was supported by a grant from the Medical Faculty of the University of Göttingen.
Abbreviations
- LRP
low-density lipoprotein receptor-related protein
- LEF
lymphocyte enhancer factor
- GSK-3β
glycogen synthase kinase-3β
- MMP
matrix metalloprotease
- Fz
Frizzled
- sFRP
secreted Fz-related proteins
- DKK
dickkopf
- JNK
Jun-N-terminal kinase
- TAM
tumor-associated macrophage
- Mφ
macrophage
- rWnt 5a
recombinant Wnt 5a.
Footnotes
Conflict of interest: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Nusse R. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- 2.Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S. A. Nat. Cell Biol. 2001;7:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- 3.Semenov M. V., Tamai K., Brott B. K., Kuhl M., Sokol S., He X. Curr. Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee A. Y., He B., You L., Xu Z., Mazieres J., Reguart N., Mikami I., Batra S., Jablons D. M. Biochem. Biophys. Res. Commun. 2004;323:1246–1250. doi: 10.1016/j.bbrc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Mikheev A. M., Mikheeva S. A., Liu B., Cohen P., Zarbl H. Carcinogenesis. 2004;25:47–59. doi: 10.1093/carcin/bgg190. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl M., Geis K., Sheldahl L. C., Pukrop T., Moon R. T., Wedlich D. Mech. Dev. 2001;106:61–76. doi: 10.1016/s0925-4773(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 7.Sheldahl L. C., Slusarski D. C., Pandur P., Miller J. R., Kuhl M., Moon R. T. J. Cell Biol. 2003;26:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capelluto D. G., Kutateladze T. G., Habas R., Finkielstein C. V., He X., Overduin M. Nature. 2002;419:726–729. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- 9.Habas R., Dawid I. B., He X. Genes Dev. 2003;15:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamanaka H., Moriguchi T., Masuyama N., Kusakabe M., Hanafusa H., Takada R., Takada S., Nishida E. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huguet E. L., Smith K., Bicknell R., Harris A. L. J. Biol. Chem. 1995;270:12851–12856. doi: 10.1074/jbc.270.21.12851. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune S., Huguet E. L., Hamby A., Poulsom R., Harris A. L. Clin. Cancer Res. 1995;1:215–222. [PubMed] [Google Scholar]
- 13.Smith K., Bui T. D., Poulsom R., Kaklamanis L., Williams G., Harris A. L. Br. J. Cancer. 1999;81:496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Sancho J. M., Aguilera O., Garcia J. M., Pendas-Franco N., Pena C., Cal S., Garcia de Herreros A., Bonilla F., Munoz A. Oncogene. 2005;24:1098–1103. doi: 10.1038/sj.onc.1208303. [DOI] [PubMed] [Google Scholar]
- 15.Wissmann C., Wild P. J., Kaiser S., Roepcke S., Stoehr R., Woenckhaus M., Kristiansen G., Hsieh J. C., Hofstaedter F., Hartmann A., et al. J. Pathol. 2003;201:204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 16.Lee A. Y., He B., You L., Dadfarmay S., Xu Z., Mazieres J., Mikami I., McCormick F., Jablons D. M. Oncogene. 2004;23:6672–6676. doi: 10.1038/sj.onc.1207881. [DOI] [PubMed] [Google Scholar]
- 17.Prange W., Breuhahn K., Fischer F., Zilkens C., Pietsch T., Petmecky K., Eilers R., Dienes H. P., Schirmacher P. J. Pathol. 2003;201:250–259. doi: 10.1002/path.1448. [DOI] [PubMed] [Google Scholar]
- 18.Van Es J. H., Giles R. H., Clevers H. C. Exp. Cell Res. 2001;10:126–134. doi: 10.1006/excr.2000.5142. [DOI] [PubMed] [Google Scholar]
- 19.Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 20.Brabletz T., Jung A., Hermann K., Gunther K., Hohenberger W., Kirchner T. Pathol. Res. Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 21.Miyazawa K., Iwaya K., Kuroda M., Harada M., Serizawa H., Koyanagi Y., Sato Y., Mizokami Y., Matsuoka T., Mukai K. Virchows Arch. 2000;437:508–513. doi: 10.1007/s004280000283. [DOI] [PubMed] [Google Scholar]
- 22.Wong G. T., Gavin B. J., McMahon A. P. Mol. Cell. Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topol L., Jiang X., Choi H., Garrett-Beal L., Carolan P. J., Yang Y. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonsson M., Andersson T. J. Cell Sci. 2001;114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- 25.Blanc E., Roux G. L., Benard J., Raguenez G. Oncogene. 2005;24:1277–1283. doi: 10.1038/sj.onc.1208255. [DOI] [PubMed] [Google Scholar]
- 26.Dejmek J., Leandersson K., Manjer J., Bjartell A., Emdin S. O., Vogel W. F., Landberg G., Andersson T. Clin. Cancer Res. 2005;11:520–528. [PubMed] [Google Scholar]
- 27.Iozzo R. V., Eichstetter I., Danielson K.G. Cancer Res. 1995;55:3495–3499. [PubMed] [Google Scholar]
- 28.Bittner M., Meltzer P., Chen Y., Jiang Y., Seftor E., Hendrix M., Radmacher M., Simon R., Yakhini Z., Ben-Dor A., et al. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 29.Weeraratna A. T., Jiang Y., Hostetter G., Rosenblatt K., Duray P., Bittner M., Trent J. M. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F., Charles K. A., Mantovani A. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Leek R. D., Harris A. L. J. Mamm. Gland Biol. Neoplasia. 2002;7:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 32.Lin E. Y., Nguyen A. V., Russell R. G., Pollard J. W. J. Exp. Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagemann T., Robinson S. C., Schulz M., Trumper L., Balkwill F. R., Binder C. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 34.Haro H., Crawford H. C., Fingleton B., Shinomiya K., Spengler D. M., Matrisian L. M. J. Clin. Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis S., Aikawa M., Szeto W., d’Amore P. A., Papkoff J. J. Cell Sci. 1999;112:3815–3820. doi: 10.1242/jcs.112.21.3815. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh S. Y., Hsieh P. S., Chiu C. T., Chen W. Y. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 37.Nozaki I., Tsuji T., Ijima O., Ohmura Y., Andou A., Miyazaki M., Shimizu N., Namba M. Int. J. Oncol. 2001;19:117–121. doi: 10.3892/ijo.19.1.117. [DOI] [PubMed] [Google Scholar]
- 38.Hoang B. H., Kubo T., Healey J. H., Yang R., Nathan S. S., Kolb E. A., Mazza B., Meyers P. A., Gorlick R. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 39.Säfholm A., Leandersson K., Dejmek J., Nielsen Ch., Villoutreix B. O., Andersson T. J. Biol. Chem. 2006;281:2740–2749. doi: 10.1074/jbc.M508386200. [DOI] [PubMed] [Google Scholar]
- 40.Hagemann Th, Wilson J., Kulbe H., Ningfeng F. L., Leinster D. A., Charles K., Klemm F., Pukrop T., Binder C., Balkwill F. J. Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 41.Nateri S. A., Spencer-Dene B., Behrens A. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- 42.Kremenevskaja N., von Wasielewski R., Rao A. S., Schofl C., Andersson T., Brabant G. Oncogene. 2005;24:2144–2154. doi: 10.1038/sj.onc.1208370. [DOI] [PubMed] [Google Scholar]
- 43.Le Floch N., Rivat C., De Wever O., Bruyneel E., Mareel M., Dale T., Gespach C. FASEB J. 2005;19:144–146. doi: 10.1096/fj.04-2373fje. [DOI] [PubMed] [Google Scholar]
- 44.Katoh M. Int. J. Mol. Med. 2001;8:657–660. doi: 10.3892/ijmm.8.6.657. [DOI] [PubMed] [Google Scholar]
- 45.Tulac S., Nayak N. R., Kao L. C., Van Waes M., Huang J., Lobo S., Germeyer A., Lessey B. A., Taylor R. N., Suchanek E., et al. J. Clin. Endocrinol. Metab. 2003;88:3860–3866. doi: 10.1210/jc.2003-030494. [DOI] [PubMed] [Google Scholar]
- 46.Sen M., Lauterbach K., El-Gabalawy H., Firestein G. S., Corr M., Carson D. A. Proc. Natl. Acad. Sci. USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shiina H., Igawa M., Breault J., Ribeiro-Filho L., Pookot D., Urakami S., Terashima M., Deguchi M., Yamanaka M., Shirai M., et al. Clin. Cancer Res. 2003;9:2121–2132. [PubMed] [Google Scholar]
- 48.Hoang B. H., Kubo T., Healey J. H., Sowers R., Mazza B., Yang R., Huvos A. G., Meyers P. A., Gorlick R. Int. J. Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 49.Behrens J., von Kries J. P., Kuhl M., Bruhn L., Wedlich D., Grosschedl R., Birchmeier W. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 50.Kaykas A., Yang-Snyder J., Heroux M., Shah K. V., Bouvier M., Moon R. T. Nat. Cell Biol. 2004;6:52–58. doi: 10.1038/ncb1081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.