Abstract
Current design of genetically engineered viruses for selective destruction of cancer cells is based on the observation that attenuated viruses replicate better in tumor cells than in normal cells. The ideal virus, however, is one that can infect only cancer cells by virtue of altered host range. Such a virus can be made more robust than the highly attenuated viruses used in clinical trials. Earlier, we reported the construction of a recombinant herpes simplex virus 1 (R5111) in which the capacity to bind heparan sulfate was disabled and which contained a chimeric IL-13-glycoprotein D that enabled the virus to infect cells expressing the IL-13α2 receptor (IL-13Rα2) commonly found on the surface of malignant glioblastomas or high-grade astrocytomas. In the earlier report, we showed that the recombinant R5111 was able to enter and infect cells via the interaction of the chimeric glycoprotein D with IL-13Rα2 but that the virus retained the capacity to bind and replicate in cells expressing the natural viral receptors HveA or nectin-1. Here, we report the construction of a recombinant virus (R5141) that can only enter and replicate in cells that express the IL-13Rα2. The recombinant R5141 does not depend on endocytosis to infect cells. It does not infect cells expressing HveA or nectin-1 receptors or cells expressing IL-13Rα2 that had been exposed to soluble IL-13 before infection. The studies described here show that the host range of herpes simplex viruses can be altered by genetic manipulation to specifically target cancer cells.
Keywords: glycoprotein D, HveA, malignant glioblastoma, nectin-1
The ideal oncolytic drug is one that fully discriminates between normal and tumor cells. Preeminent among such agents are viruses. A property of most viruses is that they have a host range that defines the species and type of cell that can become infected or replicate the virus. An additional advantage of viruses is that they can be genetically manipulated to alter their properties. Attenuated herpes simplex virus 1 (HSV-1), among other viruses, has been tested as a potential oncolytic agent with promising results (1–9). Thus viruses attenuated by deletion of the γ134.5 genes or γ134.5 and the gene encoding the major subunit of the viral ribonucleotide reductase (UL39) were found to be safe by inoculation into the central nervous system of patients with malignant glioblastoma (4, 7). The few long-term survivors after such treatment attested to the potential usefulness of these viruses but also exposed their limitation. Because the principal characteristic of viruses tested in clinical trials that enables them to discriminate between normal and tumor cells is that they cannot overcome the host defenses of the former, their ability to replicate and spread among tumor cells is severely restricted and depends in large part on the genotype of the tumor. An ideal virus would retain at least some of the properties of wild-type viruses to replicate and disseminate efficiently from cell to cell but would discriminate between normal and tumor cells by targeting surface proteins that are either unique to or grossly enriched on tumor cells. In the case of HSV-1, the main mechanism of entry into cells consists of two steps (reviewed in refs. 10–12). In the first, the viral glycoproteins B (gB) and C (gC) contained in the virion envelope bind heparan sulfate proteoglycans on cell surfaces. In the second step, the conformation of the viral glycoprotein D (gD) becomes altered as a consequence of its interaction with one of two cell surface receptors, HveA, a member of the TNF-α receptor family of proteins, or nectin-1, a member of a family of proteins that forms the intercellular networks that link cells together (10, 13, 14). The change in the conformation of gD is thought to enable a trio of glycoproteins, gH, gL, and gB, to interact with gD and enable the fusion of the envelope with the plasma membrane (15, 16). Subsequent to the fusion event, the capsid is transported to a nuclear pore where it releases the viral DNA directly into the nucleus (17). The goal of the studies described in this article was to genetically engineer HSV-1 so that it does not bind heparan sulfate proteoglycans and its entry into cells requires recognition of cell surface markers other than HveA or nectin-1.
In principle, viruses can be retargeted in two different ways. The first is to tag virion surface proteins with a single-chain antibody that recognizes a novel receptor. The extent to which such viruses interact solely with the intended receptor hinges on several factors, but primarily on the nature of the epitope recognized by the antibody (18). The second approach is to incorporate into the virion surface natural ligands for proteins unique or enriched on the tumor cell surface. We have chosen the second approach. In the first phase of these studies we constructed a virus (R5111) that was capable of entering cells via the IL-13α2 receptor (IL-13Rα2) but which retained the capacity to enter cells via HveA or nectin-1 (19). The IL-13Rα2 is monomeric and present in malignant glioblastomas and high-grade astrocytomas; it has also been reported to be present in normal testes (20, 21). We have used the same design to construct a recombinant virus capable of entering cells via the urokinase plasminogen activator receptor while still retaining the capacity to enter cells via HveA or nectin-1 receptors (22). In this article we describe the further modification of a virus that enters cells solely via the IL-13Rα2 protein. This study establishes the proof of principle that viruses can be targeted to specific single proteins on cell surfaces.
Results
Construction of R5141 Recombinant Virus.
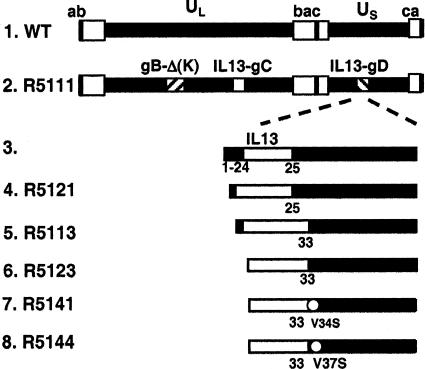
The construction of a mutant dependent on IL-13Rα2 for entry into cells took place in two stages. The first reported earlier involved the construction of a recombinant virus, R5111, that was able to enter cells via the IL-13Rα2 but retained the ability to enter cells via the HveA or nectin-1 receptors. The structure of this recombinant is shown in Fig. 1, lane 1. Briefly, in the R5111 mutant virus the polylysine tract in gB was removed and the first 132 residues of gC were replaced with the amino acid sequences encoding IL-3. The objectives were 2-fold, to remove the sites that enabled gB and gC to bind to heparin sulfate proteoglycans as described by Laquerre et al. (23) and at the same time to increase the affinity of the virus particle to the surface of cells expressing the IL-13Rα2. To specifically target the IL-13Rα2, the coding sequence of IL-13 was inserted between amino acids 24 and 25 of gD. As previously reported, the entry and initiation of infection by R5111 recombinant virus of cells expressing the IL-13Rα2 protein on their surface totally depended on the interaction of IL-13 present on the surface of the virus with the cognate receptor inasmuch as soluble IL-13 competed with the virus for the receptor (24).
Fig. 1.
Schematic representation of the sequence arrangements of the wild-type parent virus HSV-1(F) and R5111 and the chimeric gD contained in recombinant viruses R5121, R5113, R5123, R5141, and R5144. In all recombinant viruses, residues 68–78 of gB were deleted and IL-13 was inserted in place of residues 1–140 of gC. In R5111, R5121, and R5113, the signal sequence and residues 1–24 of gD were retained. In R5123, the residues 1–33 were deleted and only the signal peptide sequence of gD was retained. In R5141 and R5144, the signal peptide sequence of gD was replaced by that of IL-13. In addition, these two chimeric gDs contain additional amino acid substitutions as shown. The IL-13 inserted in R5121 and R5113 recombinant viruses carried the E13Y mutation, in which the glutamic acid was substituted by tyrosine. E13Y substitution restricts IL-13 binding to the IL-13Rα2 (52). R5121, R5141, and R5144 carried a wild-type IL-13 copy in gD.
The objective of the second stage reported here was to construct a recombinant virus that was unable to bind nectin-1 or HveA receptors. These studies were done in three phases. In the first phase we made linker insertions in the chimeric gD gene contained in the R5111 recombinant virus. The basis for the construction of this series of recombinants stemmed from reports that suggested that mutations in gD distant from the known sites of interaction of gD with HveA abolished the ability of the mutant gD to interact with nectin-1. Thus gD-carrying substitutions in residues 11, 27, 28, 29, 30, 40, and 43 were reported to bind nectin-1 but not HveA (25). In another study (26) substitutions of residue 38 disabled gD in fusion assays. Still another study (27) reported that double or triple amino acid substitutions at positions 215, 222, and 223 of gD precluded attachment to nectin-1 and corresponding inability to function in fusion assays. These results suggested that the binding sites in gD for HveA and nectin-1 did not overlap. As shown in Table 1, a common and unexpected characteristic of the R5200 series of mutants is that they retained the capacity to infect and replicate in J-nectin-1 cells but exhibited a reduced capacity to replicate in J-13R or J-HveA cell lines relative to those of the wild-type virus or the parent R5111 virus.
Table 1.
Replication of genetically engineered viruses in cell lines expressing specific receptors for viral entry
| Recombinants | Insertion (∇) Deletion (Δ) | Yields |
||
|---|---|---|---|---|
| J-nectin | J-HveA | J-13R | ||
| HSV-1(F) | WT | 6 × 108 | 5 × 108 | 2 × 101 |
| R5208 | ∇34 GKIFL | 5 × 105 | 6 × 102 | 6 × 102 |
| R5209 | ∇43 EDLP | 4 × 107 | 6 × 102 | 9 × 102 |
| R5212 | ∇77 GKIFP | 4 × 107 | 6 × 102 | 2 × 101 |
| R5213 | ∇77 EDLP | 2 × 107 | 6 × 102 | 7 × 104 |
| R5216 | ∇83 GRSS | 7 × 106 | 6 × 102 | 7 × 104 |
| R5217 | ∇84 GKIFP | 7 × 106 | 8 × 101 | 5 × 105 |
| R5220 | ∇125 GRSS | 6 × 106 | 7 × 101 | 6 × 102 |
| R5221 | ∇126 GKIFP | 6 × 106 | 7 × 101 | 6 × 103 |
| R5225 | ∇151 WKIFL | 4 × 107 | 6 × 102 | 6 × 102 |
| R5231 | ∇187 GRSS | 5 × 105 | 3 × 101 | 8 × 103 |
| R5237 | ∇243 GRSS | 6 × 107 | 5 × 104 | 3 × 103 |
| R5239 | ∇246 GKIFP | 7 × 106 | 8 × 101 | 4 × 101 |
| R5240 | ∇246 EDLP | 5 × 105 | 5 × 101 | 6 × 102 |
| R5242 | ∇277 GKIFP | 5 × 105 | 3 × 104 | 4 × 101 |
| R5121 | Δ 1–24 | 7 × 105 | 4 × 101 | 6 × 101 |
| R5113 | Δ 1–32 | 7 × 103 | 4 × 101 | 6 × 103 |
| R5123 | Δ SP, Δ 1–32 | 8 × 104 | 3 × 101 | 4 × 105 |
| R5141 | Δ SP, Δ 1–32, V34S | 8 × 101 | 6 × 101 | 6 × 106 |
| R5144 | Δ SP, Δ 1–32, V37S | 7 × 101 | 5 × 102 | 7 × 102 |
J-nectin, J-HveA, or J-13R cells grown in 25-cm2 flasks were exposed to 0.1 pfu of wild-type or recombinant virus per cell. The cultures were harvested 24 h after infection. Progeny virus was titrated on Vero-13R cells.
The construction of the recombinants in phase 2 of this study was based on the hypothesis that the functional interactions between gD and HveA or nectin-1 overlap and occur at the amino terminus of gD and that modifications of gD at sites distant from the amino terminus interfere with the interactions of the altered protein with HveA or nectin-1. The key mutations designed to test this hypothesis are illustrated in Fig. 1 and Table 1. Specifically, the key mutations are as follows.
-
(i)
In recombinant R5121 and R5113, the chimeric gD retained its signal peptide sequence but residues 1–24 or 1–32 were replaced with the coding sequence of IL-13. These recombinants failed to replicate or replicated poorly in any of the cell lines tested.
-
(ii)
In recombinant R5123, the signal peptide sequence of gD was replaced with that of IL-13. In addition, the coding sequence of IL-13 replaced the residues 1–32 of gD. We selected three clones for analysis. The clone shown in Table 1 replicated better in J-13R cells than in J-nectin-1 cells and failed to replicate in J-HveA cells.
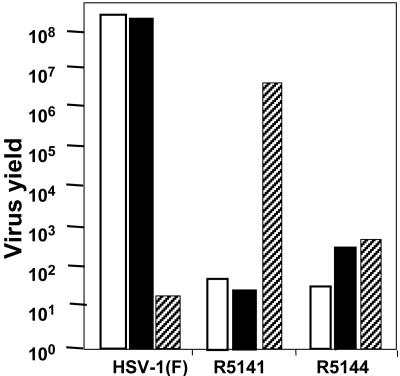
In the third phase of these studies we introduced mutations in the sequences immediately upstream from residue 33. In the chimeric gD genes constructed to make additional recombinant viruses we substituted Val-34 with Ser, Arg-35 with Asn, Arg-36 with Asp, Val-37 with Ser, or Tyr-38 with Ser, Asn, Asp, or His. The only constructs that yielded viable viruses were the substitutions of Val-34 with Ser (R5141) and Val-37 with Ser (R5144). Of the two recombinants only R5141 replicated in J-13R cells but not in J-nectin or J-HveA cells (Fig. 2 and Table 1). The recombinant was also replicated and produced plaques in the Vero-13R cell line but not in the parental Vero cells (data not shown). The plaques were smaller than those made by wild-type parent virus; they could be readily counted and indicated that the virus spread from infected to adjacent, noninfected cells. However, the yields of R5141 in IL-13Rα2-expressing cells were ≈100-fold lower than those obtained from wild-type virus in cells expressing HveA or nectin-1 receptor (Fig. 2 and Table 1). The experiments described below were designed to characterize the R5141 recombinant virus with respect to entry into cells and growth properties.
Fig. 2.
Replication of R5141, R5144 virus, and HSV-1(F) in J-nectin, J-HveA, and J-13R cells. Cells grown in 25-cm2 flasks were exposed to 0.1 pfu of the recombinant virus or wild-type HSV per cell and harvested 24 h after infection. Progeny virus was titrated on Vero-13R cells.
The Recombinant R5141 Depends on the Interaction of the IL-13 Component of Its Envelope with the IL-13 Receptor for Virus Entry into Cells.
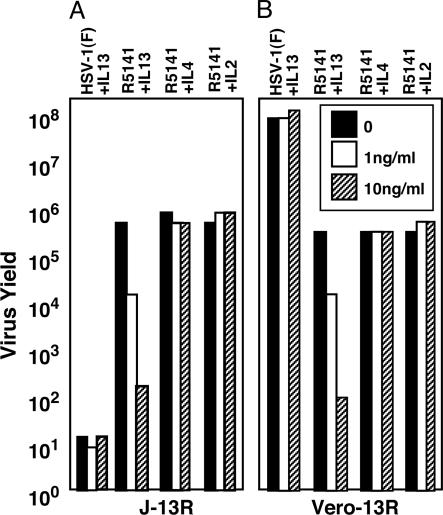
Although we earlier demonstrated that the recombinant R5111 enters cells via its interaction with IL-l3Rα2, it was essential to determine whether R5141 recombinant enters J-13R cells by the same pathway and whether it also depends on IL-13Rα2 for entry into the Vero-13R cell line. In this series of experiments J-13R or Vero-13R cells were mock-treated or exposed to 1 or 10 ng of IL-13, IL-2, or IL-4 for 1 h. The cells were then exposed to 0.1 plaque-forming units (pfu) of virus per cell. The cells were harvested 24 h after infection, and the virus was tittered in Vero-ILR cells. The results are shown in Fig. 3. The experimental design was based on the report that IL-13Rα2 is internalized after binding IL-13 and therefore the receptor would not be available for viral entry (28–30). As predicted, exposure of J-13R or Vero-13R cells to IL-13 significantly decreased viral replication, whereas IL-2 or IL-4 had no effect.
Fig. 3.
Preexposure of J-13R or Vero-13R cells to IL-13 precludes the infection and replication of R5141 mutant virus in both cell lines. Replicate cultures of J-13R cells (A) or Vero-13R cells (B) were mock-treated or exposed for 1 h to IL-13, IL-2, or IL-4 (1 ng/μl or 10 ng/μl). Then the cells were infected with 0.1 pfu of HSV-1(F) or R5141 per cell and harvested 24 h after infection. Progeny virus was titrated on Vero-13R cells.
R5141 Recombinant Virus Does Not Depend on Endocytosis for Entry into J-13R or Vero-13R Cells.
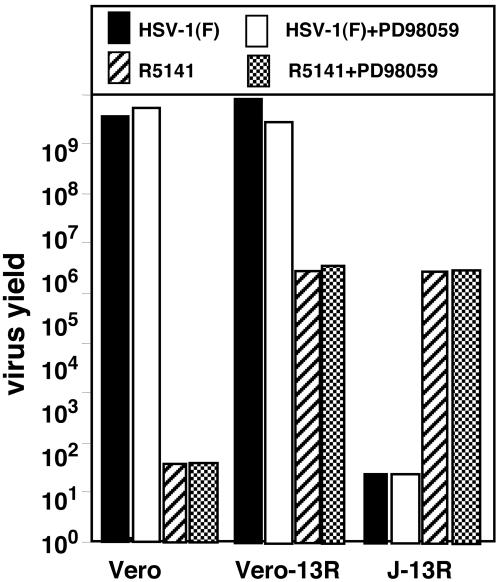
HSV-1 enters cells by one of two mechanisms. The first involves the fusion of the envelope with the plasma membrane followed by the release of capsid-tegument structures into the cytoplasm. Uptake of virions by endocytosis constitutes an alternative mode of entry, which does not require the interaction between gD and its cell surface receptor. However, subsequent events depend on the presence of at least a portion of gD in the environment of the infected cell. In the absence of gD, the endocytosed virion is degraded. Because R5141 recombinant virus carries a chimeric gD containing all but 33 residues of the native protein, it might be expected that endocytosed virions could infect cells. The question arose, therefore, whether R5141 enters cells by endocytosis. The experimental design of these studies was based on the results reported earlier that the inhibitor PD98095 blocked the replication of a gD mutant in a cell line expressing gD and exposed to gD recombinant virus (24). Consistent with the experimental design reported earlier (24), replicate cultures of Vero, J-13R, or Vero-13R cells were mock-treated or incubated in medium containing PD98095 (30 μM) for 1 h and then exposed to 0.1 pfu of R5141 recombinant virus or the wild-type HSV-1(F) virus per cell. As expected, wild-type virus replicated in Vero and Vero-13R but not in J-13R cells. The inhibitor PD980959 had no effect on the replication of HV-1(F) or R5141 virus in cells susceptible to infection (Fig. 4). We conclude that the R5141 recombinant virus did not depend on endocytosis for entry and replication in J-13R or Veo-13R cells.
Fig. 4.
R5141 recombinant virus does not depend on endocytosis for entry and replication in J-13R or Vero-IL-13R cell lines. Vero, Vero-13R, and J-13R cells were exposed to a 30 μM concentration of PD98059 inhibitor for 1 h. The cells were infected with 0.1 pfu of HSV-1(F) or R5141 per cell and then harvested 24 h after infection. Progeny virus was titrated on Vero-13R cells.
Comparison of Viral Gene Expression of R5141 Recombinant Virus in Vero and Vero-J-13 Cells.
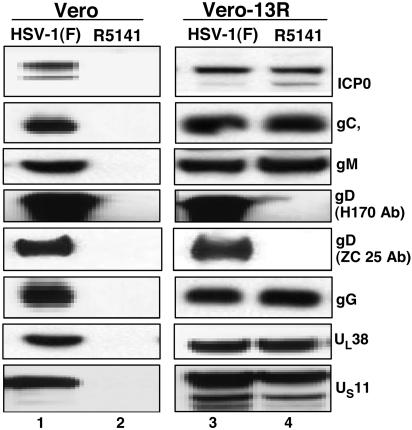
In this series of experiments Vero or Vero-13R cells grown in 25-cm2 flasks were exposed to 1.0 pfu of HSV-1 or R5141 virus per cell. The cells were harvested 24 h after infection and processed as described in Materials and Methods, and 100 mg of protein was loaded on a denaturing gel and subjected to electrophoresis, then electrically transferred onto a nitrocellulose sheet and reacted with a monoclonal antibody against infected cell protein (ICP) 0, gC, gM, gD, gG, UL38, or US11. The results shown in Fig. 5 were as follows:
Fig. 5.
Comparison of the accumulation of selected R5141 and wild-type parent proteins in Vero cells (Left) and Vero-13R cells (Right). Vero and Vero-13R cells grown in 25-cm2 flasks were exposed to 1.0 pfu of HSV-1 or R5141 virus per cell. The cells were harvested 24 h after infection, solubilized, subjected to electrophoresis in 10% denaturing polyacrylamide gels, electrically transferred onto a nitrocellulose sheet, and reacted with antibody against ICP0, gC, gM, gD (H170 and ZC25), gG, UL38, or US11. The trace amount of gD reacting with anti-gD(H170) represents spillover from an adjacent lane and is not reproducible.
-
(i)
As expected, viral proteins were not detected in Vero cells exposed to R5141 recombinant virus because the recombinant does not infect these cells.
-
(ii)
Except for the chimeric gD, R5141 recombinant virus produced in Vero-13R cells amounts of viral proteins tested in these assays that were nearly equivalent to those made by the wild-type parent virus in Vero or Vero-13R cells. Neither the antibody H170 to the amino-terminal domain deleted in R5141 recombinant nor the ZC25 antibody to the carboxyl-terminal domain detected significant amounts of the chimeic gD protein in these assays. IL-13 gD chimeric protein was readily detected in immunoprecipitates from R5141 virus-infected cells labeled with [35S]methionine (data not shown).
Discussion
Ever since humans embarked on a search for therapeutic agents, the implicit idealized objectives were to find drugs that would be maximally effective and minimally harmful. In the case of the current treatment of cancer, this objective has not been met. Aggressive treatment that produces long-term cures or significantly delays relapses is frequently associated with severe side effects. There is, in fact, no magic bullet for the simple reason that treatment directed primarily to tumor cells also affects normal cells that share properties with tumor cells. Because viruses target specific cells, they have long been viewed as potential oncolytic agents. The advent of tools designed to genetically engineer viruses suitable for human administration led to design and clinical trials of candidate therapeutic viruses. The requirements for viral therapeutic agents, that is safety and efficacy, are not different from those demanded of other therapeutic agents. Here in fact is where problems arose. To convert pathogenic agents that can cause lethal disease so as to specifically infect and destroy cancer cell, it was necessary to render them replication-defective or delete genes that enabled viruses to replicate in nondividing, resting cells, or delete genes that would enable the virus to overcome host defenses to infection. HSVs are particularly suitable for genetic engineering of such mutants because the technology for genetic engineering of such viruses has been available for the past 20+ years (31), the key function of many of the viral genes is known, and the genome contains a wide repertoire of genes suitable for genetic engineering in all of the categories listed above (32). Thus replication-incompetent viruses that remain cytotoxic, albeit unable to complete the replicative cycle, result from deletion of genes encoding the regulatory ICPs 0, 4, 22, 27, and 4 (cited in ref. 32). Replication-competent viruses restricted to reproduce solely in dividing cells result from deletion of genes encoding viral thymidine kinase or ribonucleotide reductase (33). The premier example of a gene essential to overcome host defenses and therefore a suitable target for deletion is γ134.5 described earlier in the text (34). The inherent problem in using recombinant viruses from which one or more of these genes have been deleted is that either they retain an unacceptable level of pathogenicity or they do not fully discriminate between normal and tumor cells. Although they do not replicate or spread in normal cells, the limited viral gene expression is nevertheless cytotoxic to the infected normal cells. The defect that prevents viral replication in the normal cell is also manifest to only a slightly lesser degree in tumor cells. In the end, effectiveness of a virus hinges on the genotype of the tumor cell. For example, tumor cells in which protein kinase R is activated after infection are likely to be far more resistant to the replication of Δγ134.5 mutants than tumors in which the kinase is not activated (35). Attempts to overcome this problem by insertion of genes that encode nonviral cytotoxic agents (e.g., cyclophosphamide, etc.) (36, 37), insertion of cellular genes that bolster immunity to tumors such as IL-4 or IL-12 (38–40), or combined use of ionizing radiation and virus (41) are alternatives still being tested. The pathway we have chosen is not to restrict the capacity of the virus to replicate by gene deletion but rather to retarget the virus to novel receptors present in abundant amounts on tumor cells but not on normal cells. We have selected for this purpose the IL-13Rα2 present in human malignant gliomas, high-grade astrocytomas, and less frequently in other tissues. In normal tissues it has been reported to be present in human testes, but not in other tissues. In this article we described the construction and initial characterization of a recombinant virus that targets the IL-13Rα2. The issues relevant to this study are as follows:
The R5141 recombinant virus enters cells by its interaction with the IL-13Rα2 inasmuch as depletion of the receptor from the cell surface by exposure of the cells to soluble IL-13 significantly reduces virus yields. We have also shown that the R5141 recombinant does not depend on endocytosis for entry into cells. The results indicate that entry into cells is therefore receptor-mediated at the surface of the cells exposed to this virus. The key feature that differentiates this virus from all previous attempts to construct targeted viruses (e.g., R5111, R5200 series, R5123) is that it is unable to enter cells via an interaction with the natural viral receptors HveA or nectin-1. Furthermore, the deletion of the poly(K) sequence in gB and the replacement of the amino-terminal domain of gC with IL-13 should render the R5141 recombinant incapable of binding to the heparan sulfate moiety on cell surfaces.
Elsewhere, we have reported the construction of a virus (R5181) capable of entering and replicating in cells via the urokinase plasminogen activator receptor (22). A key property of the target receptor is that it is bound to the cells surface by a glycosylphosphatidylinositol anchor. Replication of R5181 recombinant virus is abolished by digestion of cells expressing this receptor with phosphatidyl inositol-specific phospholipase C. These results suggest that the construction of viruses targeting specific cellular surface proteins is not restricted to a specific class of receptors (22).
Finally, the R5141 presents two puzzles that remain to be resolved. The first concerns the accumulation of low, virtually undetectable levels of the chimeric gD. The mechanism underlying this gross decrease in the accumulation of this protein is unclear. It is conceivable that IL-13 fused to the amino-terminal domain of gD imparts on the chimeric protein a shorter half-life or that it interacts with the receptor in cellular membranes and is degraded. An earlier report concerning a viral mutant that had a similar property concluded that the amounts of gD required for viral infection need not be very large (42). It remains to be seen whether the lower yields of R5141 relative to those of wild-type parent virus are related to the low levels of the chimeric gD.
The second problem relates to the actual mechanism that enables the entry of HSV into cells. Biochemical data (15) supported by structural studies (16) unambiguously indicate that the interaction of gD with its receptor results in a conformation change that most likely enables the interaction of gD with fusogenic viral glycoprotein and ultimately the fusion of the envelope of the virus with the plasma membrane. Because the interaction of the chimeric gD with its novel receptor occurs through an extension of gD that presumably does not impinge on the structure of the unmodified gD residues, the question arises as to how the altered interaction enables the conformational change in gD that permits the fusion of the envelope with the plasma membrane. Elucidation of this problem may enable the design and construction of more efficient chimeric gD structures.
The studies reported here pave the way for the construction of viruses that target other receptors suitable for both destruction of cancer cells or delivery of life-saving genes to cells that need them.
Materials and Methods
Cells.
Vero cells were obtained from the American Type Culture Collection and maintained in DMEM supplemented with 10% FBS. J1.1, a derivative of a baby hamster kidney (BHK) thymidine kinase-negative cell line, lacks both HveA and nectin-1 HSV-1 receptors (43). J-HveA and J-nectin-1 cell lines, expressing HveA and nectin-1, respectively, were derived from the J1.1 cell line and were kind gifts from G. Campadelli-Fiume (University of Bologna, Bologna, Italy). The cell lines J-13R and Vero-IL-13 derived by transfection of J1–1 and Vero cell lines with a plasmid encoding the IL-13Rα2 have been described (24).
Viruses.
The gD−/− mutant lacks both the gD gene and the gD protein in its envelope. gD−/− virus enters cells by endocytosis, and at high multiplicities in the absence of complementing gD it causes cells to undergo apoptosis (44). The recombinant virus R5111 has been described (19).
The R5200 series of recombinant viruses (R5208, R5209, R5212, R5213, R5216, R5217, R5220, R5221, R5225, R5231, R5237, R5239, R5239, R5240, and R5242) listed in Table 1 express mutagenized linker-insertion mutant gD originally obtained from R. J. Eisenberg and G. H. Cohen (University of Pennsylvania, Philadelphia). They were further modified by insertion of IL-13 between residues 24 and 25 as in the recombinant virus R5111. The procedures for construction of the recombinant viruses carrying the gD linker-insertion mutants have been described (45). In the recombinant R5121 virus IL-13 was inserted after the signal peptide of gD at residue 25 of gD. The sequences encoding residues 1–24 of gD were deleted. In R5113 recombinant, IL-13 was inserted between the signal peptide and residue 33 of gD, replacing residues 1–32.
Reagents.
The endocytosis inhibitor PD98059 was purchased from Cell Signaling Technology (Beverly, MA). IL-13, IL-4, and IL-2 were purchased from Research Diagnostics (Flanders, NJ).
Virus Titrations.
Replicate cultures of J-13R, J-HveA, J-nectin, and Vero-13R were exposed to 0.1 pfu of recombinant viruses or the wild-type parental HSV-1(F) virus per cell. After 24 h of incubation, the cells were harvested and disrupted by gentle sonication. Viral progeny were titrated on Vero-13R cells.
Antibodies.
Monoclonal antibodies against the amino-terminal sequence of gD clone H170 (46) or ICP0 (clone H1083) (47) were purchased from the Goodwin Institute (Plantation, FL). Monoclonal antibodies against gC (19), gM (48), gG (49), UL38 (50), and US11 protein (51) have been described. The ZC25 antibody to the carboxyl-terminal domain of gD was the kind gift of G. H. Cohen and R. J. Eisenberg.
Acknowledgments
We thank Dr. Gabriella Campadelli-Fiume for the J-1, J-nectin, and J-Hve cell lines and for advice. These studies were aided by National Cancer Institute Grants CA115662, CA83939, CA71933, CA78766, and CA88860.
Abbreviations
- HSV-1
herpes simplex virus 1
- gB
glycoprotein B
- gC
glycoprotein C
- gD
glycoprotein D
- IL-13Rα2
IL-13α2 receptor
- pfu
plaque-forming units
- ICP
infected cell protein.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Pyles R. B., Warnick R. E., Chalk C. L., Szanti B. E., Parysek L. M. Hum. Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 2.Rampling R., Cruickshank G., Papanastassiou V., Nicoll J., Hadley D., Brennan D., Petty R., MacLean A., Harland J., McKie E., et al. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 3.McKie E. A., Brown S. M., MacLean A. R., Graham D. I. Neuropathol. Appl. Neurobiol. 1998;24:367–372. doi: 10.1046/j.1365-2990.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 4.Markert J. M., Medlock M. D., Rabkin S. D., Gillespie G. Y., Todo T., Hunter W. D., Palmer C. A., Feigenbaum F., Tornatore C., Tufaro F., et al. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 5.Harrow S., Papanastassiou V., Harland J., Mabbs R., Petty R., Fraser M., Hadley D., Patterson J., Brown S. M., Rampling R. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- 6.Detta A., Harland J., Hanif I., Brown S. M., Cruickshank G. J. Gene Med. 2003;5:681–689. doi: 10.1002/jgm.396. [DOI] [PubMed] [Google Scholar]
- 7.Papanastassiou V., Rampling R., Fraser M., Petty R., Hadley D., Nicoll J., Harland J., Mabbs R., Brown M. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- 8.Shah A. C., Benos D., Gillespie G. Y., Markert J. M. J. Neurooncol. 2003;65:203–226. doi: 10.1023/b:neon.0000003651.97832.6c. [DOI] [PubMed] [Google Scholar]
- 9.Varghese S., Rabkin S. D. Cancer Gene Ther. 2002;9:967–978. doi: 10.1038/sj.cgt.7700537. [DOI] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume G., Cocchi F., Menotti L., Lopez M. Rev. Med. Virol. 2000;10:305–319. doi: 10.1002/1099-1654(200009/10)10:5<305::aid-rmv286>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Spear P. G. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 12.Spear P. G., Longnecker R. J. Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery R. I., Warner M. S., Lum B. J., Spear P. G. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 14.Spear P. G., Eisenberg R. J., Cohen G. H. Virology. 2000;275:1–9. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 15.Fusco D., Forghieri C., Campadelli-Fiume G. Proc. Natl. Acad. Sci. USA. 2005;102:9323–9328. doi: 10.1073/pnas.0503907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krummenacher C., Supekar V. M., Whitneck J. C., Connolly S. A., Eisenberg R. J., Cohen G., Wiley D. C., Carfi A. EMBO J. 2005;24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batterson W., Furlong D., Roizman B. J. Virol. 1983;45:397–407. doi: 10.1128/jvi.45.1.397-407.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang A., Chu T. H., Nocken F., Cichutek K., Dornburg R. J. Virol. 1998;72:10148–10156. doi: 10.1128/jvi.72.12.10148-10156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou G., Ye G. J., Debinski W., Roizman B. Proc. Natl. Acad. Sci. USA. 2002;99:15124–15129. doi: 10.1073/pnas.232588699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debinski W., Gibo D. M., Hulet S. W., Connor J. R., Gillespie G. Y. Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 21.Mintz A., Gibo D. M., Slagle-Webb B., Christensen N. D., Debinski W. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamiyama H., Zhou G., Roizman B. Gene Ther. 2005 Nov 17; doi: 10.1038/sj.gt.3302685. [DOI] [PubMed] [Google Scholar]
- 23.Laquerre S., Argnani R., Anderson D. B., Zucchini S., Manservigi R., Glorioso J. C. J. Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou G., Roizman B. J. Virol. 2005;79:5272–5277. doi: 10.1128/JVI.79.9.5272-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon M., Spear P. G. Proc. Natl. Acad. Sci. USA. 2004;101:17252–17257. doi: 10.1073/pnas.0407892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly S. A., Landsburg D. J., Carfi A., Whitbeck J. C., Zuo Y., Wiley D. C., Cohen G. H., Eisenberg R. J. J. Virol. 2005;79:1282–1295. doi: 10.1128/JVI.79.2.1282-1295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manoj S., Jogger C. R., Myscofski D., Yoon M., Spear P. G. Proc. Natl. Acad. Sci. USA. 2004;101:12414–12421. doi: 10.1073/pnas.0404211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami K., Taguchi J., Murata T., Puri R. K. Blood. 2001;97:2673–2679. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami K., Joshi B. H., Puri R. K. Hum. Gene Ther. 2000;11:1829–1835. doi: 10.1089/10430340050129459. [DOI] [PubMed] [Google Scholar]
- 30.Feng N., Lugli S. M., Schnyder B., Gauchat J. F., Graber P., Schlagenhauf E., Schnarr B., Wiederkehr-Adam M., Duschl A., Heim M. H., et al. Lab. Invest. 1998;78:591–602. [PubMed] [Google Scholar]
- 31.Post L. E., Roizman B. Cell. 1981;25:227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- 32.Roizman B., Knipe D. M. In: Fields Virology. 4th Ed. Knipe D. M., Howley P., Griffin D. E., Lamb R. A., Martin M. A., Roizman B., Straus S. E., editors. New York: Lippincott Williams & Wilkins; 2001. pp. 2399–2459. [Google Scholar]
- 33.Mineta T., Rabkin S. D., Martuza R. L. Cancer Res. 1994;54:3963–3966. [PubMed] [Google Scholar]
- 34.He B., Chou J., Brandimarti R., Mohr I., Gluzman Y., Roizman B. J. Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mezhir J. J., Advani S. J., Smith K. D., Darga T. E., Poon A. P. W., Schmidt H., Posner M. C., Roizman B., Weichselbaum R. R. Cancer Res. 2005;65:9479–9484. doi: 10.1158/0008-5472.CAN-05-1927. [DOI] [PubMed] [Google Scholar]
- 36.Kambara H., Saeki Y., Chiocca E. A. Cancer Res. 2005;65:11255–11258. doi: 10.1158/0008-5472.CAN-05-2278. [DOI] [PubMed] [Google Scholar]
- 37.Tyminski E., Leroy S., Terada K., Finkelstein D. M., Hyatt J. L., Dansk M. K., Poter P. M., Saeky Y., Chiocca E. A. Cancer Res. 2005;64:6850–6857. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- 38.Andreansky S., He B., van Cott J., McGhee J., Markert J. M., Gillespie G. Y., Roizman B., Whitley R. J. Gene Ther. 1998;5:121–130. doi: 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 39.Parker J. N., Gillespie G. Y., Love C. E., Randall S., Whitley R. J., Markert J. M. Proc. Natl. Acad. Sci. USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markert J. M., Parker J. N., Gillespie G. Y., Whitley R. J. Herpes. 2001;8:17–22. [PubMed] [Google Scholar]
- 41.Advani S. J., Sibley G. S., Song P. Y., Hallahan D. E., Kataoka Y., Roizman B., Weichselbaum R. R. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 42.Huber M. T., Wisner T. W., Hegde N. R., Goldsmith K. A., Rauch D. A., Roller R. J., Krummenacher C., Eisenberg R. J., Cohen G. H., Johnson D. C. J. Virol. 2001;74:10309–10318. doi: 10.1128/JVI.75.21.10309-10318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cocchi F., Menotti L., Mirandola P., Campadelli-Fiume G. J. Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou G., Galvan V., Campadelli-Fiume G., Roizman B. J. Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou G., Avitabile E., Campadelli-Fiume G., Roizman B. J. Virol. 2003;77:3759–3767. doi: 10.1128/JVI.77.6.3759-3767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arsenakis M., Tomasi L. F., Speziali V., Roizman B., Campadelli-Fiume G. J. Virol. 1986;58:367–376. doi: 10.1128/jvi.58.2.367-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ackermann M., Braun D. K., Pereira L., Roizman B. J. Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baines J. D., Roizman B. J. Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longnecker R., Roizman B. Science. 1987;236:573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- 50.Ward P. L., Ogle W. O., Roizman B. J. Virol. 1996;70:4623–4631. doi: 10.1128/jvi.70.7.4623-4631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roller R. J., Roizman B. J. Virol. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Debinski W., Thompson J. P. Clin. Cancer Res. 1999;5:3143s–3147s. [PubMed] [Google Scholar]