Abstract
Daily behavioral and physiological rhythms are linked to circadian oscillations of clock genes in the brain and periphery that are synchronized by the master clock in the suprachiasmatic nucleus. In addition, there are a number of inputs that can influence circadian oscillations in clock gene expression in a tissue-specific manner. Here we identify an influence on the circadian oscillation of the clock protein PER2, endogenous changes in ovarian steroids, within two nuclei of the limbic forebrain: the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala. We show that the daily rhythm of PER2 expression within these nuclei but not in the suprachiasmatic nucleus, dentate gyrus, or basolateral amygdala is blunted in the metestrus and diestrus phases of the estrus cycle. The blunting of the PER2 rhythm at these phases of the cycle is abolished by ovariectomy and restored by phasic estrogen replacement suggesting that fluctuations in estrogen levels or their sequelae are necessary to produce these effects. The finding that fluctuations in ovarian hormones have area-specific effects on clock gene expression in the brain introduces a new level of organizational complexity in the control of circadian rhythms of behavior and physiology.
Keywords: circadian clock, circadian rhythm, estrogen
The core molecular mechanism generating circadian rhythms within the suprachiasmatic nucleus (SCN), the master circadian clock, is based on feedback loops among several rhythmically expressed clock genes and their protein products (1, 2). Circadian rhythms in clock gene expression are observed not only in the SCN but also in other brain areas as well as peripheral tissues. These include but are not limited to the olfactory bulb, several hypothalamic nuclei, the eyes, pituitary gland, heart, and lung (3–13). Because synchronized expression of clock genes in these tissues requires an intact SCN, it has been proposed that these oscillations in clock gene expression serve to gate circadian signals from the SCN into tissue-specific rhythmic outputs (10, 12, 13).
In addition, there is growing evidence to suggest that circulating hormones as well as metabolic signals can modulate circadian oscillations of clock gene expression in some brain regions and peripheral structures (14). For example, the pineal hormone melatonin modulates the rhythm of the clock gene Per1 in the pituitary gland, striatum, and adrenal cortex (15–17). Furthermore, adrenal glucocorticoids induce Per1 expression in peripheral tissues such as the liver (18, 19) and modulate the rhythm of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminal (BNST-OV) and central nucleus of the amygdala (CEA) (12, 13). By contrast, adrenalectomy has no effect on PER2 expression in the SCN, basolateral amygdala (BLA), or dentate gyrus (DG) (12, 13), and melatonin does not affect rhythms of clock gene expression in the SCN, limbic forebrain, eye, or heart (20). The ability of circulating hormones to modulate clock gene expression within specific tissues presents a pathway for the integration and gating of circadian, hormonal, and metabolic information.
Gonadal steroids have marked and varied effects on gene expression in the brain and periphery (21, 22). In addition, circulating levels of gonadal hormones influence circadian rhythms in activity (23) and, in turn, their release is influenced by the circadian system (24, 25). Despite these interactions and the presence of clock genes throughout the reproductive axis (9, 21, 22, 26–29), there is no information on the effect of endogenous rhythms of gonadal hormones on clock gene expression in the brain or the periphery. To begin to address this issue, in the current experiments, we studied patterns of PER2 expression in the SCN and limbic forebrain across the rat estrous cycle as well as after gonadectomy in both male and female rats. Finally, we examined the effects of estrogen replacement to ovariectomized (OVX) females on rhythms of PER2. Preliminary results were published in abstract form.†
Results
PER2 Rhythms Across the Estrous Cycle.
To assess the effects of phase of the estrous cycle on rhythms of PER2 expression, female rats were randomly assigned to 1 of 16 groups with six animals per group. Daily vaginal smears were obtained for 14 days, and only rats showing regular 4- to 5-day estrus cycles were included in the experiment. Groups differed in the time of day of perfusion [Zeitgeber time (ZT) 1, ZT7, ZT13, and ZT19] and the day of the estrous cycle on which they were perfused.
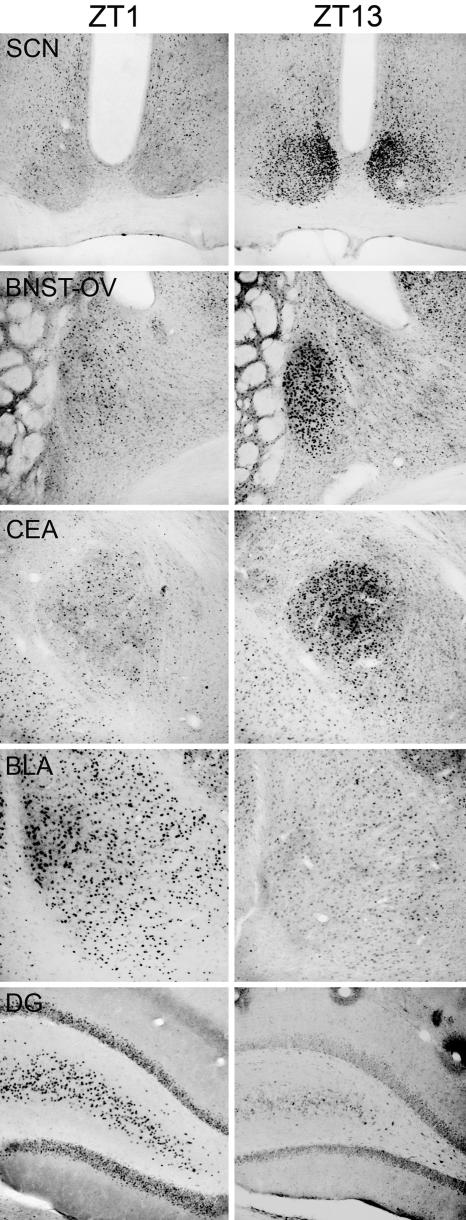
Examples of PER2 expression in the SCN, BNST-OV, CEA, BLA, and DG in female rats killed on the day of proestrus at either ZT1 or ZT13 are shown in Fig. 1. As can be seen in this figure, PER2 expression in the SCN, BNST-OV, and CEA is higher at ZT13 than at ZT1. This pattern is reversed in BLA and DG. These changes in expression are similar to those seen in male rats (12, 13).
Fig. 1.
Examples of PER2 expression in the SCN, BNST-OV, CEA, BLA, and DG at ZT1 and ZT13 in females killed on proestrus.
Fig. 2 shows the pattern of PER2 expression within all of the areas examined at each phase of the estrous cycle. As can be seen, PER2 expression in the SCN showed a similar rhythm on each day of the estrous cycle, peaking at ZT13 {significant main effect of ZT [F(3, 34) = 51.546, P < 0.01] but no main effect of the day of the estrous cycle nor a significant time–day interaction}. Bonferroni post hoc analysis revealed that PER2 expression was higher at ZT13 than at any other time point (P < 0.01).
Fig. 2.
Number of PER2-ir cells in the SCN, BNST-OV, CEA, BLA, and DG as a function of phase of the estrus cycle and ZT (means + SE are shown). ∗, Significantly different from ZT1 (P < 0.05). n = 6 per point.
Similarly in both the BLA and DG, the rhythm of expression of PER2 was consistent over the course of the estrous cycle, with peak expression occurring at ZT1 (see Fig. 2 d and e). Analysis of the data in both of these panels yielded a significant main effect of ZT [BLA, F(3, 70) = 37.261, P < 0.01; DG, F(3, 67) = 65.879, P < 0.01]. Bonferroni post hoc analyses revealed that in both structures the highest expression of PER2 was at ZT1 (P < 0.01).
In contrast to the stability of the patterns of PER2 expression observed in the SCN, BLA, and DG, within the CEA and BNST-OV PER2 expression varied as a function of day of the cycle (see Fig. 2 b and c). In both of these areas the pattern of PER2 expression on proestrus and estrus was similar to that seen in the SCN and previously reported in male rats. That is, the greatest expression of PER2 was seen at ZT13. However, on the metestrus and diestrus days of the cycle there was a marked blunting of the rhythm of PER2 expression.
Analysis of the data obtained for the BNST-OV (Fig. 2b) revealed a significant main effect for day of the cycle [F(3, 81) = 3.66, P < 0.05] and ZT [F(3, 81) = 25.725, P < 0.01], as well as a significant interaction of day of the cycle and ZT [F(9, 81) = 4.45, P < 0.01]. Simple main-effects analyses were then carried out for each of the 4 days of the cycle, and these were followed-up with pairwise comparisons (significant differences are indicated in Fig. 2). Notably, on both proestrus and estrus at ZT13, PER2 expression was significantly higher than at ZT1. In contrast, on metestrus PER2 expression at ZT19 was higher than at ZT1, and on diestrus only the ZT7 time point showed a higher expression than ZT1.
For the CEA (Fig. 2c), a two-way ANOVA revealed a significant main effect of ZT [F(3, 79) = 13.042, P < 0.01], as well as a significant time–cycle interaction [F(9, 79) = 3.153, P < 0.01] and a trend toward a significant main effect for the day of the cycle. Subsequent analysis of simple main effects showed similar results to those in the BNST-OV with the exception that no significant effect of the time of death on the day of metestrus was observed.
Effect of Gonadectomy on PER2 Expression.
The observation that PER2 expression in the BNST-OV and CEA varies as a function of the estrus cycle led us to investigate the rhythms of PER2 expression in the brains of OVX females and the influence of testicular hormones on the rhythms of PER2 expression in male rats. Three groups of rats were included in this study: OVX females (n = 12), gonadectomized males (n = 12), and intact males (n = 12). Rats from each group were then randomly assigned to subgroups based on the time of day that they were perfused (ZT1, ZT7, ZT13, or ZT19).
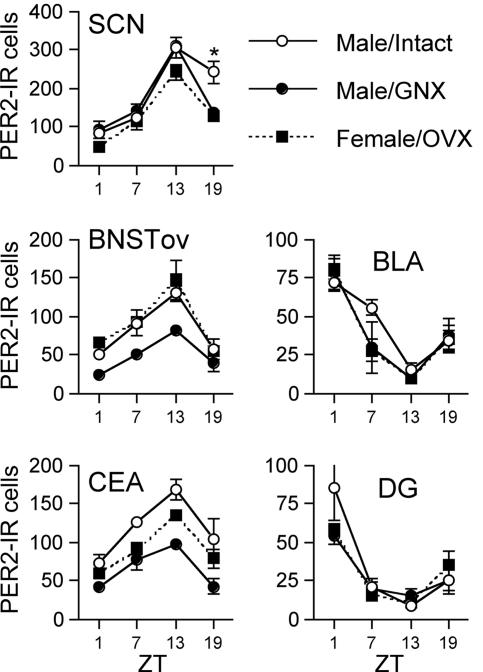
Fig. 3 shows PER2 expression as a function of time of day in each of the regions examined. As these data indicate, for all regions examined the pattern of expression of PER2 in females did not differ from that of intact males. Statistical analyses showed that in the SCN there were significant effects of both group [F(2, 24) = 7.951, P < 0.01] and ZT [F(3, 24) = 62.177, P < 0.010], as well as their interaction [F(6, 24) = 2.552, P < 0.05]. Further analysis of these effects showed that the group effect was seen only at ZT19 [F(2, 9) = 12.301, P < 0.01] and reflected the higher number of PER2-immunoreactive (ir) cells in intact males than in the other two groups (P < 0.05).
Fig. 3.
Number of PER2-ir cells in the SCN, BNST-OV, CEA, BLA, and DG in groups of intact, gonadectomized (GNX) males and OVX females as a function of ZT. ∗, Significantly different from the other two groups (P < 0.05). n = 4 per point.
In the BNST-OV and CEA the effects of both group and time were significant [BNST: group, F(2, 24) = 14.298, P < 0.01; ZT, F(3, 24) = 24.380, P < 0.01; CEA: group, F(2, 24) = 19.521, P < 0.01; ZT, F(3, 24) = 21.271, P < 0.01]. In both regions, post hoc analysis revealed that gonadectomized males had lower PER2 expression than both the OVX females and intact males (P < 0.01), and that in all three groups the highest level of PER2 expression occurred at ZT13 (P < 0.01).
In both the BLA and DG, the effect of ZT was significant [BLA, F(3, 24) = 32.109, P < 0.01; DG, F(3, 24) = 23.468, P < 0.01], and post hoc analysis revealed that ZT1 had the highest level of PER2 (P < 0.01).
Effect of Estrogen Replacement on PER2 Expression in OVX Rats.
The fact that OVX female rats showed similar patterns of PER2 expression to males in all of the regions examined suggested that hormonal fluctuations associated with the estrous cycle induced the blunting of PER2 rhythm in the BNST-OV and CEA that we observed in experiment 1. This possibility was investigated by comparing patterns of PER2 expression among groups of estrogen-replaced OVX, oil-treated OVX, and intact females. Subcutaneous injections of estrogen (2 μg of estradiol in 0.1 ml of sesame seed oil) or oil (0.1 ml) were given at ZT7 every 4 days, and the dose of estradiol was chosen to induce a peak in plasma estradiol similar to that seen in intact cycling female rats (30). This injection protocol was followed for 20 days (five cycles). Rats in the intact group were perfused on either proestrus or metestrus; those in the OVX plus estrogen groups were perfused either on the day of estradiol treatment (E0) or 1 day (E24), 2 days (E48), or 3 days (E72) after estradiol treatment. All rats including those in the OVX plus oil group were perfused at either ZT1 or ZT13. Thus, rats in the E0 group killed at ZT1 were killed 90 h after estradiol treatment whereas the ZT13 group were killed 6 h after injection.
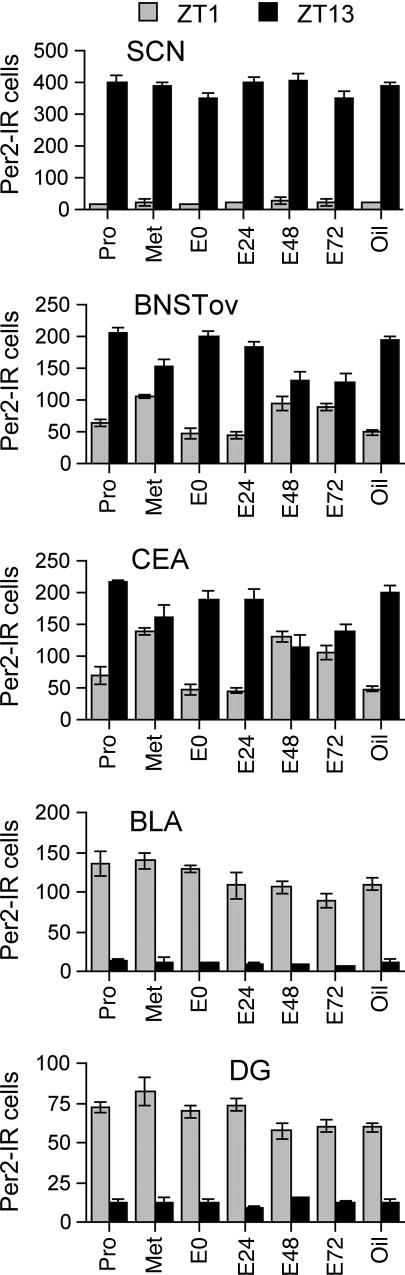
Fig. 4 shows the number of PER2-ir cells at ZT1 and ZT13 for each treatment group and for each region examined. As expected, in the SCN, PER2 expression was much higher at ZT13 than at ZT1 regardless of treatment, and these data are reflected in a significant main effect of time [F(1, 41) = 2045.657, P < 0.01] and the absence of any other significant effects.
Fig. 4.
Number of PER2-ir cells in the SCN, BNST-OV, CEA, BLA, and DG at ZT1 and ZT13 in groups of intact females on proestrus (Pro) and metestrus (Met) and in OVX females killed on the day of estrogen injection (E0) or 24 (E24), 48 (E48), or 72 (E72) h after estradiol injection or treatment with oil (Oil). n = 4 per point.
Consistent with previous reports and with the results of experiments 1 and 2, PER2 expression in the BLA and DG showed the opposite pattern from the SCN and was higher at ZT1 than at ZT13 [significant main effect for time: DG, F(1, 41) = 742.811, P < 0.01; BLA, F(1, 41) = 648.959, P < 0.01]. Unlike the SCN, treatment did appear to reduce peak expression of PER2 (see Fig. 4) resulting in significant main effects for treatment (DG, F(6, 41) = 2.489, P = 0.04; BLA, F(6, 41) = 3.247, P < 0.01), a significant treatment–time interaction for the DG [F(6, 41) = 3.673, P < 0.01], and a trend toward a significant interaction in the BLA [F(6, 41) = 2.08, P = 0.0766]. Further analysis of these data showed that treatment only affected PER2 expression at ZT1 and that peak levels in the E48, E72, and oil groups were lower than in the intact and E0 groups.
In the CEA and BNST-OV, PER2 expression at both ZT1 and ZT13 varied across treatment groups (see Fig. 4) with the result that the differences in PER2 expression between these time points was decreased in the E48, E72, and intact metestrus groups. Analysis of these data yielded significant effects of time [BNST-OV, F(1, 41) = 416.07, P < 0.01; CEA, F(1, 41) = 189.19, P < 0.01] as well as a significant time–treatment interaction [BNST-OV, F(6, 41) = 17.917, P < 0.01; CEA, F(6, 41) = 18.299, P < 0.01]. There was also a significant main effect of treatment in the CEA [F(1, 41) = 2.436, P < 0.01], but this effect was only a trend in the BNST-OV [F(1, 41) = 2.222, P = 0.061]. Further investigation of these results included using both simple main-effects analyses and pairwise post hoc tests. In both areas, at ZT1, the E48, E72, and intact metestrus groups showed higher levels of PER2 expression than the other four groups. Conversely, at ZT13, the E48, E72, and intact metestrus groups showed lower levels of PER2 expression than the other four groups in both of the regions examined.
Discussion
The results reported here show that phase of the reproductive cycle has a profound effect on the oscillation of clock genes within the brain. In female rats, there is a marked blunting of the circadian rhythm in the expression of the clock protein PER2 within the BNST-OV and CEA on the metestrus and diestrus phases of the estrus cycle. By contrast, rhythms of PER2 expression within the SCN, DG, and BLA were unaffected by stage of cycle. Interestingly, a daily rhythm in PER2 expression in the BNST-OV and CEA does not depend on the presence of estrogen because OVX females showed the same pattern of PER2 expression as that seen in intact females on the day of proestrus and estrus. Rather, the results of experiment 3 suggest that high levels of estrogen such as those seen on the morning of proestrus are sufficient to set off a cascade of events that in turn lead to the blunting of the rhythm in PER2 expression, which is reflected primarily in a reduction of expression at ZT13. Whether the subsequent decline in estrogen levels is necessary and whether the intervening steps on the cascade of events that lead to changes in PER2 levels occur within the CEA and the BNST-OV or are mediated through other structures remains to be determined. Estrogen has multiple effects throughout the brain. For example, it regulates neuropeptide expression in a variety of areas including the BNST and CEA (31, 32). Together with our earlier results showing a blunting of the rhythms of PER2 expression in the BNST-OV and CEA after adrenalectomy or lesions of the SCN, these data do suggest that putative clock cells in both of these areas are influenced by both peripheral and central signals.
Although we observed no changes in the rhythm of PER2 expression within the SCN, BLA, and DG as a function of estrous cycle phase or between gonadectomized and intact rats, there were some effects of gonadal steroids on the amount of PER2 expressed in these structures. In males, gonadectomy reduced overall PER2 expression in the SCN as well as in the BNST-OV and CEA, whereas in females, higher estrogen levels were associated with increased expression in the BLA and DG but only at ZT1. Thus, in these structures PER2 expression at ZT1 was similar in the E48, E72, and oil groups (see Fig. 4). These data are consistent with earlier results showing both organizational and activational effects of gonadal hormones on phase and amplitude of locomotor activity (23) and effects of tonic estrogen replacement to OVX rats on clock gene expression in the SCN, kidney, liver, and uterus (33).
An obvious issue raised by the current data concerns the functional significance of estrous-cycle-dependent changes in the daily pattern of expression of PER2 within the BNST-OV and CEA. These two structures are similar in cytoarchitecture (34) as well as neurochemistry (35, 36), and have reciprocal connections (37–39). In addition, both of these areas play key roles in autonomic regulation, feeding, and stress responsivity (38, 40, 41). Furthermore, recent studies have demonstrated effects of gonadal hormones on measures of light-enhanced startle, an anxiety-like behavior known to be mediated by the BNST (32, 42, 43). How modulation of the pattern of PER2 expression in the BNST-OV and CEA might be associated with changes in these functions remains to be determined. It is possible that alterations in the pattern of oscillations in PER2 are associated with changes in neuronal response to different types of inputs, with the processing of information within these structures, and/or with the modulation of their output pathways. For example, cyclic variation in GABAergic tone or in neuropeptide content associated with changes in PER2 expression within the BNST-OV and CEA could contribute to the variation in stress responsivity observed across the estrous cycle (44, 45).
The ability of fluctuations in estrogen levels to modulate rhythms of PER2 expression may not be limited to the BNST-OV and CEA or to this one clock gene. Indeed, the recent work of Nakamura et al. (33) showed that PER1 rhythms in liver, kidney, and uterus were sensitive to modulation by tonic estrogen. It is of considerable interest to consider the possibility that endogenous fluctuations in ovarian hormone levels may modulate clock gene expression in a number of peripheral tissues. Moreover, given the proposed association between clock gene expression, the cell cycle, and tumor growth (46–49), this is one route through which ovarian hormones might influence susceptibility to cancer development. Other naturally occurring changes in circulating ovarian hormones across the lifespan might also be expected to change the coupling of clock gene expression across tissues and brain areas. Extending the current results to study the effects of estrous cycle stage and other reproductive states such as pregnancy and lactation as well as puberty and senescence on PER2 expression in peripheral tissues and other brain areas would shed light on this relationship.
The finding that circadian oscillators in select regions of the limbic forebrain are modulated by endogenous changes in ovarian hormones adds a previously unrecognized level of complexity to the organization of the circadian system. In particular, it shows that there are sex differences in circadian oscillations in the brain in intact males and females at some stages of the reproductive cycle. The challenge now is to identify the associated effects on physiological and behavioral parameters.
Materials and Methods
Animals.
All rats were obtained from Charles River Breeding Laboratories and weighed between 150 and 175 g on arrival. The rats were housed four per cage and were given ad libitum access to food and water. All rats were entrained to a 12 h:12 h light/dark cycle (for half of the rats the lights were on from 800 hours to 2000 hours, and for the remainder the lights were on from 2000 hours to 800 hours). The rats were given a 2-week period to entrain to their light/dark cycle. All procedures were approved by the Concordia University Animal Care Committee under the guidelines of the Canadian Council on Animal Care.
Surgical Procedures.
For both males and females, gonadectomy was carried out under ketamine/xylazine anesthesia (5.7 mg of ketamine and 0.86 mg of xylazine/100 g of body weight). Ovaries were removed through bilateral dorsolateral incisions of the skin and peritoneum and testes through a single ventral incision. After closing incisions in both muscle and skin, rats were given an intramuscular injection of Procillin (MTC Bimeda, Cambridge, ON, Canada), an antibiotic (0.1 ml), and Anafen (Ketoprofen injectable; Merial, Baie d’Urfé, QC, Canada) (10 mg/ml) for pain relief (0.5 ml/100 g). All rats were allowed 3 weeks to recover from surgery and to entrain to the light/dark cycle.
Hormone Replacement.
After 2 days of recovery from surgery, OVX rats were treated either with estrogen (2 μg of estradiol in 0.1 ml of sesame seed oil) or with 0.1 ml of sesame seed oil alone. Food intake and body weight were measured daily to verify the expected anorectic action of estradiol, and daily vaginal smears were taken from intact rats.
Tissue Preparation and Immunocytochemistry.
Rats were injected with an overdose of sodium pentobarbital (100 mg/kg) and perfused intracardially with 300 ml of cold saline (0.9% NaCl) followed by 300 ml of cold 4% paraformaldehyde in a 0.1 M phosphate buffer (pH 7.3). After perfusion, brains were postfixed in 4% paraformaldehyde and stored at 4°C overnight. Serial coronal brain sections (50 μm) were collected from each animal by using a vibratome.
Free-floating sections were washed in cold 50 mM Tris-buffered saline (TBS) (pH 7.6) and incubated at room temperature for 30 min in a quenching solution made of TBS and 3% wt/wt hydrogen peroxide (H2O2). After the quenching phase, sections were rinsed in cold TBS and incubated for 1 h at room temperature in a preblocking solution made of 0.3% Triton X-100 in TBS (Triton-TBS), 3% normal goat serum, and 5% milk buffer. After the preblocking phase, sections were transferred directly into an affinity-purified rabbit polyclonal antibody raised against PER2 (Alpha Diagnostic, San Antonio, TX) diluted 1:1,000 with a solution of Triton-TBS with 3% normal goat serum in milk buffer. Sections were incubated with the primary antibody for 48 h at 4°C. After incubation in the primary antibody, sections were rinsed in cold TBS and incubated for 1 h at 4°C with a biotinylated anti-rabbit IgG made in goat (Vector Laboratories), diluted 1:200 with Triton-TBS with 2% normal goat serum. After incubation with secondary antibody, sections were rinsed in cold TBS and incubated for 2 h at 4°C with an avidin–biotin–peroxidase complex (VECTASTAIN Elite ABC kit; Vector Laboratories). After incubation with the ABC reagents, sections were rinsed with cold TBS, rinsed again with cold 50 mM Tris·HCl (pH 7.6), and rinsed again for 10 min with 0.05% 3,3′-diaminobenzidine in 50 mM Tris·HCl. Sections were then incubated on an orbital shaker for 10 min in 3,3′-diaminobenzidine/Tris·HCl with 0.01% H2O2 and 8% NiCl2. After this final incubation, sections were rinsed in cold TBS, wet-mounted onto gel-coated slides, dehydrated through a series of alcohols, soaked in CitriSolv (Fisher), and cover-slipped with Permount (Fisher).
Data Analysis.
Evidence of PER2 immunoreactivity was examined under a light microscope, and images from BNST-OV, SCN, CEA, BLA, and DG were captured by using a Sony XC-77 video camera, a Scion LG-3 frame grabber (Scion, Frederick, MD), and nih image 1.63 software (http://rsb.info.nih.gov/nih-image). A 400 × 400-μm frame was used to capture images of the SCN, BNST-OV, CEA, and BLA, whereas a 200 × 400-μm frame was used to capture images of the DG. The number of cells expressing PER2 immunoreactivity was then counted by using image sxm 1.75 software. For each brain region of interest, a mean number of PER2-ir cells was calculated for each animal by quantifying the average of the six images that showed the largest number of stained cells. For all analyses, the alpha level was set at 0.05. Means and standard deviations were calculated for each group of intact females, and two-way ANOVAs were conducted to determine the effects of both the day of the cycle and the ZT on the number of PER2-ir cells in each region of interest. One-way ANOVAs to assess simple main effects and Bonferroni post hoc tests were conducted as appropriate.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Council of Canada and by funding from the Concordia University Research Chairs Program (to S.A.).
Abbreviations
- BLA
basolateral amygdala
- BNST-OV
oval nucleus of the bed nucleus of the stria terminal
- CEA
central nucleus of the amygdala
- DG
dentate gyrus
- OVX
ovariectomized
- PER2-ir
PER2-immunoreactive
- SCN
suprachiasmatic nucleus
- TBS
Tris-buffered saline
- ZT
Zeitgeber time.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Shearman L. P., Sriram S., Weaver D. R., Maywood E. S., Chaves I., Zheng B., Kume K., Lee C. C., van der Horst G. T., Hastings M. H., Reppert S. M. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 2.Reppert S. M. Semin. Perinatol. 2000;24:243–246. doi: 10.1053/sper.2000.9122. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S., Shigeyoshi Y., Ishida Y., Fukuyama T., Yamaguchi S., Yagita K., Moriya T., Shibata S., Takashima N., Okamura H. J. Comp. Neurol. 2001;430:518–532. [PubMed] [Google Scholar]
- 4.Granados-Fuentes D., Prolo L. M., Abraham U., Herzog E. D. J. Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schibler U., Sassone-Corsi P. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 6.Hastings M. H., Reddy A. B., Maywood E. S. Nat. Rev. Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 7.Hastings M., Maywood E. S. BioEssays. 2000;22:23–31. doi: 10.1002/(SICI)1521-1878(200001)22:1<23::AID-BIES6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Abe M., Herzog E. D., Yamazaki S., Straume M., Tei H., Sakaki Y., Menaker M., Block G. D. J. Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kriegsfeld L. J., Korets R., Silver R. Eur. J. Neurosci. 2003;17:212–220. doi: 10.1046/j.1460-9568.2003.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto K., Nagase T., Fukui H., Horikawa K., Okada T., Tanaka H., Sato K., Miyake Y., Ohara O., Kako K., Ishida N. J. Biol. Chem. 1998;273:27039–27042. doi: 10.1074/jbc.273.42.27039. [DOI] [PubMed] [Google Scholar]
- 11.Shieh K. R. Neuroscience. 2003;118:831–843. doi: 10.1016/s0306-4522(03)00004-6. [DOI] [PubMed] [Google Scholar]
- 12.Lamont E. W., Robinson B., Stewart J., Amir S. Proc. Natl. Acad. Sci. USA. 2005;102:4180–4184. doi: 10.1073/pnas.0500901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amir S., Lamont E. W., Robinson B., Stewart J. J. Neurosci. 2004;24:781–790. doi: 10.1523/JNEUROSCI.4488-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter J., Reick M., McKnight S. L. Annu. Rev. Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- 15.Messager S., Garabette M. L., Hastings M. H., Hazlerigg D. G. NeuroReport. 2001;12:579–582. doi: 10.1097/00001756-200103050-00029. [DOI] [PubMed] [Google Scholar]
- 16.Uz T., Akhisaroglu M., Ahmed R., Manev H. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 17.von Gall C., Garabette M. L., Kell C. A., Frenzel S., Dehghani F., Schumm-Draeger P. M., Weaver D. R., Korf H. W., Hastings M. H., Stehle J. H. Nat. Neurosci. 2002;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- 18.Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schutz G., Schibler U. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 19.Balsalobre A., Marcacci L., Schibler U. Curr. Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 20.Oishi K., Murai I., Sakamoto K., Otsuka H., Miyake Y., Nagase T., Ishida N. Brain Res. 2000;885:298–302. doi: 10.1016/s0006-8993(00)02982-6. [DOI] [PubMed] [Google Scholar]
- 21.Metz R. P., Qu X., Laffin B., Earnest D., Porter W. W. Dev. Dyn. 2006;235:263–271. doi: 10.1002/dvdy.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller B. H., Olson S. L., Turek F. W., Levine J. E., Horton T. H., Takahashi J. S. Curr. Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albers H. E. Am. J. Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- 24.Wiegand S. J., Terasawa E. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand S. J., Terasawa E., Bridson W. E. Endocrinology. 1978;102:1645–1648. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- 26.Chappell P. E., White R. S., Mellon P. L. J. Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson M. H., Lim A., Fernando D., Day M. L. Reprod. Biomed. Online. 2002;4:140–145. doi: 10.1016/s1472-6483(10)61931-1. [DOI] [PubMed] [Google Scholar]
- 28.Kennaway D. J., Varcoe T. J., Mau V. J. Mol. Hum. Reprod. 2003;9:503–507. doi: 10.1093/molehr/gag067. [DOI] [PubMed] [Google Scholar]
- 29.Sellix M. T., Egli M., Poletini M. O., McKee D. T., Bosworth M. D., Fitch C. A., Freeman M. E. Am. J. Physiol. 2005 doi: 10.1152/ajpregu.00555.2005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asarian L., Geary N. Horm. Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 31.Simerly R. B., Young B. J., Capozza M. A., Swanson L. W. Proc. Natl. Acad. Sci. USA. 1989;86:4766–4770. doi: 10.1073/pnas.86.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasnow A. M., Schulkin J., Pfaff D. W. Horm. Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T. J., Moriya T., Inoue S., Shimazoe T., Watanabe S., Ebihara S., Shinohara K. J. Neurosci. Res. 2005;82:622–630. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- 34.Cassell M. D., Freedman L. J., Shi C. Ann. N.Y. Acad. Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- 35.Ju G., Han Z. S. Neurosci. Lett. 1989;99:246–250. doi: 10.1016/0304-3940(89)90454-0. [DOI] [PubMed] [Google Scholar]
- 36.Ju G., Swanson L. W. J. Comp. Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- 37.Dong H. W., Petrovich G. D., Swanson L. W. Brain Res. Brain Res. Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- 38.Dong H. W., Petrovich G. D., Watts A. G., Swanson L. W. J. Comp. Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- 39.Gray T. S. Ann. N.Y. Acad. Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- 40.Herman J. P., Figueiredo H., Mueller N. K., Ulrich-Lai Y., Ostrander M. M., Choi D. C., Cullinan W. E. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Van de Kar L. D., Blair M. L. Front. Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 42.Toufexis D., Davis C., Hammond A., Davis M. J. Neurosci. 2005;25:9010–9016. doi: 10.1523/JNEUROSCI.0127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toufexis D. J., Davis C., Hammond A., Davis M. J. Neurosci. 2004;24:10280–10287. doi: 10.1523/JNEUROSCI.1386-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holdcroft A., Sapsed-Byrne S., Ma D., Hammal D., Forsling M. L. Br. J. Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 45.Viau V., Meaney M. J. J. Neuroendocrinol. 2004;16:72–78. doi: 10.1111/j.1365-2826.2004.01122.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen S. T., Choo K. B., Hou M. F., Yeh K. T., Kuo S. J., Chang J. G. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 47.Fu L., Lee C. C. Nat. Rev. Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 48.Reddy A. B., Wong G. K., O’Neill J., Maywood E. S., Hastings M. H. Mutat. Res. 2005;574:76–91. doi: 10.1016/j.mrfmmm.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 49.Stevens R. G. Epidemiology. 2005;16:254–258. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]