Abstract
Induced resistance protects plants against a wide spectrum of diseases; however, it can also entail costs due to the allocation of resources or toxicity of defensive products. The cellular defense responses involved in induced resistance are either activated directly or primed for augmented expression upon pathogen attack. Priming for defense may combine the advantages of enhanced disease protection and low costs. In this study, we have compared the costs and benefits of priming to those of induced direct defense in Arabidopsis. In the absence of pathogen infection, chemical priming by low doses of β-aminobutyric acid caused minor reductions in relative growth rate and had no effect on seed production, whereas induction of direct defense by high doses of β-aminobutyric acid or benzothiadiazole strongly affected both fitness parameters. These costs were defense-related, because the salicylic acid-insensitive defense mutant npr1-1 remained unaffected by these treatments. Furthermore, the constitutive priming mutant edr1-1 displayed only slightly lower levels of fitness than wild-type plants and performed considerably better than the constitutively activated defense mutant cpr1-1. Hence, priming involves less fitness costs than induced direct defense. Upon infection by Pseudomonas syringae or Hyaloperonospora parasitica, priming conferred levels of disease protection that almost equaled the protection in benzothiadiazole-treated wild-type plants and cpr1 plants. Under these conditions, primed plants displayed significantly higher levels of fitness than noninduced plants and plants expressing chemically or cpr1-induced direct defense. Collectively, our results indicate that the benefits of priming-mediated resistance outweigh the costs in environments in which disease occurs.
Keywords: induced resistance, innate immunity, plant defense
Plants resist the attacks of harmful microorganisms and insects through constitutive and inducible defenses. It is generally believed that inducible defenses have evolved to save energy under enemy-free conditions, but costs still arise upon activation of these defenses under hostile conditions. These costs can result from allocation of limited resources to defensive compounds or toxicity of the defense to the plant's own metabolism (1). In addition, costs can arise from external factors, when the defensive trait affects a beneficial interaction with another organism in the environment. It is therefore reasonable to assume that plants express their inducible defenses only if the benefits (i.e., protection against the attackers) outweigh the costs of the resistance.
Various studies have demonstrated costs related to jasmonic acid (JA)-inducible defense against herbivory. These costs can affect plant growth and reproductive traits (2–4). There are also studies that demonstrated ecological benefits of JA-inducible defense. Agrawal (5) showed that induction of defense in wild radish against insects correlated with enhanced seed production. Additionally, JA-induced defense in wild populations of Nicotiana attenuata conferred enhanced seed production in populations exposed to herbivory (3). Hence, costs of JA-inducible defenses are outweighed by the benefits of protection when plants are attacked by herbivores. A cost–benefit balance of salicylic acid (SA)-inducible defenses against pathogens has also been supposed. In wheat, Heil et al. (6) demonstrated costs of SA-inducible defenses on growth and seed set. In Arabidopsis, Cipollini (7) showed that exogenously applied SA reduced seed production. Recently, Heidel et al. (8) performed a field experiment with two sets of Arabidopsis genotypes: one group that is blocked in SA-inducible defenses and another group that constitutively expresses SA-inducible defenses. Both classes of genotypes were negatively affected in growth and seed set, suggesting that plant fitness reaches an optimum at a certain intermediate level of resistance that balances fitness and defense.
Upon appropriate stimulation, plants can increase their level of resistance against future pathogen attack. This phenomenon is known as induced resistance. Based on differences in signaling pathways and spectra of effectiveness, different types of induced resistance have been defined. The classic form of induced resistance is referred to as systemic acquired resistance (SAR) and occurs in distal plant parts upon localized infection by a necrosis-inducing pathogen (9). SAR is controlled by a signaling pathway that depends on endogenous accumulation of SA and the defense regulatory protein NPR1 (10) and is predominantly effective against biotrophic pathogens (11). Selected strains of nonpathogenic rhizobacteria can also induce systemic resistance, which is referred to as induced systemic resistance (ISR) (12). In Arabidopsis, ISR triggered by Pseudomonas fluorescens WCS417r functions independently of SA but requires NPR1 and responsiveness to JA and ethylene (13). P. fluorescens WCS417r-mediated ISR has a different spectrum of effectiveness than SAR, and is predominantly effective against pathogens that are sensitive to JA- and ethylene-dependent basal resistance (11). A third type of induced resistance is activated upon application of the chemical β-aminobutyric acid (BABA). The signaling pathway controlling BABA-induced resistance (BABA-IR) (14) differs from that of SAR and ISR. Although BABA-IR against Pseudomonas syringae depends solely on SA and NPR1 (15), against pathogenic fungi and oomycetes it is controlled by a pathway that involves abscisic acid- and phosphoinositide-dependent signaling (16, 17). BABA-IR is effective against biotrophic and necrotrophic pathogens, as well as certain types of abiotic stress (14–20).
For a long time, it was assumed that protection by induced resistance is based on direct activation of defenses by the resistance-inducing agent. Accumulation of pathogenesis-related proteins is an example that occurs directly upon induction of SAR. However, the suggested contribution of pathogenesis-related proteins to resistance is uncertain and appears insufficient to explain the broad spectrum of protection by SAR (21). Moreover, both rhizobacteria-mediated ISR and BABA-IR are not associated with direct activation of defense-related genes (12, 14). Interestingly, plants expressing SAR, ISR, or BABA-IR exhibit a faster and stronger activation of specific defense responses after they have been infected by a pathogen. This capacity for augmented defense expression is called priming (22).
Since the discovery of priming in plant cell suspension cultures by Kauss et al. (23), priming has been demonstrated in different plant species against pathogens, insects, and abiotic stress (22). Hence, priming appears to be a common feature of a plant's immune system that offers protection against a wide spectrum of environmental stresses. In Arabidopsis, Kohler et al. (24) demonstrated that SAR-induced Arabidopsis expressed augmented levels of the defense-related PAL gene upon infection by P. syringae. Van Wees et al. (25) and Verhagen et al. (26) demonstrated that treatment of Arabidopsis with ISR-inducing P. fluorescens WCS417r bacteria did not directly activate defense-related genes; however, Arabidopsis was primed for enhanced expression of JA- and ethylene-inducible genes upon infection by P. syringae. Treatment with BABA primes Arabidopsis for SA-dependent defenses (15, 20) and enhanced formation of callose-rich papillae that functions independently from SA and NPR1 (16). Recently, we showed that these forms of priming require specific cellular signaling components (17), suggesting a regulation mechanism that is exclusively dedicated to priming. Priming accelerates and increases the plant's ability to activate the defense that is best adapted to cope with a certain stress situation. In this perspective, priming represents an important ecological adaptation to resist environmental stress.
As most studies on costs and benefits of induced resistance have focused on situations in which the defense is activated directly by the inducing agent, the possibility of studying costs and benefits of priming has so far been overlooked. This lack of data prompted us to determine the costs and benefits of priming in Arabidopsis and compare those to the costs and benefits of induced direct defense. By using BABA as a chemical inducer of priming and a constitutive priming mutant of Arabidopsis, we compared fitness parameters in the absence and presence of pathogen attack. We demonstrate that priming involves considerably fewer costs than induction of direct defense. In addition, we demonstrate that the benefits of priming outweigh the costs when infection by pathogens occurs.
Results
Effectiveness of Chemically Induced Priming and Direct Defense.
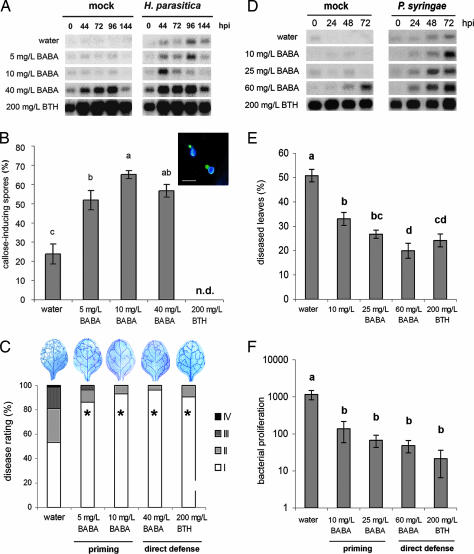
To compare the effectiveness of priming and direct defense, 3- and 6-week-old Arabidopsis plants (Col-0) were treated with increasing concentrations of BABA or benzothiadiazole (BTH). Two days later, the 3-week-old plants were mock or challenge inoculated with Hyaloperonospora parasitica, whereas the 6-week-old plants were mock-inoculated or challenge-inoculated with P. syringae. To determine the level of priming and/or direct defense, leaves were collected at different time points after inoculation for quantification of PR-1 gene activation and callose deposition. In 3-week-old plants, treatment with 5 and 10 mg/liter BABA did not activate the PR-1 gene, whereas treatment with 40 mg/liter BABA and 200 mg/liter BTH triggered PR-1 expression directly (Fig. 1A). After challenge with H. parasitica, PR-1 was induced transiently in water-treated control plants, reaching a maximum at 96 h after inoculation. Treatment with 5 mg/liter BABA did not accelerate this pathogen-induced activation of PR-1. However, treatment with 10 mg/liter BABA conferred enhanced levels of PR-1 expression at 44 and 72 h after inoculation, indicating that this treatment primed Arabidopsis for SA-inducible defenses. All treatments with BABA primed Arabidopsis for callose deposition, as evidenced by enhanced numbers of callose-rich papillae at the sites of spore germination (Fig. 1B). Resistance against H. parasitica was quantified by determining disease symptom severity and pathogen colonization at 8 days after inoculation. Compared with water-treated control plants, all treatments with BABA and BTH strongly reduced colonization by H. parasitica and disease severity (Fig. 1C). Apparently, BABA-induced priming is equally effective against H. parasitica as induction of direct defense by BABA or BTH. In 6-week-old Arabidopsis, treatment with 10 or 25 mg/liter BABA did not induce PR-1 expression directly, but it did prime plants for earlier and stronger PR-1 expression upon P. syringae infection (Fig. 1D). Treatment with BTH induced PR-1 directly in noninfected plants of this age. In addition, 60 mg/liter BABA induced PR-1 expression in noninfected plants, although it did so much later and to a lower extent than BTH (Fig. 1D). Induced resistance against P. syringae was quantified by determining the percentage of diseased leaves and the extent of bacterial proliferation in the leaves at 3 days after inoculation. Compared with control plants, priming-inducing concentrations of BABA significantly reduced the level of disease and bacterial growth (Fig. 1 E and F). Activation of direct defense with 60 mg/liter BABA or 200 mg/liter BTH induced only slightly higher levels of resistance, indicating that priming is almost as effective against P. syringae as induction of direct defense.
Fig. 1.
Chemical induction of priming and direct defense against H. parasitica WACO9 (A–C) and P. syringae pv. tomato DC3000 (D–F). Col-0 plants were soil-drenched with increasing concentrations of BABA or sprayed with BTH and pathogen-inoculated 2 days later. (A) PR-1 gene expression in 3-week-old control plants or BABA- or BTH-treated plants at different time points after inoculation. hpi, hours postinoculation. (B) Callose deposition 2 days after H. parasitica inoculation. (Inset) A representative example of H. parasitica spores triggering callose deposition in epidermal cells. (Scale bar, 20 μm.) n.d., not determined. (C) Induced resistance against H. parasitica at 8 days after inoculation. Asterisks indicate statistically different distributions of disease severity classes compared with the water control (χ2 test; α = 0.05). Colonization by the pathogen was visualized by lactophenol/trypan blue staining and light microscopy. (D) PR-1 gene expression in 6-week-old control plants or BABA- or BTH-treated plants at different time points after inoculation. (E) Induced resistance against P. syringae. Shown are means ± SEM (n = 15–20) of the percentage of leaves with symptoms at 3 days after inoculation. Different letters indicate statistically significant differences (least significant difference test; α = 0.05). (F) Growth of P. syringae over a 3-day time interval. Shown are means ± SD (n = 5–10). Different letters indicate statistically significant differences (least significant difference test; α = 0.05). All experiments shown were repeated with comparable results.
Costs and Benefits of Chemically Induced Priming and Direct Defense.
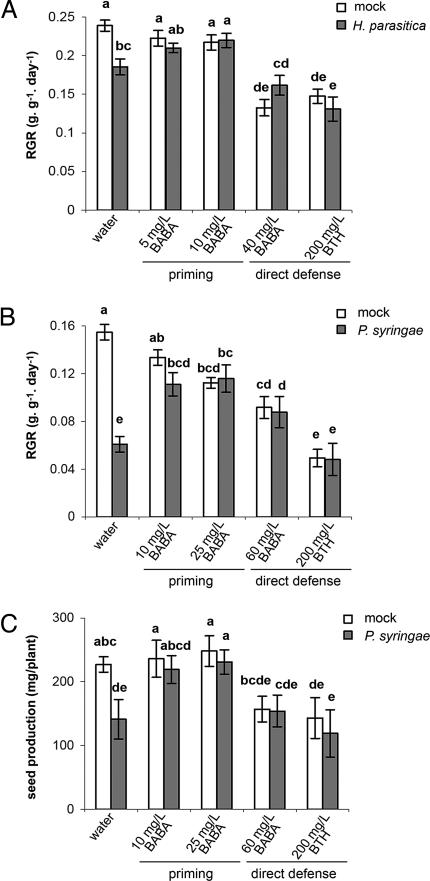
Costs and benefits of BABA- and BTH-induced resistance on plant growth were analyzed by quantifying the relative growth rate (RGR). In 3- to 4-week-old plants, induction of priming by 5 or 10 mg/liter BABA did not lead to statistically significant reductions in RGR in the noninfected plants (Fig. 2A and B). In contrast, the direct defense-inducing treatments, 40 mg/liter BABA and 200 mg/liter BTH, reduced RGR by 44% and 39%, respectively (Fig. 2 A). In 6- to 7-week old plants, induction of priming by 10 mg/liter BABA had no significant effect on RGR, whereas 25 mg/liter BABA resulted in a 27% reduction of RGR (Fig. 2B). The direct defense-inducing treatments 60 mg/liter BABA and 200 mg/liter BTH caused significantly stronger reductions in RGR (41% and 71%, respectively). Hence, induction of direct defense involves higher costs on plant growth than induction of priming. Upon inoculation with H. parasitica, control plants were significantly reduced in RGR in comparison with noninfected control plants (24%) (Fig. 2A). Infection with P. syringae caused an even stronger reduction in RGR (62%) (Fig. 2B). These disease-related costs were absent in all BABA- and BTH-treated plants (Fig. 2 A and B). Interestingly, of all pathogen-inoculated plants, the primed plants displayed the highest growth rates (Fig. 2 A and B), which indicates that priming yields optimal levels of plant growth under conditions of disease pressure.
Fig. 2.
Costs and benefits of chemically induced priming and direct defense in the absence and presence of H. parasitica (A) or P. syringae (B and C). Plants were treated as described in the legend of Fig. 1. (A) RGR of mock- and H. parasitica-inoculated plants (3–4 weeks old) over the 12-day period from chemical treatment. Shown are mean values ± SEM (n = 8–12). (B) RGR of mock- and P. syringae-inoculated plants (6–7 weeks old) over the 12-day period from chemical treatment. (C) Seed production of mock- and P. syringae-inoculated plants. Shown are mean values ± SEM (n = 8–12) of the seed weight per plant. Different letters indicate statistically significant differences (least significant difference test; α = 0.05). All experiments shown were repeated with comparable results.
To further examine the impact of priming and direct defense on plant fitness, seed production was determined in mock- and P. syringae-inoculated plants. In mock-inoculated plants, the priming-inducing treatments did not affect seed production, whereas the direct defense-inducing treatments, 60 mg/liter BABA and 200 mg/liter BTH, reduced seed production by 31% and 37%, respectively (Fig. 2C). Inoculation of control plants with P. syringae reduced seed production by 38% (Fig. 2C). In contrast, inoculation with P. syringae had no significant effect on seed production in plants treated with priming- or direct defense-inducing concentrations of BABA and BTH. Most importantly, the primed plants produced the highest amount of seeds of all P. syringae-inoculated plants. Hence, priming provides enhanced fitness under conditions of disease pressure.
Fitness Reduction by BABA and BTH Is Caused by NPR1-Dependent Defenses.
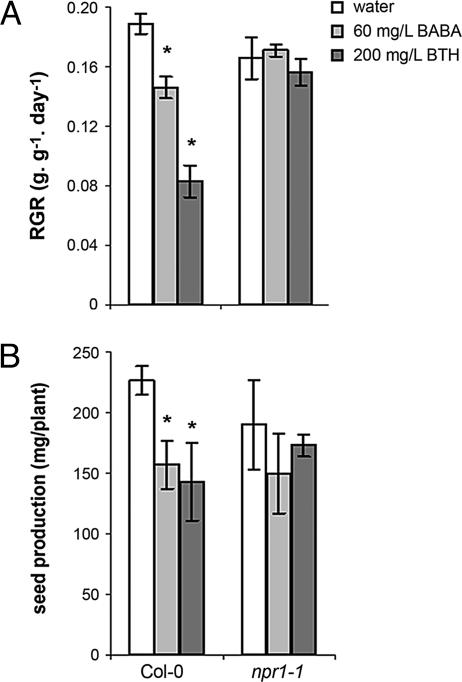
To exclude the possibility that the fitness-reducing effects of high concentrations of BABA and BTH are caused by direct phytotoxicity of the chemicals, RGR and seed production were quantified in npr1-1 plants, which are unable to express SA-dependent defenses (27). In contrast with wild-type plants, npr1-1 was not affected in growth or seed production after treatment with 60 mg/liter BABA or 200 mg/liter BTH (Fig. 3), indicating that the BABA- and BTH-induced reductions in plant fitness are not caused by direct phytotoxicity but are due to costs of NPR1-dependent resistance mechanisms.
Fig. 3.
Effects of direct defense-inducing amounts of BABA and BTH on RGR (A) and seed production (B) in Col-0 and npr1-1. Six-week-old plants were soil-drenched with water or 60 mg/liter BABA or were sprayed with 200 mg/liter BTH. See the legend of Fig. 2 for further details. Asterisks indicate statistically significant differences compared with the water control (α = 0.05, Student's t test).
Effectiveness of edr1-Induced Priming and cpr1-Induced Direct Defense.
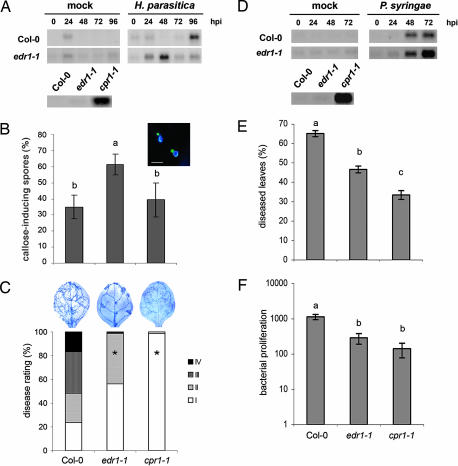
The edr1-1 mutant has been described as having an enhanced response to infection by biotrophic pathogens (28) and could be regarded as a constitutive priming mutant. In contrast, the cpr1-1 mutant displays constitutive expression of SA-inducible defenses (29). To confirm these phenotypes in our bioassay systems, both mutants were tested for PR-1 expression, papillae formation, and resistance against H. parasitica and P. syringae. Noninfected edr1 plants did not express elevated levels of PR-1 gene expression (Fig. 4A). However, after inoculation with either H. parasitica or P. syringae, edr1 activated this marker gene faster and/or stronger than wild-type plants (Fig. 4 A and D), confirming that edr1 is constitutively primed for SA-inducible defenses. In addition, edr1 deposited more callose-rich papillae at 2 days after inoculation with H. parasitica than did wild-type and cpr1 plants (Fig. 4B). These findings indicate that edr1 is also constitutively primed for enhanced callose deposition. The cpr1 mutant showed enhanced PR-1 expression in the absence of a pathogen (Fig. 4 A and D), confirming its constitutive defense phenotype. Upon infection with P. syringae or H. parasitica, cpr1 and edr1 displayed enhanced levels of resistance compared with wild-type plants (Fig. 4 C, E, and F). However, the level of resistance against both pathogens was consistently higher in cpr1 than in edr1.
Fig. 4.
Priming in the edr1-1 mutant and constitutive direct defense in the cpr1-1 mutant against H. parasitica (A–C) and P. syringae (D–F). (A) PR-1 gene expression in 3-week-old plants at different time points after inoculation. hpi, hours postinoculation. (B) Callose deposition at 2 days after challenge with H. parasitica. (C) Induced resistance against H. parasitica 8 days after inoculation. (D) PR-1 gene expression in 6-week-old plants at different time points after inoculation. (E) Induced resistance against P. syringae. (F) Growth of P. syringae over a 3-day time interval. See the legend of Fig. 1 for details.
Costs and Benefits of edr1-Induced Priming and cpr1-Induced Direct Defense.
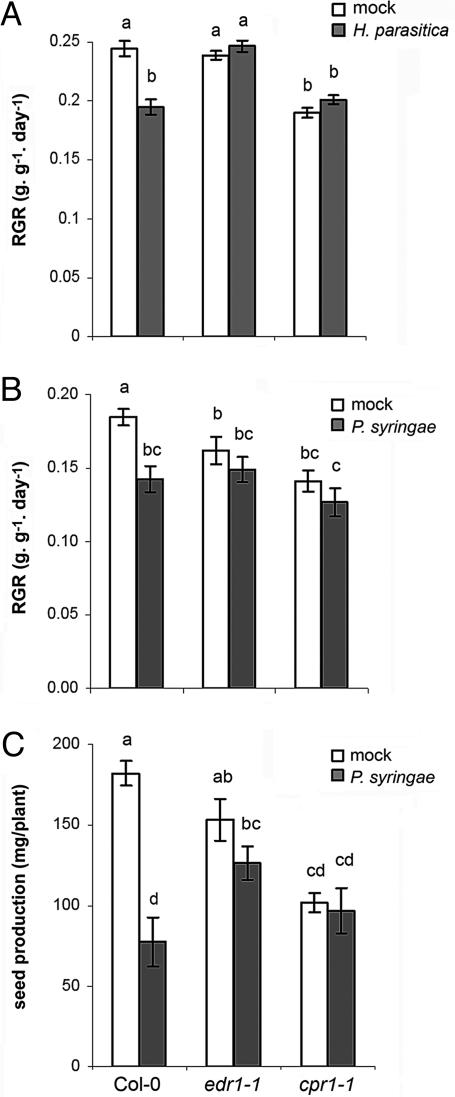
Costs of edr1-induced priming and cpr1-induced direct defense were quantified by RGR and seed production. Noninfected, 3- to 4-week-old edr1 plants showed similar growth rates as wild-type plants (Fig. 5A), whereas 6- to 7-week-old edr1 plants grew significantly slower than the wild-type (Fig. 5B), indicating that edr1-inducing priming involves costs on plant growth only at the relatively old age of a plant. The edr1 mutant did not show a significant reduction in seed production (Fig. 5C). Compared with the wild type, cpr1 displayed strongly reduced RGR, irrespective of its age, and produced significantly less seeds than wild-type and edr1 plants (Fig. 5). After inoculation with H. parasitica or P. syringae, wild-type plants were significantly reduced in RGR compared with noninfected plants (Fig. 5 A and B). Inoculation with P. syringae also decreased seed production in wild-type plants (Fig. 5C). These disease-related effects were considerably less pronounced in the more resistant edr1 and cpr1 plants (Fig. 5). Upon infection by H. parasitica, edr1 showed a higher RGR than wild-type and cpr1 plants (Fig. 5A). This effect was not evident among the P. syringae-infected plants (Fig. 5B). However, seed production upon infection with P. syringae was significantly higher in edr1 than in wild-type plants, whereas that of cpr1 did not significantly differ from the wild-type (Fig. 5C). Hence, edr1-induced priming yields a higher level of fitness than cpr1-induced defense upon infection by H. parasitica or P. syringae.
Fig. 5.
Costs and benefits of edr1-induced priming and cpr1-induced defense in the absence and presence of H. parasitica (A and B) and P. syringae (C). (A) RGR in 3- to 4-week-old plants over the 10-day period after mock or challenge inoculation with H. parasitica. Shown are mean RGR values ± SEM (n = 8–12). (B) RGR in 6- to 7-week-old plants over the 12-day period after mock or challenge inoculation with P. syringae. (C) Seed production by mock- and P. syringae-inoculated plants. Shown are mean values ± SEM (n = 8–12) of the seed weight per plant. See the legend of Fig. 2 for further details.
Discussion
In this study, we have shown that priming by BABA or the edr1 mutation is associated with only marginal reductions in growth, whereas induction of direct defense by high concentrations of BABA, BTH, or the cpr1 mutation, causes much stronger reductions in plant growth and even reduced seed production (Figs. 2 and 5). Thus, induction of priming involves fewer costs than direct defense. Despite the fact that chemically and cpr1-induced direct defense yielded high levels of resistance (Figs. 1 and 4), the costs on plant growth and seed production were in the same order of magnitude as the disease-related costs in noninduced plants (Figs. 2 and 5). Therefore, it can be concluded that the costs of induced direct defense are not outweighed by the benefits of the enhanced protection. In contrast, primed plants were almost equally resistant to P. syringae and H. parasitica but suffered from considerably fewer fitness costs. Moreover, after pathogen infection, primed plants displayed higher levels of fitness than noninduced plants and plants expressing direct defense, which strongly indicates that the benefits of induced resistance through priming outweigh the costs under conditions of disease pressure.
An important challenge for the future will be to assess the role of priming under field conditions. However, at this stage, there are no clear genetic, molecular, or physiological markers available to quantify the state of priming. Therefore, the only way to experimentally determine priming is by measuring the speed and intensity by which defense is activated after disease exposure. Such experiments require two conditions: (i) no disease pressure before the measurements of defense and (ii) sufficient disease pressure during the measurements of defense. Unfortunately, both conditions are very difficult to control under field conditions.
Interestingly, the effects of the edr1 mutation on plant growth were age-dependent: 3- to 4-week-old edr1 plants displayed similar growth rates as wild-type plants (Fig. 5A), whereas 6- to 7-week-old edr1 plants were significantly reduced in growth compared with wild-type plants (Fig. 5B). Hence, costs of edr1-induced priming are only apparent in relatively old plants. This age-dependency might be explained by the enhanced senescence phenotype of the edr1 mutant (30).
Because the defense arsenal in primed plants remains dormant until pathogen infection, priming does not confer major fitness costs under pathogen-free conditions (Figs. 2 and 5). But even upon infection, the augmented defense expression in primed plants remains mostly localized at the sites of pathogen attack. For instance, BABA-induced priming leads to augmented callose deposition only in cells that were in contact with the invading pathogen (15, 16). Hence, priming provides the plant with a timely and spatially efficient strategy to deploy its defensive mechanisms. Yet, priming caused low but statistically significant reductions in plant fitness (Figs. 2 and 5). These priming-related costs could be explained by the observations that Arabidopsis shows enhanced expression of signaling-related genes upon induction of priming (ref. 31 and J.T. and S. van der Ent, unpublished results). Because the defense is not activated before pathogen attack, it is assumed that the corresponding signal transduction pathways remain inactive until the plant is exposed to pathogen attack. Nevertheless, enhanced expression of inactive signaling proteins could already involve small allocation costs. Hence, only if the environment imposes relatively high levels of disease pressure will the plant benefit from priming.
In this study, we have shown that induced resistance through induction of active defense is not an optimal strategy for disease protection, because the associated costs outweigh the benefits of protection. Conversely, induced resistance by means of priming offers an efficient form of plant protection with significant benefits under conditions of disease occurrence. Hence, knowledge about the molecular mechanisms underlying priming can be instrumental in the development of new concepts for disease control, because it provides broad-spectrum resistance without major trade-offs on growth and fruit and seed set. Our data show that priming has significant effects on plant growth and seed production that depend on the extent of disease pressure. Given the fact that growth and seed set are crucially important for the ecological performance of plants, it is plausible that priming plays an important role in nature. Hence, investigating the evolutionary and ecological function of priming will be an exciting challenge for future research.
Materials and Methods
Plants and Pathogens.
Arabidopsis Col-0 mutants npr1-1 (27), cpr1-1 (29), and edr1-1 (28) were kindly provided by X. Dong (Duke University, Durham, NC) and R. Innes (Indiana University, Bloomington, IN), respectively. Seedlings were grown in sand for 2 weeks and subsequently transferred to 60-ml pots containing a potting soil–sand (12:5) mixture as described in ref. 13. Plants were cultivated in a growth chamber with a 9-h day (24°C) and 15-h (20°C) night cycle at 60–70% relative humidity. P. syringae pv. tomato strain DC3000 (32) and H. parasitica strain WACO9 were cultured as described in ref. 11.
RNA Extraction and Blotting.
For RNA extraction, leaves from 4–10 plants were collected. RNA extraction, RNA blotting, and labeling of the PR-1 probe was performed as described in ref. 33. Equal loading was verified by ethidium bromide staining of the gel.
P. syringae Bioassays.
For the chemically induced resistance assays, 6-week-old plants were soil-drenched with water (control) or a BABA solution. BTH was administered by spraying the leaves with a BTH solution containing wettable powder. Two days later, plants were inoculated by dipping the leaves in a suspension of P. syringae, as described in ref. 17. Mock inoculation was performed by dipping the leaves into a similar solution without bacteria. Three days after inoculation, the percentage of leaves with symptoms was determined per plant (n = 15–20). Leaves showing water-soaked lesions surrounded by chlorosis were scored as diseased. Bacterial proliferation over a 3-day time interval was determined as described in ref. 17. Plant material for RGR analysis was collected at the day of chemical treatment and 12 days later. During the experiments with edr1 and cpr1, material was collected 1 day before pathogen inoculation and 12 days later. Plants started to flower in the period of RGR analysis after inoculation, enabling disease progression into the flower stalk.
H. parasitica Bioassays.
For the chemically induced resistance assays, BABA and BTH were applied to 3-week-old plants as described above. Two days later, H. parasitica inoculation was performed by spraying the leaves with 10 mM MgSO4 containing 5 × 104 sporangia per milliliter. To ensure infection, plants were put at 17°C and kept at 100% relative humidity for 24 h after inoculation. At 6 days after inoculation, plants were returned to 100% relative humidity to trigger sporulation. Disease symptoms were scored in 40 plants per treatment (≈200 leaves) at 8 days after inoculation. Disease rating was expressed as severity of disease on each leaf: I, no sporulation; II, trailing necrosis; III, <50% of the leaf area covered by sporangia; IV, >50% of the leaf area covered with sporangia, with additional chlorosis. Leaves from five plants were stained with lactophenol trypan blue and examined microscopically (34). Callose staining was performed as described in ref. 17. Callose deposition was quantified as the proportion of the attempted penetration sites per leaf with callose depositions. For RGR analysis, plant material was collected at the day of chemical treatment and 10 days later. During the experiments with edr1 and cpr1, plant material was collected at 1 day before pathogen inoculation and 10 days later.
Fitness Parameters.
RGR (g·g−1·day−1) was calculated according to RGR = (lnW2 − lnW1)/t2 − t1, where W1 and W2 is plant dry weight at time points t1 and t2, respectively. At each time point, rosette dry weights from 8–12 randomly collected plants were determined (dried for 48 h at 70°C). After time point t2, plants were transferred to a 16-h day/8-h night cycle. Seeds from 8–12 plants per treatment were collected in Aracon tubes (Beta Developments, Gent, Belgium). Plants were watered regularly until they had fully senesced. Seed production was expressed as the average seed weight per plant.
Acknowledgments
We thank R. Innes for providing edr1-1 seeds and H. Poorter for useful suggestions about RGR analysis. This work was supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek Grants 863.04.019 (to J.T.) and 865.04.002 (to C.M.J.P.).
Abbreviations
- BABA
β-aminobutyric acid
- BABA-IR
BABA-induced resistance
- BTH
benzothiadiazole
- ISR
induced systemic resistance
- JA
jasmonic acid
- RGR
relative growth rate
- SA
salicylic acid
- SAR
systemic acquired resistance.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Heil M. Curr. Opin. Plant. Biol. 2002;5:345–350. doi: 10.1016/s1369-5266(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A. A., Strauss S. Y., Stout M. J. Evolution. 1999;53:1093–1104. doi: 10.1111/j.1558-5646.1999.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin I. T. Proc. Natl. Acad. Sci. USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Dam N. M., Baldwin I. T. Funct. Ecol. 2001;15:406–415. [Google Scholar]
- 5.Agrawal A. A. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- 6.Heil M., Hilper A., Kaiser W., Linsenmair K. E. J. Ecol. 2000;88:645–654. [Google Scholar]
- 7.Cipollini D. F. Oecologia. 2002;131:514–520. doi: 10.1007/s00442-002-0909-5. [DOI] [PubMed] [Google Scholar]
- 8.Heidel A. J., Clarke J. D., Antonovics J., Dong X. Genetics. 2004;168:2197–2206. doi: 10.1534/genetics.104.032193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryals J. A., Neuenschwander U. H., Willits M. G., Molina A., Steiner H.-Y., Hunt M. D. Plant Cell. 1996;8:1808–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong X. N. Curr. Opin. Plant Biol. 2004;7:547–552. doi: 10.1016/j.pbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Ton J., Van Pelt J. A., Van Loon L. C., Pieterse C. M. J. Mol. Plant–Microbe Interact. 2002;15:27–34. doi: 10.1094/MPMI.2002.15.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Van Loon L. C., Bakker P. A., Pieterse C. M. J. Annu. Rev. Phytopathol. 1998;36:453–483. doi: 10.1146/annurev.phyto.36.1.453. [DOI] [PubMed] [Google Scholar]
- 13.Pieterse C. M. J., Van Wees S. C. M., Van Pelt J. A., Knoester M., Laan R., Gerrits H., Weisbeek P. J., Van Loon L. C. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jakab G., Cottier V., Toquin V., Rigoli G., Zimmerli L., Metraux J.-P., Mauch-Mani B. Eur. J. Plant Pathol. 2001;107:29–37. [Google Scholar]
- 15.Zimmerli L., Jakab G., Métraux J.-P., Mauch-Mani B. Proc. Natl. Acad. Sci. USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ton J., Mauch-Mani B. Plant J. 2004;38:119–130. doi: 10.1111/j.1365-313X.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- 17.Ton J., Jakab G., Toquin V., Flors V., Iavicoli A., Maeder M. N., Metraux J.-P., Mauch-Mani B. Plant Cell. 2005;17:987–999. doi: 10.1105/tpc.104.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen Y. R. Plant Dis. 2002;86:448–457. doi: 10.1094/PDIS.2002.86.5.448. [DOI] [PubMed] [Google Scholar]
- 19.Jakab G., Ton J., Flors V., Zimmerli L., Metraux J.-P., Mauch-Mani B. Plant Physiol. 2005;139:267–274. doi: 10.1104/pp.105.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerli L., Métraux J.-P., Mauch-Mani B. Plant Physiol. 2001;126:517–523. doi: 10.1104/pp.126.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Loon L. C. Eur. J. Plant Pathol. 1997;103:753–765. [Google Scholar]
- 22.Conrath U., Pieterse C. M. J., Mauch-Mani B. Trends Plant Sci. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- 23.Kauss H., Theisinger-Hinkel E., Mindermann R., Conrath U. Plant J. 1992;2:655–660. [Google Scholar]
- 24.Kohler A., Schwindling S., Conrath U. Plant Physiol. 2002;128:1046–1056. doi: 10.1104/pp.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Wees S. C. M., Luijendijk M., Smoorenburg I., Van Loon L. C., Pieterse C. M. J. Plant Mol. Biol. 1999;41:537–549. doi: 10.1023/a:1006319216982. [DOI] [PubMed] [Google Scholar]
- 26.Verhagen B. W. M., Glazebrook J., Zhu T., Chang H.-S., Van Loon L. C., Pieterse C. M. J. Mol. Plant–Microbe Interact. 2004;17:895–908. doi: 10.1094/MPMI.2004.17.8.895. [DOI] [PubMed] [Google Scholar]
- 27.Cao H., Bowling S. A., Gordon A. S., Dong X. Plant Cell. 1994;6:1583–1592. doi: 10.1105/tpc.6.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frye C. A., Innes R. W. Plant Cell. 1998;10:947–956. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowling S. A., Guo A., Cao H., Gordon A. S., Klessig D. F., Dong X. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang D., Christiansen K. M., Innes R. W. Plant Physiol. 2005;138:1018–1026. doi: 10.1104/pp.105.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maleck K., Levine A., Eulgem T., Morgan A., Schmid J., Lawton K. A., Dangl J. L., Dietrich R. A. Nat. Genet. 2000;26:403–410. doi: 10.1038/82521. [DOI] [PubMed] [Google Scholar]
- 32.Whalen M. C., Innes R. W., Bent A. F., Staskawicz B. J. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ton J., De Vos M., Robben C., Buchala A., Métraux J.-P., Van Loon L. C., Pieterse C. M. J. Plant J. 2002;29:11–21. doi: 10.1046/j.1365-313x.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- 34.Koch E., Slusarenko A. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]