Abstract
Control of nosocomial transmission of methicillin-resistant Staphylococcus aureus (MRSA) has been unsuccessful in most countries. Yet, some countries have maintained low endemic levels by implementing nationwide MRSA-specific infection control measures, such as “search & destroy” (S&D). These strategies, however, are not based on well designed studies, and their use in countries with high levels of endemicity is controversial. We present a stochastic three-hospital model and an analytical one-hospital model to quantify the effectiveness of different infection control measures and to predict the effects of rapid diagnostic testing (RDT) on isolation needs. Isolation of MRSA carriers identified by clinical cultures is insufficient to control MRSA. However, combined with proactive search (of high-risk patients on admission and/or contacts of index patients), it will maintain prevalence levels <1%. Concerted implementation of S&D in countries with high nosocomial endemicity reduces nosocomial prevalence to <1% within 6 years. Stepwise implementation of control measures can reduce isolation capacities needed. RDT can reduce isolation needs by >90% in low-endemic settings and by 20% in high-endemic settings. Surveillance of colonization and improved hand hygiene can markedly increase control efficacy. These findings strongly suggest that: (i) causality exists between S&D and low MRSA prevalence; (ii) isolating MRSA carriers identified by clinical cultures as a single measure is insufficient for control; (iii) a combined approach of isolation and screening confers efficacy; and (iv) MRSA-prevalence levels can be reduced to <1% in high-endemic settings by S&D or a stepwise approach to interventions. RDT can markedly enhance feasibility.
Keywords: infection control, mathematical model, search & destroy, antibiotic resistance
Nosocomial infections with methicillin-resistant Staphylococcus aureus (MRSA) are a major healthcare problem in most developed countries (1). Despite an increasing number of reports on infections caused by so-called community-associated MRSA (2), the majority of MRSA infections are still hospital-related (3). Asymptomatic carriage of MRSA is now endemic among hospitalized patients in many countries with reported proportions of staphylococcal bloodstream infections caused by MRSA as high as 44% in the U.K., 20% in Germany, 30% in Belgium [European Antimicrobial Resistance Surveillance System (EARSS) data from www.rivm.nl/earss], and 50% in the U.S. [National Nosocomial Infections Surveillance System (NNIS) from www.cdc.gov/ncidod/dhqp/nnis_pubs.html].
Scandinavian countries and The Netherlands, however, have maintained prevalence levels of nosocomial MRSA infections <1% (1, 4–8), presumably because of nationwide policies to identify MRSA carriage and specific infection control measures for managing colonized patients. This so-called “search & destroy” (S&D) policy was implemented in the 1980s. Although successful, this strategy is not based on well designed studies (9), and the relative importance of individual components has never been determined.
The negative aspects of this policy are the associated extra costs (8), a potentially reduced quality of patient care (10), a reduction in hospital admission capacity because of isolation of patients in single bedrooms, and the need to temporarily close wards in case of patient-to-patient transmission. Moreover, because of the diagnostic delay of currently used conventional microbiological culture techniques, 95% of patients suspected of MRSA carriage are unnecessarily isolated for, on average, 5 days (11). Implementation of S&D raises moral and legal issues, including whether healthcare workers (HCWs) could be compelled to undergo medical treatment or be furloughed from working for the benefit of their patients (12).
Herein we use two different compartmentalized models to analyze transmission dynamics of MRSA, to quantify the effectiveness of individual components of S&D, and to determine short-term and long-term effects of rapid diagnostic testing (RDT). First, the efficacy of infection control measures is analyzed in settings of low endemicity (e.g., The Netherlands) and subsequently in settings of high endemicity (e.g., the U.S. and U.K.).
Simulation Model
All parameter estimates are based on data obtained in the University Medical Center Utrecht (UMCU), unless specified otherwise (Table 1). The UMCU is a tertiary referral center with all medical specialties represented. S&D for MRSA has been executed since 1986 (8, 13) by the Department of Hospital Hygiene & Infection Prevention, with prospective monitoring of all relevant data.
Table 1.
Parameters in the models
| Parameter | Symbol | Value | Source |
|---|---|---|---|
| Fraction superspreaders | 0.01 | A | |
| Relative infectivity of superspreaders | 10 | A | |
| Interward transfer (per patient/per day) | 0.02 | A | |
| Average LOS in ICU | 3 days | U | |
| Average LOS in regular ward | 7 days | U | |
| Average LOS in simplified model | d | 8 days | U |
| Admission from other hospitals | 5% | U | |
| High-risk patients | 10% | A | |
| Rel. admission rate of high-risk patients | 10 | A | |
| Staff:patient ratio in ICU | 1:1 | U | |
| Staff:patient ratio in regular ward | 5:18 | U | |
| Ratio of cross-transmission to HCW transmission | 8:1 | A | |
| Mass action term unit vs. hospital | p:1 − p | 0.95:0.05 | A |
| Transmission risk ICU:ward | 3:1 | A | |
| Admission from endemic settings | 0.38% | U | |
| Patients from endemic setting MRSA+ | 4.7% | [11] | |
| Extramural acquisition from different populations | 5% | A | |
| Duration of colonization in extramural populations | 1/ξ | 370 days | [20] |
| Detection rate of MRSA (per day) | ν | 0.03 | U |
| Fraction failure to isolate | 1-g | 0.12 | U |
| Successful decontamination | φ | 0.25 | [24, 25] |
| Duration of preventive isolation | 5 days | U | |
| Reduction of transmission by isolation | π | 1 | A |
U, parameters estimated from data from University Medical Center Utrecht; A, parameters based on assumptions and, therefore, subjected to a sensitivity analysis; LOS, length of stay.
Our discrete time stochastic simulation model comprises three hospitals, each with 36 18-bed general wards and 5 9-bed intensive care units (ICUs) with 100% bed occupancy. Each hospital has its corresponding catchment population of 200,000 individuals. Within hospitals, patients can be either MRSA carriers or noncarriers. Patients are identified as MRSA carriers, suspected of MRSA carriership (with yet uncertain colonization status), or not suspected of MRSA carriership.
In the extramural setting, only individuals who have been identified as MRSA carriers during a previous hospitalization are considered as possible MRSA carriers; i.e., there is no active search for carrier status in the community. All noncolonized individuals are susceptible to colonization. Within hospitals, a small fraction (1%) of colonized patients is considered “superspreader,” and such patients are 10 times more infectious than other colonized patients during their entire future stay in the hospital. Patients are admitted to either a general or an ICU ward. Patient flow is from an ICU to a regular ward to an extramural population and from a regular ward to an extramural population, with a possibility of interward transfer (P = 0.02 per patient per day) (Fig. 1a).
Fig. 1.
Patient dynamics (a) and MRSA dynamics (b) within a hospital. Solid arrows, relatively frequent processes; dotted arrows, relatively infrequent ones.
Length of stay does not depend on colonization status and is exponentially distributed with a mean of 3 and 7 days for ICU and regular wards, respectively. Five percent of admissions are from other hospitals, and all patients are discharged to their own extramural population. A high-risk population (10% of total catchment population), characterized by a higher hospitalization rate (1 per year vs. 0.1 per year), constitutes, on average, half of the hospital population. All deaths are immediately replaced by noncolonized individuals, who enter the extramural population in the same community and the same group (high-risk group or non-high-risk group), thereby ensuring a constant population size. During hospitalization, 2.9% of the hospitalized patients die. The death rate within ICUs is higher as compared with regular wards (ratio 3:1). In the extramural population, life expectancy, given that the patient is not hospitalized, is exponentially distributed. The overall life expectancy (including death during hospitalization) is 20 and 78 years for the high-risk and non-high-risk population, respectively.
Two types of HCWs (HCW1 and HCW2) are incorporated. HCW1 interact with patients in a single ward. Staff:patient ratios are 1:1 in ICU and 5:18 in general wards. Patient contacts of HCW2 (n = 80) are not restricted. Total number of HCW per hospital is 1,525, with 305 working per 8-hour shift. Initial prevalence levels of MRSA in hospitals and communities are 0.0004 for countries with low-endemic levels (14) and 0.15 and 0.02 for countries with high-endemic levels. For simplicity, we define low- and high-endemicity settings as prevalence levels <2% and >10%, respectively.
The model was investigated with Monte Carlo simulations. All analyses are based on 1,000 simulations for a 30-year time period after a burn-in period of 10 years to guarantee the correct distribution over the high-risk and the non-high-risk group.
Dynamics of MRSA
Nosocomial acquisition of MRSA occurs through patient-to-patient transmission (i.e., cross-transmission via transiently colonized HCWs) or through transmission of MRSA from persistently colonized HCWs (Fig. 1b), with cross-transmission occurring eight times more frequently than transmission from a persistently colonized HCW in high-endemicity settings. Transmission occurs according to the mass action principle, with mass action being 20 times higher for transmission within a single unit (for both transmission possibilities) as compared with the global mass action term for the hospital at large (e.g., see ref. 15). As compared with regular wards, transmission risk is higher in ICUs (ratio 3:1) because of higher patient susceptibility, more intense patient–HCW contacts, and higher antibiotic use, which may give resistant bacteria an advantage in colonizing patients compared with nonresistant bacteria.
Once treated in isolation, transmission and acquisition (in case of false alarm) are equally prevented with efficacy π. Hospitals can “infect” each other by transfer of MRSA-colonized patients. To mimic the Dutch situation, 0.0038 of admissions come from endemic settings (i.e., foreign hospitals), with 4.7% being colonized with MRSA (11). The ultimate average nosocomial endemic MRSA prevalence without interventions is set at 15%. This prevalence is based on determination of endemic prevalences (16–19) as well as on proportions of MRSA (40% to 50%) among staphylococcal bloodstream infections (EARSS and NNIS data), with 30% of the hospital population carrying S. aureus.
In the extramural setting, colonization will disappear at a fixed rate, with an average duration of colonization, in absence of readmission, of 370 days (20). Patient-to-patient transmission of hospital-related MRSA strains in the extramural setting is considered of minor importance (21) and, therefore, is neglected.
The value of the transmission parameters was determined by fitting the endemic curve to observed curves in the U.S. and the U.K., leading to nosocomial prevalence levels of 15%. The relative importance of the individual transmission routes, as described here, however, was maintained. The compartmental model structure and the depletion of susceptibles within a unit complicate definition and calculation of RA, defined as the average number of secondary cases resulting from one primary case when other patients are susceptible during a single hospital admission of the primary case (22). Hence, we obtained several values for RA, depending on the initial ward and the superspreader status of the patient.
Interventions
The per patient probability per day to detect MRSA colonization (or infection) by way of clinical microbiological cultures is 0.03, which is based on the average time between admission and detection of unexpected colonization (M.J.M.B. and M.C.J.B., unpublished data). Measures of S&D include the following [guidelines from the Dutch Workingparty Infection Prevention at www.wip.nl/UK/free_content/Richtlijnen/MRSA(1).pdf]:
Identified MRSA carriers are treated in single rooms with barrier precautions (i.e., isolation).
High-risk patients (previously identified MRSA carriers or those transferred from endemic settings) are screened for MRSA colonization upon admission and precautionarily isolated, with 0.88 efficacy; i.e., 12% are not isolated (based on ref. 23). Isolation instructions are only withdrawn upon negative results of conventional microbiological cultures.
All patients in a single ward will be screened for MRSA colonization in case of an unexpected finding of MRSA colonization (i.e., in a patient not treated in isolation).
In addition to measure III, all HCWs in the affected ward will be screened for MRSA colonization, and colonized HCWs will be furloughed from working until decontamination has been achieved.
Wards will be closed for new admissions when there is evidence of MRSA transmission among patients (>1 MRSA carrier) and will remain closed until isolation capacity is sufficient for all carriers.
MRSA colonization is eradicated at the end of hospitalization with an efficacy of 0.25 (24, 25).
Hand disinfection is not specifically mentioned as an intervention, because adherence should be high, regardless of colonization status of patients. Yet, the effects of increased adherence to hand hygiene in general can be analyzed by reducing the likelihood of transmission with the same factor for all transmission routes.
RDT
Conventional microbiological cultures are assumed to be 100% reliable but require quite some time (up to 5 days; ref. 11). RDT will determine colonization status within a few hours and will reduce unnecessary precautionary isolation days of high-risk patients screened on admission. It will also lead to a faster detection of spread among contact patients and HCWs, however, possibly at the cost of misclassification. The effects of RDT were analyzed for two scenarios, namely when used as the only tool or when used in combination with conventional cultures. Evaluation is based on the number of isolation days needed for infection control. In the combined approach, the isolation status of patients, as initiated upon RDT results, may be changed after, on average, 5 days, on the basis of conventional culture results.
Quantifying Effects of Individual Components
A second, simplified model (one hospital with an infinite number of wards and with only cross-transmission as the transmission route; see Supporting Text, which is published as supporting information on the PNAS web site) was used to verify (by way of comparing outcomes) that the simulation model is correctly implemented (and vice versa that no mistakes are made in the analysis of the simplified model). In addition, this analytical formulation adds to conceptual clarity and allows quantification of the effect of individual parameters. In this formulation, we introduce the critical basic reproduction ratio (R0c) of a single outbreak of MRSA that leads to an effective R value of 1 when readmission is taken into account and a control measure is implemented. Below this value, transmission is insufficient to generate a large outbreak. Above this level, however, transmission control will fail, and intramural and extramural prevalence levels will rise.
Results
Low-Endemicity Settings.
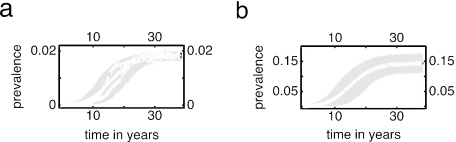
Starting from low MRSA-prevalence levels and without any infection control measure, average nosocomial endemic MRSA prevalence will increase in all simulations from almost absent (<1%) to saturation (15%) within a period of ≈10 years (Fig. 2). This rise may be delayed considerably because of stochastic effects (up to 10 years). Therefore, the mean prevalence in 1,000 simulations rises more slowly to saturation than the prevalence in a typical individual simulation (Fig. 2a). The extramural prevalence remains <2%, in line with longitudinal epidemiological observations in both the U.S. and U.K. (26). Ultimately, MRSA prevalence will be 9% within the high-risk population and 18% in the extramural and intramural populations, respectively.
Fig. 2.
Extramural (a) and intramural (b) MRSA prevalence starting from low endemicity. The black line denotes the mean result of 1,000 simulations with 90% of the simulations falling in the gray region. The two colored lines in a represent individual simulations. The prevalence in these simulations rises faster than the mean over 1,000 simulations.
Effect of Individual Components of S&D.
The isolation of identified carriers as a single control measure (measure I, with 100% efficacy) will not be sufficient to maintain endemic levels <1%, although endemic levels will rise slowly (to 1.5% in 30 years). The addition of either screening and precautionary isolation of high-risk patients (measure II) or screening of contact patients in case of the identification of an unexpected index patient (measure III) will be sufficient to maintain low endemicity (Fig. 3a). Additional measures such as screening of HCWs in case of the identification of an unexpected index patient (measure IV), temporary closing of wards (measure V), and eradication of carriage (measure VI) offer little extra benefit. Stealth dynamics and stochastic control failure, as described by Cooper et al. (22), are equally relevant in our simulations but are not described in detail here. In addition, the efficacy of intervention measures depends critically on interventions taken by other hospitals, also confirming previous findings (27, 28).
Fig. 3.
Effect of intervention strategies on nosocomial prevalence levels when isolation is 100% effective. (a) Initial low prevalence. (b) Initial high prevalence. For description of interventions, see Interventions.
Sensitivity Analysis.
Results of the simplified one-hospital model confirm the results of simulations of the three-hospital model. When starting from low-endemic levels, R0c are 1.2 for isolating patients identified upon clinical cultures (measure I) and 1.4 and 1.5 when combining measure I either with screening high-risk patients on admission (measures I and II) or with screening contact patients from index cases (measures I and III), respectively. A combination of all these interventions (measures I through III) will raise R0c to 2.1. In sensitivity analyses, the frequency of detecting MRSA colonization by way of clinical cultures is the most critical parameter (Fig. 4). For combined interventions, R0c increases dramatically when detection rates increase to values >0.1 per day, which implies that newly colonized patients are, on average, detected within 10 days. Such a value would be attained by obtaining surveillance cultures on a weekly basis. Changing other variables, such as unit size in the hospital structure, efficacy of decolonization, or high-risk population size, within reasonable ranges, hardly affects efficacy of infection control measures. The latter may sound surprising, because a large core group size corresponds to more readmission and, hence, a higher basic reproduction ratio (R0). However, our claim is that the R0 value of a disease, which can be controlled by a set of measures, is not so much influenced by the core group size. For the same R0 value, the transmissibility of the disease should be lower when the core group size is larger. Only when the core group size is very small does isolating patients with a history of MRSA upon admission become less effective, whereas the screening of contact patients becomes more effective. A higher relative influence of persistently colonized HCWs on the spread of MRSA has no major consequences when duration of persistent colonization (1 year) is shorter than the time scale of a decrease in the prevalence levels within hospitals (several years), although the effect of screening of HCWs in the affected wards (measure IV) will increase.
Fig. 4.
Changes in critical reproduction ratio (R0c) for several combinations of intervention measures according to changes in model parameters. Default values of the parameter are shown in Table 1. (a) Relative increase in critical transmission parameter βc for a hospital with wards of size N compared with a hospital consisting of wards of infinite size (i.e., without depletion of susceptibles). (b–e) Critical reproduction ratio R0c for one of the intervention strategies as function of one of the parameters. (b) Effect of improvement of hand hygiene. (c) Core group size. (d) Detection rate of colonized patients (one per day). (e) Mean length of colonization. (See Figs. 7–10, which are published as supporting information on the PNAS web site, for more information.)
RDT.
The average number of patients in isolation per day in the University Medical Center Utrecht from 1999 to 2004 was 1.88, which coincides well with the prediction of the simulation model (2.0). If RDT would perform as well as conventional culture techniques (sensitivity = 1; specificity = 1), the number of isolation days needed for the full S&D policy in a low-endemicity setting would be reduced by >90%. Obviously, even with less-optimal test characteristics, RDT will reduce the number of isolation days in the short term, because it may take years to establish high-endemic levels, even without infection control measures.
RDT will reduce unnecessary precautionary isolation days of high-risk patients. However, with suboptimal test characteristics, RDT may fail to identify some of the MRSA carriers among contact patients (because of false-negative RDT results) and may identify noncarriers as MRSA carriers (because of false-positive RDT results). For certain test characteristics, both effects will balance in the long run (30 years). For instance, with 80% sensitivity, specificity should be at least 15% and 60% for the scenarios with and without conventional cultures being used as back-up, respectively (Fig. 5a). Because of the introduction of RDT, the prevalence level may slightly increase but always will remain <1.5%.
Fig. 5.
Effect of RDT. (a) Effect in low-endemicity settings with S&D. RDT will reduce unnecessary precautionary isolation days of high-risk patients. However, with suboptimal test characteristics, RDT may fail to identify some of the MRSA carriers among contact patients (because of false-negative RDT results) and may identify noncarriers as MRSA carriers (because of false-positive RDT results). Both effects will balance for certain test characteristics, and these lines are depicted. The upper line represents the scenario in which conventional cultures are used as back-up; the lower line is the scenario without back-up cultures. Above the lines, the use of RDT will increase the number of isolation days needed. Below the lines, reduction of isolation days will dominate and the net effect will thus be fewer isolation days. (b and c) Effect of RDT with measures I + II + III for four culture regimes: No RDT, RDT with sensitivity and specificity of 90%, RDT with sensitivity and specificity of 90% with backup of standard culture methods, and RDT with sensitivity of 90% and specificity of 100% with backup of standard culture methods. For the first 5 years, efficacy of isolation is 50%; afterward, efficacy is 80%. (b) Effect on the prevalence level in hospitals. (c) Expected isolation (iso) days.
Control Strategies for High-Endemicity Settings.
In a high-endemicity setting, any intervention (measures I–VI) will reduce endemic prevalence, although average time to reach a certain threshold level will differ considerably (Fig. 3b). Typically, a two-phase reduction is seen. During the first phase (first weeks), endemic levels will rapidly decrease as a result of lower transmission of MRSA because of interventions. After the initial phase, endemic levels will further decrease because of the feedback-loop dynamics with the community, which are as follows: reduced nosocomial spread leads to a lower extramural prevalence, lower fractions of patients colonized on admission, and lower endemic levels in the hospital.
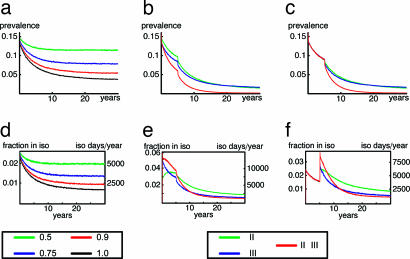
The isolation of MRSA patients identified by clinical cultures as the only intervention can reduce endemic prevalence to 5% within 15 years. Yet, the efficacy of isolation will be crucial (Fig. 6a). With isolation efficacy of 50%, ultimate prevalence levels will remain >10%. Addition of other preventive measures will markedly enhance MRSA control. Screening of contact patients (with precautionary isolation) of an index case (measures I + III) is more effective than screening of high-risk patients on admission (measures I + II), because it will take, on average, 8 and 15 years, respectively, to reach endemic levels <1% (Fig. 3b). Again, we assumed 100% isolation efficacy. The application of the full S&D policy (measures I–VI) will reduce endemic prevalence levels to <1% within 6 years.
Fig. 6.
Effect of (stepwise) introduction of intervention measures for high-endemic settings. (Upper) Nosocomial prevalence. (Lower) Number of isolation days. (a and d) Isolation of patients with a positive clinical culture for different levels of isolation efficacy. (b and e) Increment of isolation efficacy from 50% to 80% after 5 years for several intervention measures. (c and f) First 5 years, isolation of colonized patients (measure I) with 80% efficacy. Additional interventions are implemented after 5 years (isolation efficacy remains at 80%).
Precautionary isolation will require a large isolation capacity in high-endemic settings (Fig. 6d) and, therefore, may not be feasible. Instead, several MRSA patients could be cohorted in designated rooms, although this measure will probably reduce the efficacy of isolation. Hence, we propose two alternative approaches for high-endemicity settings, namely cohorting multiple patients and gradual implementation of intervention measures. (Screening of HCWs, eradication of colonization, and closing of wards added little to ultimate prevalence levels and, therefore, are not shown.) In the first approach, the initial efficacy of isolation is set at 50% and only after 5 years is the efficacy increased to 80%, e.g., by switching from cohorting of multiple MRSA patients in single rooms to isolating individual patients in single bedrooms. In this scenario, combinations of measures I + II, I + III, and I + II + III will reduce prevalence levels to levels <2% within 30, 31, and 18 years, respectively (Fig. 6b). Regimens, including screening of contact patients (measure III), require the highest isolation capacity (Fig. 6e) but will lead to a faster decline in prevalence levels as compared with measures I + II. The total number of isolation days per hospital required over 30 years is 132,000, 102,000 and 112,000 for measures I + II, I + III, and I + II + III, respectively.
In the second approach, only measure I is used with an efficacy of 80% during the first 5 years. After 5 years, the policy is extended to measures I + II, I + III, or I + II + III (efficacy of isolation remains at 80%). Naturally, only the combined approach of three measures will reduce prevalence levels to <1% (Fig. 6c). This policy would reduce the required isolation capacity at the start of interventions from 6% of hospital beds in approach 1 to 3% in approach 2. After 5 years, 3% of hospital beds would be needed for isolation with both approaches (Fig. 6f). The total number of isolation days per hospital over 30 years with this approach would be 109,000, 82,000, and 84,000 for measures I + II, I + III, and I + II + III, respectively.
RDT in High-Endemicity Settings.
The effects of RDT were determined for the two stepwise approaches introduced in the Control Strategies for High-Endemicity Settings. The screening of high-risk patients and contact patients is based on RDT with sensitivity of 90% and specificity of either 90% or 100%. RDT with a specificity of 100% reduces peak isolation capacity by 20% (Fig. 5c) and reduces prevalence levels faster than when infection control is based on conventional cultures (Fig. 5b). A specificity of 90%, however, would increase the number of isolation beds needed, as compared with the strategy without RDT. The use of RDT in combination with conventional microbiological culture methods offers little additional benefit.
Discussion
The results of our simulation model do the following:
strongly suggest causality between S&D and the low prevalence of MRSA in hospitals and community;
indicate that isolating MRSA carriers identified by clinical cultures as a single infection control measure, although useful, is unlikely to be sufficient to eliminate or prevent endemicity;
suggest that a combined approach of screening high-risk patients upon admission plus the screening of contact patients when an index patient is identified is responsible for the success of S&D; and
suggest that with full S&D, or one with a stepwise approach toward this policy, high nosocomial endemicity levels can be reduced to levels <1% within 6–12 years.
Furthermore, RDT is likely to reduce isolation needs considerably in low-endemicity settings and will enhance the decline in prevalence levels in high endemicity settings, although without reducing isolation needs on the short term.
Our findings strongly support the use of S&D to control nosocomial spread of MRSA and indicate that elimination of MRSA in high-endemicity settings is likely achievable with targeted approaches (29). Successful elimination can be enhanced by a concerted approach of healthcare organizations, and feasibility of these measures can be improved by using RDT. In our model, we sought to mimic hospital and population structures as reliably as possible, by using empirical data from a single medical center where possible. Naturally, model construction will always involve simplifications, but valuable information on trends and dynamics can be gained. Because nationwide prospective trials to evaluate these measures will never be performed, mathematical modeling, taking intramural and extramural dynamics and stochastic effects into account, offers the best approach.
Two other mathematical models have been used to investigate transmission dynamics of MRSA with and without infection control measures and with integration of intramural and extramural transmission dynamics, although with important differences in modeling approaches (22, 28, 29). Unique in our approach is the compartmental structure of hospitals, which reflects reality more closely and allows modeling of specific intervention measures. Smith et al. (28), Pan et al. (29), and Cooper et al. (22) used models in which hospitals were reflected as a single unit with transmission occurring according to the mass-action principle. The representation of individual wards already affects transmission dynamics, which mimics clinical observations that outbreaks predominantly occur within single wards. Other unique aspects of our approach are that persistent carriership among HCWs and heterogeneity in infectivity of patients have been taken into account and that a simplified one-hospital model was used to quantify effectiveness of each of the components of S&D. The latter also allowed sensitivity analyses of other critical variables. Further differences include the way in which intervention strategies are evaluated.
Smith et al. (28) and Pan et al. (29) used a deterministic model with a single unspecified intervention strategy that affects the transmission rate (e.g., improved hand hygiene), whereas Cooper et al. (22) considered isolation of patients as identified by clinical cultures as the only intervention measure. Screening on admission of high-risk patients was evaluated in a simulation model of a single ward without feedback loop and with a constant fraction of patients colonized on admission, thereby neglecting interaction between intramural and extramural dynamics (30).
Cooper et al. (22) concluded from their model that prompt and effective isolation of MRSA carriers (identified as such by clinical cultures) can contribute to control by reducing transmission, but its efficacy would be limited by shortage of isolation capacity (22). Our predictions about this single intervention are even less optimistic. Although this measure will maintain low MRSA levels in low-endemicity settings for some time, it is unlikely to eliminate MRSA in high-endemic settings (although substantial reduction can be obtained). Indeed, the only randomized trial on this intervention in such a setting failed to control transmission of MRSA (17).
Two important differences in parameter values between Cooper's model (22) and ours must be discussed. First, we have used a lower detection rate of MRSA through clinical isolates (0.03 vs. 0.1; ref. 22), motivated by data from our own hospital. A higher detection rate, for instance through surveillance cultures, would dramatically enhance efficacy of control measures (Fig. 4d). The second difference is that we did not assume that MRSA colonization prolongs the length of stay. The additional length of stay attributable to MRSA bacteremia in studies adequately controlling for duration of exposure, have ranged from 0 to 2 days (33). Yet, the majority of colonized patients will not develop systemic infections, and colonization in itself is unlikely to prolong the length of stay.
Increased adherence to hand hygiene practices clearly would effect transmission of MRSA, because it would reduce the general transmission parameter and, thereby, prevalence levels (see Fig. 9) and critical basic reproduction ratios (Fig. 4b). Because unchanged hand disinfection practices were assumed in all analyses of our study, our findings must be considered as conservative estimates of efficacy that could be enhanced by simultaneous enforcement of better hand hygiene.
Decolonization of MRSA carriers within hospitals is only successful occasionally (P = 0.25; refs. 24 and 25). In essence, decolonization after discharge and screening of previously colonized patients upon readmission have similar effects, because both measures prevent colonized patients to transmit when readmitted. Therefore, as part of S&D, more effective decolonization after hospital discharge would only have moderate effects on transmission dynamics, even though the number of isolation days needed could be reduced considerably. Naturally, more successful eradication of MRSA carriage during hospital stay would dramatically contribute to the efficacy of control measures.
Although we have tried to stay with our model as close to real MRSA dynamics as possible, it should be explicitly stated that our findings represent average behavior of 1,000 simulations, with large variations attributable to stochastic processes. The latter is especially important for low-endemicity settings, because interventions may fail to control MRSA. Moreover, we had to make “guesstimates” for some parameters (Table 1) such as the fraction of superspreaders (0.01) and their increased capacity to spread MRSA (10 times), the intrahospital transfer rate (0.02 per patient per day), the proportion of the high-risk population (10%), the likelihood that transmission occurred more efficiently (8 times) between patients than from persistently colonized HCWs and more efficiently (20 times) within a single ward than between wards and more efficiently in ICUs than in general wards (3 times), and, finally, the extramural interaction fraction between catchment populations (0.05). Also the assumption of exponentially distributed lifespans may be unrealistic for a developed country. The importance of such assumptions has been determined in sensitivity analyses, however, and variation of these parameters turned out to have no significant effects on simulation results (data not shown).
When ignoring depletion of susceptibles, RA values range from 0.75 for nonsuperspreaders admitted to a regular ward to 11 for superspreaders admitted to the ICU leading to a basic reproduction ratio (R0) in our model of 1.2. In real life, however, specific subgroups, e.g., hemodialysis patients, may have even higher admission rates and, consequently, higher propensity to carry MRSA. As a matter of fact, interventions that control MRSA for the population at large may not be effective in subpopulations. In our model, we focused on hospital-related MRSA. Findings change drastically when community-associated MRSA becomes a major source of nosocomial MRSA infections (data not shown).
An important finding of our simulations was that, in addition to isolating identified carriers, screening and precautionary isolation of contacts in case of an index patient was more effective than screening of high-risk patients on admission. An intuitive explanation is that the probability to detect colonized patients increases with the size of a nosocomial outbreak. Indeed, screening on admission will reduce the number of outbreaks but not affect the outbreak size, whereas screening contact patients (with precautionary isolation) will interrupt transmission and thus will prevent outbreaks from becoming large.
Implementation of RDT has the potential to dramatically reduce isolation needs and, thus, increase the cost effectiveness of S&D in countries with low-endemic levels. But as long as the sensitivity of these tests in low-endemicity settings has not been determined, a combined approach with conventional cultures must be advised. Sensitivity and specificity levels of RDT of >90% have been reported from high-endemicity settings (32). In high-endemic countries, implementation of RDT can reduce isolation needs by 20%, even without the simultaneous use of conventional cultures.
In conclusion, our findings provide a theoretical basis for the success of MRSA control in countries using a nationwide S&D policy and suggest that successful control would not be jeopardized if some measures were abandoned or if numbers of high-risk patients increased. In such settings, RDT would significantly reduce isolation needs. Our findings also suggest that effective control is possible in countries where MRSA already is an important nosocomial pathogen. The combination of concerted action, a stepwise approach, and rapid detection of carriership offers the best guarantee for success, which could be enhanced by improving compliance with hand hygiene. Considering the burden of disease caused by MRSA, the time seems right for action. And if not for MRSA, lessons can be drawn for future pathogens, such as vancomycin-resistant S. aureus.
Supplementary Material
Acknowledgments
We thank Hajo Grundmann, Richard Wenzel, Robert Weinstein, Jan Kluytmans, and Ben Cooper for stimulating discussions. Financial support was provided by Nederlandse Organisatie voor Wetenschappelijk Onderzoek Grants ZonMW 2100.0051 and CLS 635.100.002.
Abbreviations
- HCW
healthcare worker
- ICU
intensive care unit
- MRSA
methicillin-resistant S. aureus
- RDT
rapid diagnostic testing
- S&D
search & destroy.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Tiemersma E. W., Bronzwaer S. L., Lyytikainen O., Degener J. E., Bruinsma N., Monen J., Witte W., Grundmann H. Emerging Infect. Dis. 2004;10:1627–1634. doi: 10.3201/eid1009.040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zetola N., Francis J. S., Nuermberger E. L., Bishai W.R. Lancet Infect. Dis. 2005;5:275–286. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 3.Salgado C. D., Farr B. M., Calfee D. P. Clin. Infect. Dis. 2003;36:131–139. doi: 10.1086/345436. [DOI] [PubMed] [Google Scholar]
- 4.Faria N. A., Oliviera D. C., Westh H., Monnet D. L., Larsen A. R., Skov R., de Lencastre H. J. Clin. Microbiol. 2005;43:1836–1842. doi: 10.1128/JCM.43.4.1836-1842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastila S., Sammalkorpi K. T., Vuopio-Varkila J., Kontiainen S., Ristola M. A. J. Hosp. Infect. 2004;58:180–186. doi: 10.1016/j.jhin.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 6.Bjorholt I., Haglind E. Eur. J. Clin. Microbiol. Infect. Dis. 2004;23:688–695. doi: 10.1007/s10096-004-1198-1. [DOI] [PubMed] [Google Scholar]
- 7.Christensen A., Scheel O., Urwitz K., Bergh K. Scand. J. Infect. Dis. 2001;33:663–666. doi: 10.1080/00365540110026944. [DOI] [PubMed] [Google Scholar]
- 8.Vriens M., Blok H., Fluit A., Troelstra A., Van Der Werken C., Verhoef J. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:782–786. doi: 10.1007/s10096-002-0811-4. [DOI] [PubMed] [Google Scholar]
- 9.Voss A. Br. Med. J. 2004;329:521. doi: 10.1136/bmj.329.7465.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelfox H. T., Bates D. W., Redelmeier D. A. J. Am. Med. Assoc. 2003;290:1899–1905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser A. M., Schultsz C., Kruithof G. J., Debets-Ossenkoop Y., Vandenbroucke-Grauls C. Clin. Microbiol. Infect. 2004;10:972–979. doi: 10.1111/j.1469-0691.2004.01000.x. [DOI] [PubMed] [Google Scholar]
- 12.Gebhardt D. O. E. J. Med. Ethics. 2003;29:212. doi: 10.1136/jme.29.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenbroucke-Grauls C. M., Frenay H. M., Klingeren B. van, Savelkoul T. F., Verhoef J. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:6–11. doi: 10.1007/BF01967090. [DOI] [PubMed] [Google Scholar]
- 14.Wertheim H. F., Vos M. C., Boelens H. A., Voss A., Vandenbroucke-Grauls C. M., Meester M. H., Kluytmans J. A., van Keulen P. H., Verbrugh H. A. J. Hosp. Infect. 2004;56:321–325. doi: 10.1016/j.jhin.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Ball F., Mollison D., Scalia-Tomba G. Ann. Appl. Probab. 1997;7:46–89. [Google Scholar]
- 16.Nijssen S., Bonten M. J. M., Weinstein R. A. Clin. Infect. Dis. 2005;40:405–409. doi: 10.1086/427281. [DOI] [PubMed] [Google Scholar]
- 17.Cepeda J. A., Whitehouse T., Cooper B., Hails J., Jones K., Kwaku F., Taylor L., Hayman S., Cookson B., Shaw S., et al. Lancet. 2005;365:295–304. doi: 10.1016/S0140-6736(05)17783-6. [DOI] [PubMed] [Google Scholar]
- 18.Hori S., Sunley R., Tami A., Grundmann H. J. Hosp. Infect. 2002;50:25–29. doi: 10.1053/jhin.2001.1130. [DOI] [PubMed] [Google Scholar]
- 19.Grundmann H., Hori S., Winter B., Tami A., Austin D. J. J. Infect. Dis. 2002;185:481–488. doi: 10.1086/338568. [DOI] [PubMed] [Google Scholar]
- 20.Scanvic A., Denic L., Gaillon S., Giry P., Andremont A., Lucet J. Clin. Infect. Dis. 2001;32:1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 21.Eveillard M., Martin Y., Hidri N., Boussougant Y., Joly-Guillou M. L. Infect. Control Hosp. Epidemiol. 2004;25:114–120. doi: 10.1086/502360. [DOI] [PubMed] [Google Scholar]
- 22.Cooper B. S., Medley G. F., Stone S. P., Kibbler C. C., Cookson B. D., Roberts J. A., Duckworth G., Lai R., Ebrahim S. Proc. Natl. Acad. Sci. USA. 2004;101:10223–10228. doi: 10.1073/pnas.0401324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blok H., Gigengack-Baars A., Mascini E. M., Troelstra A. Infectieziekten Bull. 2000;11:165–169. [Google Scholar]
- 24.Fitzpatrick F., Murphy O. M., Brady A., Prout S., Fenelon L. E. J. Hosp. Infect. 2000;46:271–279. doi: 10.1053/jhin.2000.0838. [DOI] [PubMed] [Google Scholar]
- 25.Harbarth S., Dharan S., Liassine N., Herrault P., Auckenthaler R., Pittet D. Antimicrob. Agents Chemother. 1999;43:1412–1416. doi: 10.1128/aac.43.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reacher M. H., Shah A., Livermore D. M., Wale M. C., Graham C., Johnson A. P., Heine H., Monnickendam M. A., Barker K. F., James D. Br. Med. J. 2000;320:213–216. doi: 10.1136/bmj.320.7229.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D. L., Dushoff J., Perencevich E. N., Harris A. D., Levin S. A. Proc. Natl. Acad. Sci. USA. 2004;101:3709–3714. doi: 10.1073/pnas.0400456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith D. L., Levin S. A., Laxminarayan R. Proc. Natl. Acad. Sci. USA. 2005;102:3153–3158. doi: 10.1073/pnas.0409523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan A., Carnevale G., Catenazzi P., Colombini P., Crema L., Dolcetti L., Ferrari L., Mondello P., Signorini L., Tinelli C., et al. Infect. Control Hosp. Epidemiol. 2005;26:127–133. doi: 10.1086/502515. [DOI] [PubMed] [Google Scholar]
- 30.Raboud J., Saskin R., Simor A., Loeb M., Green K., Low D. E., McGeer A. Infect. Control Hosp. Epidemiol. 2005;26:607–615. doi: 10.1086/502589. [DOI] [PubMed] [Google Scholar]
- 31.Cosgrove S. E., Qi Y., Kaye K. S., Harbarth S., Karchmer A. W., Carmeli Y. Infect. Control Hosp. Epidemiol. 2005;26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 32.Warren D. K., Liao R. S., Merz L. R., Eveland M., Dunne W.M., Jr. J. Clin. Microbiol. 2004;42:5578–5581. doi: 10.1128/JCM.42.12.5578-5581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.