Abstract
Bone mineral composition, crystallinity, and bone mineral content of osteoporotic patients are different from those of normal subjects. We review the evidence that these mineralization parameters contribute to the strength (fracture resistance) of bone and the methods that have been used to examine them. A specific example is provided from analysis of biopsies from the Multiple Outcomes in Raloxifene Evaluation trial. For the analyses, randomly selected biopsies from placebo, low-dose, and high-dose groups (n = 5 per group) obtained at time zero and 2 years after treatment were examined by infrared imaging spectroscopy. In all cases, comparable increases in mineral content were found, but there were no significant variations in mineral crystallinity.
Osteoporosis is a devastating disease that affects more than 10 million people in the United States, with annual costs in excess of 13.5 billion dollars.123 According to the US Surgeon General’s Report,123 by the year 2020 1/2 of all Americans older than 50 years will be at risk of an osteoporotic fragility fracture. Osteoporosis is characterized by low bone mass and structural deterioration of bone, leading to bone fragility and an increased tendency to fracture. Fracture resistance is determined by the strength of the bone, which in turn depends on its geometric properties (size, shape, and connectivity), the activities of the cells in the tissue, and the material properties of the tissue.36,73,109 The material properties of bone include the mineral content,73 mineral composition and mineral crystal size,27 and matrix content and composition.35 The most frequently used clinical indication of osteoporosis and fracture risk, bone mineral density (BMD), is also the most readily accessible non-invasive measure of bone mineral content.85 The purpose of this review is to describe the additional properties that may be predictive of mechanical strength obtained by analyses of bone tissue specimens. Methods of analysis and recent data obtained by these methods also are reviewed to show how material properties are altered in osteoporosis.
Specific questions addressed are how the composition of bone is altered in osteoporosis; how mineral crystal composition and size vary in osteoporosis; how spectroscopic analyses can be used to characterize these alterations in properties with high spatial resolution; and how therapies currently in clinical use affect these properties.
The Composition of Bone
Bone is a composite consisting, in decreasing order, of mineral (an analogue of geologic hydroxyapatite [HA]), an organic matrix, cells, and water.14 The organic matrix predominately is Type I collagen but includes a small percentage of noncollagenous proteins. These constituents are distributed in different patterns in various types of bone. Classical chemical analyses of ash content (percent mineral after the water and organic components are burned-off)14,71,88,99,124,128; mineral ion composition;13,21,22,56,71,88,83,120 electron microscopic and xray diffraction analysis of bone mineral crystal size6,8,11,23,55,56,57,107,118,122; and vibrational spectroscopic analysis of mineral content (mineral to matrix ratio), carbonate content, and acid phosphate content15,16,23,24,30,77 have been used to analyze homogenized biopsy and cadaver tissues and bones from animal models of osteoporosis.13,71,88,99,125 Newer techniques, such as backscatter electron imaging,8,18 atomic force microscopy,58,119 small angle neutron or xray scattering,108,109,110 nuclear magnetic imaging,20,28,128 Fourier transform infrared (FTIR)imaging, and Raman microscopic imaging,16,24,75 more recently have been used or have the potential to be used in the analyses of mineral properties in osteoporotic tissues. These chemical analyses show an age-dependent and site-dependent variation in mineral properties in healthy individuals, which are not apparent in osteoporotic tissues.15 They also have shown alteration in collagen composition in osteoporotic patients.5 Each of these parameters can have substantial effects on the mechanical performance of bone. We focus on the mineral changes in osteoporosis.
How Mineral Properties Affect Mechanical Strength
Mineral content in vertebrae and long bones is correlated with a variety of whole bone mechanical properties (stiffness, strain, ultimate load, etc.).60,82 Currey34,35 showed that the observed torsional strength is proportional to and most dependent on mineral content. More recent analyses using microcomputerized tomography show that correlations improve when microarchitecture and mineral content are included in the regression.63 However, even when mineral content and microarchitecture are considered, only about 80% of the variance is accounted for, indicating there are additional factors that must contribute to bone strength.
Bones are known to become more brittle when the mineral content exceeds a critical value50 and to be less able to bear load when the mineral content is too low.114 Bone mineral density is related directly to mechanical strength, and the decreased bone mineral density associated with fracture risk in patients with osteoporosis25,42,67,69,76 is confirmed by decreases in the distribution of mineral density determined by density fractionation in tissues from animal models of osteoporosis23,55,66 and spectroscopically determined decreases in mineral to matrix ratio in osteoporotic tissues.9,11,48,61,62,81,91,93 Variation in mineral content in osteoporosis is important, but there are other mineral properties that also contribute to the loss of mechanical strength in osteoporotic bones.
The HA crystals found in bone are nanocrystalline and contain a large number of imperfections and impurities.14 Hydroxyapatite crystal size and perfection first were suggested to contribute to the mechanical strength of bones in the early 1980s.27 Increased bone mineral particle size is associated with increased bone fragility27; crystals that are too small do not reinforce the bone composite, suggesting there also is an optimal size range for bone mineral crystals.50 Compositional changes may be caused by alterations in crystal size (smaller crystals have more surface area to adsorb foreign ions) or may be indicative of the size changes. In osteoporosis and in aging, there are reports of increased magnesium content and decreased fluoride, acid phosphate, boron, strontium, and carbonate contents.1 The significance of these impurities to the mechanical competence of the bones is not yet known.
Spectroscopic Analysis of Bone Material Properties
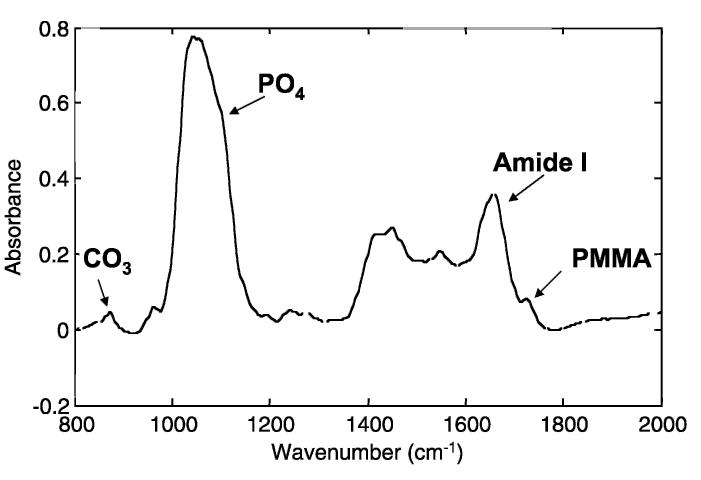
Vibrational spectroscopy has been used extensively for the analysis of mineral properties in bones, teeth, and other mineralized tissues. The wavelength of many infrared and Raman absorption bands are characteristic of specific types of chemical bonds, and vibrational spectroscopy has been used to confirm the identity of particular compounds in mineralized tissues. In infrared bands, asymmetric vibrations are most intense; in the Raman spectrum the strongest (and sharper) bands are the symmetric vibrations. In both, band shapes and positions are sensitive to the molecular environment; examining spectral details at discrete sites in the tissue provides insight into environmental changes at well-defined anatomic locations. Raman bands are sharper and better defined, while the infrared bands are more intense (Fig 1). Vibrations that seem strong in Raman generally are weak in the infrared and vice versa, making these two techniques complimentary. For bone, similar mineral parameters generally are evaluated for both; however, these are based on different vibrations (Table 1). Classically, these spectroscopic techniques were applied to homogenized bones; Cohen and Kitzes30 were among the first to document changes in bone mineral content and composition in osteoporosis. The introduction of multichannel array detectors allowed the development of infrared imaging and Raman imaging microspectroscopy in which multiple spectra are recorded simultaneously.74 An image cube is generated in which the x-axis and y-axis correspond to the x-position and y-position in the tissue sample, and the z-axis is the intensity of a particular vibration (Fig 2A), a ratio of two peak areas (Fig 2B), or a ratio of two intensities (Figs 2C, D). Pixel histograms provide statistical information on the distribution of values in any given sample (Figs 2E-G).
Fig 1.
A typical spectrum of trabecular bone, taken from a biopsy in a placebo-treated patient in the MORE study is shown. The spectral features of interest are indicated. The absorbance is a dimensionless ratio.
Table 1.
Parameters Analyzed in Vibrational Spectroscopic Analysis of Bone
| Parameter | Infrared | Raman | Comments |
|---|---|---|---|
| Mineral:matrix ratio | Integrated area of v1, v3 (900-1200 cm-1) phosphate/amide I (1575-1720 cm-1)101 | Integrated area of v1 (920-980 cm-1) phosphate band/amide I (∼1665 cm-1) or hydroxyproline (876 cm-1) band117 | Linearly related to ash weight44,101 |
| Carbonate:amide I | Integrated area of 855-890 cm-1carbonate band/amide I band78,104 | Integrated area of peaks centered at 1070 cm-1/1665 cm-11,98,117 | Can also be expressed as carbonate to phosphate area. |
| Crystallinity | Curvefit 1030:1020 cm-1subband area or 1030:1020 intensity area17 | Line broadening of v1 phosphate band38 | The 1030 band is seen in stoichiometric hydroxyapatite and the 1020 band in nonstoichiometric hydroxyapatite.The ratio was correlated to mineral crystal size based on xray diffraction line broadening analysis.48 |
| Acid phosphate | Subband at 1117 cm-115,105normalized to 960 cm-1subband or phosphate band | 1007 or 873 band normalized to v1 or amide I bands98 | |
| Collagen maturity | Curvefit 1660 cm-1/1690 cm-1subband area ratio or intensity ratio17,97 | Factor analysis of amide I band117 |
Fig 2A-G.
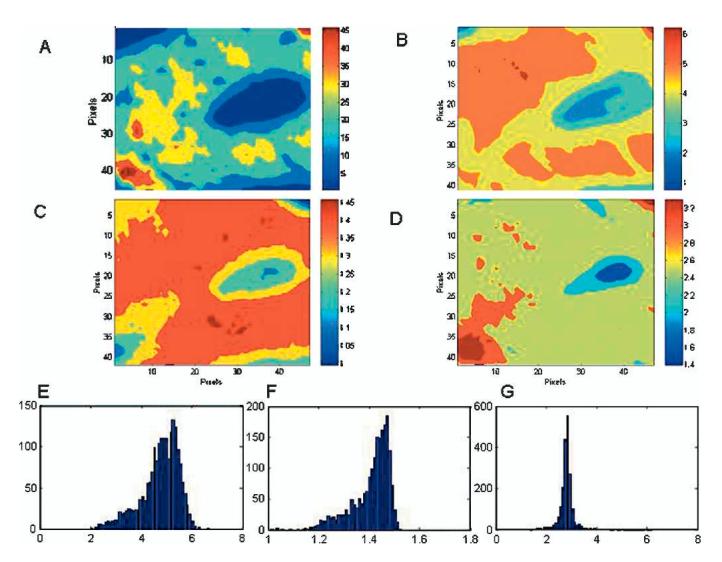
Images here were taken from the same placebo-control sample as shown in Figure 1 using infrared imaging spectroscopy. (A) The distribution of mineral intensities as revealed by the integrated area under the mineral phosphate band (900-1200 cm-1) in the raw data. (B) The ratio of the integrated areas of the mineral phosphate band to the amide I peak shows the anatomic distribution of the mineral/matrix ratio. The axes are in pixels, where each pixel is 6.25 μm. (C) The ratio of the intensity of subbands at 1030 cm-1 and 1020 cm-1 shows the distribution of the crystallinity parameter. (D) The ratio of intensity of subbands at 1660 and 1690 cm-1 show a parameter related to the ratio of nonreducible and reducible collagen cross-links. (E) Pixel distribution for the image of mineral/matrix ratio in Figure 2B is shown. Note the distribution is skewed to the left. (F) Pixel distribution for the image of crystallinity in Figure C is shown. The distribution is skewed to the left, showing the presence of relatively more small crystal-lites than larger ones. (G) Pixel distribution for the image of collagen cross link ratio shown in Figure D is shown. Note the peak is sharp, showing limited variation in collagen maturity in the controls. The bin size for these histograms was set at 50.
These spectroscopic imaging techniques (Table 2) have been applied to bone biopsies from patients and animals with osteoporosis and osteomalacia and treated versus with nontreated subjects.9,15,16,75,89,91,92,93,94,95 In general, in the osteoporotic tissues the mineral content (degree of mineralization) is decreased,10 the HA crystal size and perfection is increased, the carbonate content is increased, and the acid phosphate content is decreased.15 Treatment with fluoride increases crystal length and decreases width,7,47,121 and treatment with several of the bisphosphonates has been reported to have no effect23,53 or to increase crystal width slightly.122 Strontium decreases bone mineral crystal size,56 but strontium ralenate increases BMD and mineral distribution.11 Parathyroid hormone restores the mineral content and crystallinity to normal values in a monkey model of osteoporosis93 whereas hormone replacement therapy in perimenopausal women increases mineral content and decreases crystal size.92
Table 2.
Infrared Analyses of Changes in Bone Mineral Properties
| Species | Property | Change in Property |
|---|---|---|
| Canine osteoporosis81,101 | Mineral:matrix (cortical) | Decreased |
| Postmenopausal women15,16,91,92,94,95 | Mineral:matrix (cortical and trabecular) | Decreased |
| Crystallinity (cortical) | Increased | |
| Crystallinity (trabecular) | Increased | |
| Carbonate:phosphate (trabecular) | Increased | |
| Collagen maturity | Increased | |
| Ovariectomized monkey48,93 | Mineral:matrix (trabecular) | Decreased |
| Crystallinity (trabecular) | Increased | |
| Biglycan-deficient mouse129 | Mineral:matrix | Decreased |
| Interleukin-deficient mouse39 | Crystallinity | Increased |
| Ovariectomized rat4,9,87,97,102,111 | Mineral:matrix | Decreased |
| Disuse osteoporosis in rat31 | Crystallinity | No change |
Spectroscopic parameters that are usually evaluated in the infrared spectra of bone include the mineral to matrix peak area ratio (which is correlated with ash weight),44,101 the 1660/1690 cm-1 peak intensity ratio (which is related to variations in collagen cross-linking),95,96 the 1030 cm-1/1020 cm-1 peak intensity ratio (which is correlated with mineral crystal size and perfection as determined by xray diffraction line broadening),49 the carbonate/phosphate or carbonate/amide I ratio (which indicates the amount of carbonate substitution for phosphate or hydroxide in the mineral crystals),15,98 and the acid phosphate content (a parameter that decreases as the crystals become more mature).15
Changes in Mineral Properties in Osteoporosis before and after Therapeutic Treatment
Using the techniques discussed above, mineral changes have been noted after treatment of osteoporosis in a variety of animal models and in a limited number of studies of patient biopsies from clinical trials. Fluoride was the first therapy reported to have an effect on mineral properties. Given with calcium and vitamin D, fluoride increases bone density83 but also increases the incidence of hip (but not vertebral) fractures.106 Fluoride therapy increases HA crystal length, decreases crystal width, and increases mineral content.6,26,121 The decreased crystal width is correlated with decreased ability of the tissue to withstand load (increased risk of fracture).26
Hormone replacement therapy was used for the treatment and prevention of osteoporosis before fluoride treatment.102,115 Estrogen and progesterone have been shown in animal models to improve mechanical strength and create a broader distribution of mineral crystals sizes.21,65,116 Using infrared imaging, we previously examined biopsies from perimenopausal women who were treated with hormone replacement therapy after their initial biopsies. A second biopsy obtained after 2 years showed an apparent increase in mineral content, a decrease in the average crystal size, and a broadening of the population of crystal sizes in each sample.92 This is in agreement with the finding that estrogen treatment accelerates fracture healing in ovariectomized rats, while at the same time creating a population of new crystals and newly synthesized collagen.90
In related studies, several growth factors and phytoestrogens were evaluated in rodent models of osteoporosis.19,32,68,100 These agents increase density but have no effect on mechanical properties in monkeys.103 Effects of most of these agents on mineral characteristics have not been reported but effects on collagen have been.84 In one study, mineral properties including crystal size in homogenized bones from male rats treated short-term with a synthetic isoflavone derivative did not differ from controls; however, the mechanical strength increased.29 This indicates that information may be lost when homogenized tissues are examined. Similarly, the cytokine insulin line growth factor-1 (IGF-1) has variable effects on bone density, does not increase mechanical strength,31,111 however, mice overexpressing IGF-binding proteins show substantial alterations in crystallite size and perfection.3
Rodent models, although widely used, are not thought to be good models of human osteoporosis. With age, human bones become thinner and more brittle while rodent bones become thicker and stronger.4 Mineral properties in ovariectomized rodents also are not comparable with those in humans.97 However, the FDA accepts small animal models for initial evaluation of osteoporotic therapies; therefore, there are a large number of studies in rats and mice. Lessons about the effects of therapies on bone properties can be approximated from these studies as long as it is recognized that these models do not generally mimic human osteoporosis.
The bisphosphonates used in the treatment of osteoporosis (didronel, ibandronate, pamidronate, alendronate, risedronate, tiludronate, zoledronic acid, etc.) all have nonhydrolyzable P-C-P bonds and in general, high affinities for the HA crystal surface.59,64 They have direct inhibitory effects on osteoclast action but as they also bind to HA, they can regulate mineral crystal growth and dissolution.45,112 Each of the bisphosphonates increase bone density and sometimes increase mechanical strength or microcrack density,41,52,70,72,81 but their effects on mineral properties have only been evaluated in a limited number of instances. Bohic et al9 used 31P NMR, vibrational spectroscopy, and chemical analyses to characterize the effects of the bisphosphonate tiludronate on HA properties. Comparing treated and untreated ovariectomized rats they found no change in the NMR spectra, no change in the acid phosphate, carbonate content, or mineral content, by Raman and infrared spectroscopy. In dogs, high-dose alendronate (5× normal) and high-dose risedronate did not have any effect on mineral crystal properties in the homogenized vertebrae despite large changes in mechanical properties.23,123 The authors of one study suggested that the failure to find a difference was because of low sensitivity of the measurement.23 As noted previously, homogenization of the tissue before analysis could have masked changes at the sites of newly formed or newly remodeled bone. In contrast, intravenous pamidronate given to dogs for a 1-year period caused a dose dependent increase in bone mineral density and a decrease in crystallite size and HA structure.57 Very few studies of mineral properties have been reported in biopsies from humans treated with any of the bisphosphonates. These limited studies in patients and baboons treated with alendronate showed that an increase in the degree of mineralization paralleled a rise in the mechanical strength in the cortical and cancellous bone.46,57,78
The dog studies mentioned previously suggest a species-dependent difference in response to therapy. Calcitonin, which blocks osteoclastic resorption, has been reported to improve trabecular bone mechanics in all but one model.126,127 In beagles treated with human calcitonin for a 16-week period, comparison with controls showed tibia tested in torsion failed at lower load and had less torsional stiffness.101 Cancellous bone in treated animals was similarly weaker, cortical and cancellous bones had reduced BMD, and mineral/matrix ratio and greater carbonate/phosphate ratio. Patients with osteoarthritis, in contrast, have an improvement in crystallinity (based on xray diffraction) indicative of new bone formation after calcitonin treatment.80 In an animal model of osteoarthritis, infrared microspectroscopy failed to show any differences in the mineral crystal properties of the thickened calcified cartilage plate.79 Unfortunately, with the exception of one rodent study,51 there are no reports of mineral properties in patients or animals with osteoporosis treated with calcitonin. In the ovariectomized rats treated with calcitonin, the bone mineral calcium to phosphate ratio and the crystallite size were similar to those in the control untreated rats. The reduced bone formation and mineral content noted in the Calcitonin-treated dogs and their increased carbonate to phosphate ratios are in agreement with the mineral changes noted in animal models of osteoporosis.
Aging monkeys lose bone mass and have fractures when studied in their natural environments.54 The changes noted in their trabecular bone during aging and after ovariectomy mimic those observed in humans.48,55,65,66 There have been three reports of the effects of antiresorptive and anabolic therapies on mineral properties in ovariectomized monkeys.48,61,93 Nandrolone decanoate, a resorption inhibitor, was shown to improve the mechanical properties and increase mineral content; however they have no effect on carbonate, acid phosphate, or collagen cross-links.48,61 Parathyroid hormone in low and high doses caused notable changes in the distribution of mineral parameters in treated ovariectomized monkeys after 18 months relative to ovariectomized negative controls.93 The mineral/matrix ratio and the crystallinity shifted to lower values, indicative of the formation of new bone, and mechanical strength was increased in the animals treated with parathyroid hormone.113
To illustrate how these techniques can be applied to the evaluation of biopsies obtained in therapeutic trials and how such data correlates with other methods, we present data from an evaluation of raloxifene, a selective estrogen receptor modulator. The changes in spectroscopic parameters at 6μ m spatial resolution in patients treated with raloxifene or placebo controls were evaluated in a randomly selected subset of biopsies from a large trial evaluating raloxifene therapy in postmenopausal women.33,43 In this trial, the treatment of osteoporosis with two different doses of raloxifene was examined in a cohort of 7705 postmenopausal women.43 Raloxifene reduced the risk of vertebral fracture in the postmenopausal women and quantitative microradiographic analyses of bone biopsies from this trial showed an increase in mineral content with treatment of 2.1% (60 mg/d group) and 2.4% (120 mg/d group). Increase in mineral density as measured by dual energy xray absorptiometry (DEXA) in the spine was 0.5% in the placebo group, 3.1% in the 60 mg/day group, and 3.2% in the 120 mg/day group. Vertebral fracture risk was reduced in both experimental groups relative to the placebo, 0.7 (60 mg/d) and 0.5 to 0.8 (120 mg/d); the prevalence of fractures at other sites did not change. The purpose of our study was to evaluate the mineral changes in a randomly selected group of iliac crest biopsies from this study.
MATERIALS AND METHODS
We retrospectively analyzed randomly selected iliac crest biopsies (designated by a statistician from Eli Lilly, Co.) from three groups of patients (n = 5 per group) enrolled in the MORE (Multiple Outcomes in Raloxifene Evaluation) trial. In this trial, there were two experiment groups, treated with 60 mg or 120 mg of raloxifene for 2 years, and a placebo group. All patients were supplemented with calcium and vitamin D. Biopsies obtained at baseline and 2 years after treatment were embedded in polymethylmethacrylate (PMMA), cut in 2 μm-thick sections, and spectra recorded in imaging mode using a Fourier Transform Infrared Imaging (FTIRI) spectrometer (Spotlight, Perkin Elmer, Shelton, CT). For each biopsy, spectra from six 200 × 200 μm regions were collected, three in the cortical bone and three in the trabecular at a spectral resolution of 8 cm-1. The spectra were baselined linearly and the contribution of the embedding media2 was subtracted spectrally using commercial software (ISYS, Spectral Dimensions, Olney, MD). Spectroscopic parameters examined were mineral/matrix peak area ratio, 1660/1690 cm-1peak intensity ratio (collagen cross-linking), and 1030 cm-1/1020 cm-1peak intensity ratio (mineral crystallinity). The change in pixel population averages for each of the parameters was compared with one-way analysis of variance (ANOVA) (Instat, GraphPad Corp., Carlsbad, CA).
RESULTS
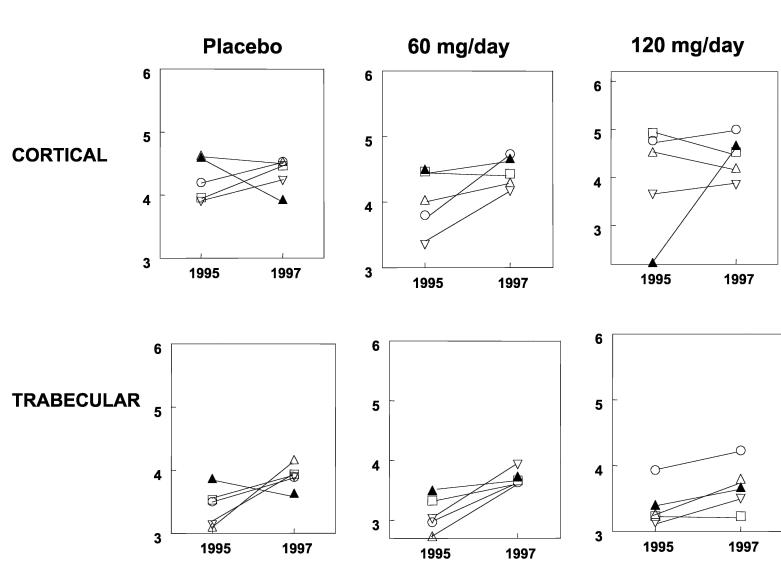
All three groups in the MORE study showed increases in the mineral/matrix ratio in trabecular and cortical bone (Fig 3, Table 3), but no changes were found among groups. Crystallinity and the collagen cross-link parameter did not differ throughout the 2-year period and did not differ between treatment groups (Table 3).
Fig 3.
Changes in mineral/matrix ratio in individual patients after two years of treatment with raloxifene is shown in this graph. The y-axis shows the mineral/matrix ratio (ratio of the integrated areas of the phosphate band to that of the amide I band) with each unique symbol in a given figure representing the same patient at baseline (1995) and 2 years after treatment (1997) for cortical and trabecular bone. The solid lines connect the baseline trabecular or cortical data to the 2-year time point for each patient.
Table 3:
Mineral and Matrix Properties before and after 2 Years of Treatment
| Cortical | Trabecular | ||||
|---|---|---|---|---|---|
| Crystallinity | Collagen Cross-Links | Mineral:Matrix | Crystallinity | Collagen Cross Links | Mineral:Matrix |
| Placebo | |||||
| 1.433 | 3.967 | 4.633 | B 1.300 | 3.333 | 3.100 |
| 1.200 | 3.733 | 4.533 | A 1.200 | 3.367 | 4.167 |
| 1.500 | 3.167 | 3.900 | B 1.200 | 2.900 | 3.133 |
| 1.200 | 3.500 | 4.233 | A 1.333 | 2.667 | 3.900 |
| 1.200 | 3.433 | 4.200 | B 1.100 | 2.800 | 3.500 |
| 1.200 | 3.533 | 4.533 | A 1.267 | 3.367 | 3.867 |
| 1.300 | 3.600 | 3.967 | B 1.100 | 2.833 | 3.533 |
| 1.500 | 3.200 | 4.467 | A 1.233 | 3.233 | 3.933 |
| 1.433 | 3.667 | 4.600 | B 1.267 | 3.333 | 3.867 |
| 1.400 | 4.033 | 3.933 | A 1.333 | 3.333 | 3.633 |
| 60 mg raloxifene | |||||
| 1.267 | 3.733 | 4.033 | B 1.200 | 3.033 | 2.733 |
| 1.233 | 3.433 | 4.300 | A 1.267 | 3.450 | 3.733 |
| 1.300 | 2.300 | 3.350 | B 1.200 | 2.250 | 3.025 |
| 1.467 | 2.700 | 4.167 | A 1.200 | 2.433 | 3.933 |
| 1.400 | 2.433 | 3.800 | B 1.367 | 2.267 | 2.967 |
| 1.433 | 3.533 | 4.733 | A 1.333 | 3.100 | 3.633 |
| 1.533 | 3.600 | 4.467 | B 1.267 | 3.067 | 3.333 |
| 1.367 | 3.500 | 4.433 | A 1.300 | 2.967 | 3.667 |
| 1.367 | 3.500 | 4.500 | B 1.233 | 2.933 | 3.500 |
| 1.367 | 3.633 | 4.667 | A 1.367 | 3.300 | 3.733 |
| 120 mg raloxifene | |||||
| 1.433 | 3.433 | 4.533 | B 1.333 | 2.900 | 3.267 |
| 1.400 | 3.300 | 4.200 | A 1.233 | 3.367 | 3.800 |
| 1.400 | 2.750 | 3.650 | B 1.300 | 2.100 | 3.133 |
| 1.450 | 2.600 | 3.875 | A 1.300 | 2.633 | 3.500 |
| 1.500 | 2.967 | 4.767 | B 1.433 | 2.533 | 3.933 |
| 1.433 | 2.700 | 5.000 | A 1.233 | 2.700 | 4.233 |
| 1.267 | 2.967 | 4.933 | B 1.333 | 2.400 | 3.233 |
| 1.400 | 3.133 | 4.533 | A 1.200 | 2.567 | 3.233 |
| 1.533 | 3.233 | 2.233 | B 1.100 | 3.200 | 3.400 |
| 1.533 | 2.933 | 4.467 | A 1.300 | 2.633 | 3.367 |
B = Before; A = After
DISCUSSION
These experimental data reveal how mineral analyses can be used to probe the effects of therapies on osteoporotic bone mineral properties. Although the 2-year treatment with raloxifene did not cause a significant change in the infrared parameters when the three treatment groups were compared, the treatments (including supplementation with calcium and vitamin D in the placebo group) resulted in improvement of mineral properties with time. These data are in agreement with the mean degree of mineralization of bone data, which similarly showed no changes between the raloxifene groups and the placebo groups.12 The effects seen in the placebo group confirm previous observations that this supplementation can reduce some of the bone loss in menopausal women.12,37,86 The distribution of mineral was not different when the groups were compared, suggesting that the decrease in fracture risk compared with the placebo group in the larger population study was not caused by changes in the mineral parameters. It is probable that the decreased bone turnover in the raloxifene-treated groups reduced perforation of bone plates and therefore the bone strength on the tissue mechanical level. However, longer-term studies and a larger sample size probably will be needed to validate that possibility.
From the data presented in this review, it seems that a broad distribution of mineral crystal sizes, increased mineral content, and improved microarchitecture are associated with improved mechanical properties. Dual energy xray absorptiometry measurements can only provide information on BMD, and this is the most extensively used surrogate in clinical trials.40 However, when biopsies are available, measurement of crystal and architectural properties can provide information that may reveal the efficacy of therapeutics. The observation that the biopsies in the MORE study (placebo and treatment) showed improvement in mineral parameters after 2 years makes longer-term analyses of similar parameters essential and suggests a need for evaluation of mineral properties in additional therapeutic trials.
Footnotes
One or more of the authors (ALB) has received funding from NIH grants AR043125 and AR046121. Each author certifies that he or she has no commercial associations that might pose a conflict of interest with the submitted article.
Each author certifies that his or her institution has approved the human protocol for theinvestigations reported in the review and that all investigations were conducted in conformity with ethical principles of research and that informed consent was obtained.
References
- 1.Akkus O, Adar F, Schaffler MB. Age-related changes in physico-chemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone. 2004;34:443–453. doi: 10.1016/j.bone.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio S, Doty SB, Camacho NP, Paschalis EP, Spevak L, Mendelsohn R, Boskey AL. Optimal methods for processing mineralized tissues for Fourier transform infrared microspectroscopy. Calcif Tissue Int. 2002;70:422–429. doi: 10.1007/s00223-001-1016-z. [DOI] [PubMed] [Google Scholar]
- 3.Atti E, Boskey AL, Canalis E. Over-expression of IGF-binding protein 5 alters mineral and matrix properties in mouse tibia: An infrared imaging study. Calcif Tissue Int. 2004;76:187–193. doi: 10.1007/s00223-004-0076-2. [DOI] [PubMed] [Google Scholar]
- 4.Bagi CM, Ammann P, Rizzoli R, Miller SC. Effect of estrogen deficiency on cancellous and cortical bone structure and strength of the femoral neck in rats. Calcif Tissue Int. 1997;61:336–344. doi: 10.1007/s002239900344. [DOI] [PubMed] [Google Scholar]
- 5.Bailey AJ, Wotton SF, Sims TJ, Thompson PW. Biochemical changes in the collagen of human osteoporotic bone matrix. Connect Tissue Res. 1993;29:119–132. doi: 10.3109/03008209309014239. [DOI] [PubMed] [Google Scholar]
- 6.Baud CA, Very JM, Courvoisier B. Biophysical study of bone mineral in biopsies of osteoporotic patients before and after long-term treatment with fluoride. Bone. 1988;93:61–65. doi: 10.1016/8756-3282(88)90117-2. [DOI] [PubMed] [Google Scholar]
- 7.Blank RD, Baldini TH, Kaufman M, Bailey S, Gupta R, Yershov Y, Boskey AL, Coppersmith SN, Demant P, Paschalis EP. Spectroscopically determined collagen Pyr/deH-DHLNL cross-link ratio and crystallinity indices differ markedly in recombinant congenic mice with divergent calculated bone tissue strength. Connect Tissue Res. 2003;44:134–142. doi: 10.1080/03008200390223918. [DOI] [PubMed] [Google Scholar]
- 8.Bloebaum RD, Skedros JG, Vajda EG, Bachus KN, Constantz BR. Determining mineral content variations in bone using backscattered electron imaging. Bone. 1997;20:485–490. doi: 10.1016/s8756-3282(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 9.Bohic S, Rey C, Legrand A, Sfihi H, Rohanizadeh R, Martel C, Barbier A, Daculsi G. Characterization of the trabecular rat bone mineral: effect of ovariectomy and bisphosphonate treatment. Bone. 2000;26:341–348. doi: 10.1016/S8756-3282(99)00276-8. [DOI] [PubMed] [Google Scholar]
- 10.Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 11.Boivin G, Deloffre P, Perrat B, Panczer G, Boudeulle M, Mauras Y, Allain P, Tsouderos Y, Meunier PJ. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J Bone Miner Res. 1996;11:1302–1311. doi: 10.1002/jbmr.5650110915. [DOI] [PubMed] [Google Scholar]
- 12.Boivin G, Lips P, Ott SM, Harper KD, Sarkar S, Pinette KV, Meunier PJ. Contribution of raloxifene and calcium and vitamin D3 supplementation to the increase of the degree of mineralization of bone in postmenopausal women. J Clin Endocrinol Metab. 2003;884:199–205. doi: 10.1210/jc.2002-022020. [DOI] [PubMed] [Google Scholar]
- 13.Boivin G, Meunier PJ. The mineralization of bone tissue: a forgotten dimension in osteoporosis research. Osteoporos Int. 2003;14:S19–S24. doi: 10.1007/s00198-002-1347-2. [DOI] [PubMed] [Google Scholar]
- 14.Boskey AL. Bone Mineralization. In: Cowin SC, editor. Bone Biomechanics. CRC Press; Boca Raton: 2001. pp. 5.1–5.34. Ed 3. [Google Scholar]
- 15.Boskey A, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high-turnover and low-turnover osteoporosis: an ft-ir microspectroscopic investigation. Osteoporos Int. 2005 doi: 10.1007/s00198-005-1992-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boskey AL, Mendelsohn R. Infrared Analysis of Bone in Health and Disease. J Biomed Opt. 2004 doi: 10.1117/1.1922927. In press. [DOI] [PubMed] [Google Scholar]
- 17.Boskey AL, Moore DJ, Amling M, Canalis E, Delaney AM. Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res. 2003;18:1005–1011. doi: 10.1359/jbmr.2003.18.6.1005. [DOI] [PubMed] [Google Scholar]
- 18.Boyde A, Kingsmill VJ. Age changes in bone. Gerodontology. 1998;15:25–34. doi: 10.1111/j.1741-2358.1998.00025.x. [DOI] [PubMed] [Google Scholar]
- 19.Branca F. Dietary phyto-oestrogens and bone health. Proc Nutr Soc. 2003;62:877–887. doi: 10.1079/PNS2003309. [DOI] [PubMed] [Google Scholar]
- 20.Brown CE, Srinivasan R, Sigmann P, Myklebust JB, Battocletti JH. Comparison of the compression strength of human vertebral bodies with the mass and density of apatite: A study by 31P NMR spectroscopy. Clin Chem. 1988;34:2114–2117. [PubMed] [Google Scholar]
- 21.Broulik PD, Rosenkrancova J, Ruzicka P, Sedlacek R. Effects of triiodothyronine and estrogen administration on bone mass, mineral content and bone strength in male rats. Horm Metab Res. 2003;35:527–531. doi: 10.1055/s-2003-42653. [DOI] [PubMed] [Google Scholar]
- 22.Burnell JM, Baylink DJ, Chestnut CH, III, Mathews MW, Teubner EJ. Bone matrix and mineral abnormalities in postmenopausal osteoporosis. Metabolism. 1982;31:1113–1120. doi: 10.1016/0026-0495(82)90161-5. [DOI] [PubMed] [Google Scholar]
- 23.Burr DB, Miller L, Grynpas M, Li J, Boyde A, Mashiba T, Hirano T, Johnston CC. Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone. 2003;33:960–969. doi: 10.1016/j.bone.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Carden A, Morris MD. Application of vibrational spectroscopy to the study of mineralized tissues (review) J Biomed Opt. 2000;5:259–268. doi: 10.1117/1.429994. [DOI] [PubMed] [Google Scholar]
- 25.Cefalu CA. Is bone mineral density predictive of fracture risk reduction. Curr Med Res Opin. 2004;20:341–349. doi: 10.1185/030079903125003062. [DOI] [PubMed] [Google Scholar]
- 26.Chachra D, Turner CH, Dunipace AJ, Grynpas MD. The effect of fluoride treatment on bone mineral in rabbits. Calcif Tissue Int. 1999;4:345–351. doi: 10.1007/s002239900630. [DOI] [PubMed] [Google Scholar]
- 27.Chatterji S, Wall JC, Jeffery JW. Age-related changes in the orientation and particle size of the mineral phase in human femoral cortical bone. Calcif Tissue Int. 1981;33:567–574. doi: 10.1007/BF02409493. [DOI] [PubMed] [Google Scholar]
- 28.Cho G, Wu Y, Ackerman JL. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science. 2003;300:1123–1127. doi: 10.1126/science.1078470. [DOI] [PubMed] [Google Scholar]
- 29.Civitelli R. In vitro and in vivo effects of ipriflavone on bone formation and bone biomechanics. Calcif Tissue Int. 1997;61:S12–S14. doi: 10.1007/s002239900378. [DOI] [PubMed] [Google Scholar]
- 30.Cohen L, Kitzes R. Infrared spectroscopy and magnesium content of bone mineral in osteoporotic women. Isr J Med Sci. 1981;17:1123–1125. [PubMed] [Google Scholar]
- 31.Conover CA, Johnstone EW, Turner RT, Evans GL, John Ballard FJ, Doran PM, Khosla S. Subcutaneous administration of insulin-like growth factor (IGF)-II/IGF binding protein-2 complex stimulates bone formation and prevents loss of bone mineral density in a rat model of disuse osteoporosis. Growth Horm IGF Res. 2002;12:178–183. doi: 10.1016/s1096-6374(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 32.Coxam V. Prevention of osteopaenia by phyto-oestrogens: Animal studies. Br J Nutr. 2003;89:S75–S85. doi: 10.1079/BJN2002798. [DOI] [PubMed] [Google Scholar]
- 33.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 34.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Phil Tran R Soc London. 1984;B304:509–518. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 35.Currey JD. The many adaptations of bone. J Biomech. 2003;36:1487–1495. doi: 10.1016/s0021-9290(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 36.Dalle Carbonare L, Giannini S. Bone microarchitecture as an important determinant of bone strength. J Endocrinol Invest. 2004;27:99–105. doi: 10.1007/BF03350919. [DOI] [PubMed] [Google Scholar]
- 37.Dawson-Hughes B, Dallal GE, Krall EA, Harris S, Sokoll LJ, Falconer G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Ann Intern Med. 1991;115:505–512. doi: 10.7326/0003-4819-115-7-505. [DOI] [PubMed] [Google Scholar]
- 38.de Mul FF, Hottenhuis MH, Bouter P, Greve J, Arends J, ten Bosch JJ. Micro-Raman line broadening in synthetic carbonated hydroxyapatite. J Dent Res. 1986;65:437–440. doi: 10.1177/00220345860650031301. [DOI] [PubMed] [Google Scholar]
- 39.Dresner-Pollak R, Gelb N, Rachmilewitz D, Karmeli F, Weinreb M. Interleukin 10-deficient mice develop osteopenia, decreased bone formation, and mechanical fragility of long bones. Gastroenterology. 2004;127:792–801. doi: 10.1053/j.gastro.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Eastell R, Blumsohn A. The value of biochemical markers of bone turnover in osteoporosis. J Rheumatol. 1997;24:1215–1217. [PubMed] [Google Scholar]
- 41.Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- 42.Ettinger MP. Aging bone and osteoporosis: Strategies for preventing fractures in the elderly. Arch Intern Med. 2003;163:2237–2246. doi: 10.1001/archinte.163.18.2237. [DOI] [PubMed] [Google Scholar]
- 43.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 44.Faibish D, Gomez A, Boivin G, Binderman I, Boskey A. Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone. 2005;36:6–12. doi: 10.1016/j.bone.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez D, Vega D, Goeta A. Alendronate zwitterions bind to calcium cations arranged in columns. Acta Crystallogr C. 2003;59:m543–m545. doi: 10.1107/s0108270103025599. [DOI] [PubMed] [Google Scholar]
- 46.Follet H, Boivin G, Rumelhart C, Meunier PJ. The degree of mineralization is a determinant of bone strength: a study on human calcanei. Bone. 2004;34:783–789. doi: 10.1016/j.bone.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Fratzl P, Roschger P, Eschberger J, Abendroth B, Klaushofer K. Abnormal bone mineralization after fluoride treatment in osteoporosis: A small-angle x-ray-scattering study. J Bone Miner Res. 1994;9:1541–1549. doi: 10.1002/jbmr.5650091006. [DOI] [PubMed] [Google Scholar]
- 48.Gadeleta SJ, Boskey AL, Paschalis E, Carlson C, Menschik F, Baldini T, Peterson M, Rimnac CM. A physical, chemical, and mechanical study of lumbar vertebrae from normal, ovariectomized, and nandrolone decanoate-treated cynomolgus monkeys (Macaca fascicularis) Bone. 2000;27:541–550. doi: 10.1016/s8756-3282(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 49.Gadaleta SJ, Paschalis EP, Betts F, Mendelsohn R, Boskey AL. Fourier transform infrared spectroscopy of the solution-mediated conversion of amorphous calcium phosphate to hydroxyapatite: New correlations between X-ray diffraction and infrared data. Calcif Tissue Int. 1996;58:9–16. doi: 10.1007/BF02509540. [DOI] [PubMed] [Google Scholar]
- 50.Gao H, Ji B, Jager IL, Arzt E, Fratzl P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc Natl Acad Sci U S A. 2003;100:5597–5600. doi: 10.1073/pnas.0631609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giardino R, Fini M, Nicoli Aldini N, Gnudi S, Biagini G, Gandolfi MG, Mongiorgi R. Effects of synthetic salmon calcitonin and alendronate on bone quality in ovariectomized rats. Minerva Med. 1997;88:469–477. [PubMed] [Google Scholar]
- 52.Glatt M, Pataki A, Evans GP, Hornby SB, Green JR. Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int. 2004;15:707–715. doi: 10.1007/s00198-004-1588-3. [DOI] [PubMed] [Google Scholar]
- 53.Grynpas MD, Acito A, Dimitriu M, Mertz BP, Very JM. Changes in bone mineralization, architecture and mechanical properties due to long-term (1 year) administration of pamidronate (APD) to adult dogs. Osteoporos Int. 1992;2:74–81. doi: 10.1007/BF01623840. [DOI] [PubMed] [Google Scholar]
- 54.Grynpas MD, Holmyard D. Changes in quality of bone mineral on aging and in disease. Scanning Microsc. 1988;2:1045–1054. [PubMed] [Google Scholar]
- 55.Grynpas MD, Huckell B, Pritzker KP, Hancock RG, Kessler MJ. Bone mineral and osteoporosis in aging rhesus monkeys. P R Health Sci J. 1989;8:197–204. [PubMed] [Google Scholar]
- 56.Grynpas MD, Marie PJ. Effects of low doses of strontium on bone quality and quantity in rats. Bone. 1990;11:313–319. doi: 10.1016/8756-3282(90)90086-e. [DOI] [PubMed] [Google Scholar]
- 57.Grynpas MD, Rey C. The effect of fluoride treatment on bone mineral crystals in the rat. Bone. 1992;13:423–429. doi: 10.1016/8756-3282(92)90085-b. [DOI] [PubMed] [Google Scholar]
- 58.Hassenkam T, Fantner GE, Cutroni JA, Weaver JC, Morse DE, Hansma PK. High-resolution AFM imaging of intact and fractured trabecular bone. Bone. 2004;35:4–10. doi: 10.1016/j.bone.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Hirabayashi H, Fujisaki J. Bone-specific drug delivery systems: Approaches via chemical modification of bone-seeking agents. Clin Pharmacokinet. 2003;42:1319–1330. doi: 10.2165/00003088-200342150-00002. [DOI] [PubMed] [Google Scholar]
- 60.Hornby SB, Evans GP, Hornby SL, Pataki A, Glatt M, Green JR. Long-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult rats. Calcif Tissue Int. 2003;72:519–527. doi: 10.1007/s00223-002-2015-4. [DOI] [PubMed] [Google Scholar]
- 61.Huang RY, Miller LM, Carlson CS, Chance MR. Characterization of bone mineral composition in the proximal tibia of cynomolgus monkeys: Effect of ovariectomy and nandrolone decanoate treatment. Bone. 2002;30:492–497. doi: 10.1016/s8756-3282(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 62.Huang RY, Miller LM, Carlson CS, Chance MR. In situ chemistry of osteoporosis revealed by synchrotron infrared microspectros-copy. Bone. 2003;33:514–521. doi: 10.1016/s8756-3282(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 63.Hudelmaier M, Kuhn V, Lochmuller EM, Well H, Priemel M, Link TM, Eckstein F. Can geometry-based parameters from pQCT and material parameters from quantitative ultrasound (QUS) improve the prediction of radial bone strength over that by bone mass (DXA) Osteoporos Int. 2004;15:375–381. doi: 10.1007/s00198-003-1551-8. [DOI] [PubMed] [Google Scholar]
- 64.Jarvis MF, Burns CJ, Pauls HW, Assal A, Kim JS, Cheney DL, Youssefyeh RD. Characterization of the bisphosphonate recognition site on hydroxyapatite using radioligand binding techniques with [14C] citric acid. Calcif Tissue Int. 1993;52:372–377. doi: 10.1007/BF00310202. [DOI] [PubMed] [Google Scholar]
- 65.Jerome CP. Hormonal therapies and osteoporosis. ILAR J. 2004;45:170–178. doi: 10.1093/ilar.45.2.170. [DOI] [PubMed] [Google Scholar]
- 66.Jerome CP, Carlson CS, Jayo MJ, Register TC, Weaver DS, Lees CJ, Adams MR. Histomorphometric and mineral density fractionation studies of lumbar vertebrae of intact and ovariectomized (OVX) monkeys. Bone Miner. 1994;26:275–278. doi: 10.1016/s0169-6009(08)80175-4. [DOI] [PubMed] [Google Scholar]
- 67.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 68.Kim HJ, Bae YC, Park RW, Choi SW, Cho SH, Choi YS, Lee WJ. Bone-protecting effect of safflower seeds in ovariectomized rats. Calcif Tissue Int. 2002;71:88–94. doi: 10.1007/s00223-001-1080-4. [DOI] [PubMed] [Google Scholar]
- 69.Koh LK, Ng DC. Osteoporosis risk factor assessment and bone densitometry: Current status and future trends. Ann Acad Med Singapore. 2002;31:37–42. [PubMed] [Google Scholar]
- 70.Koivukangas A, Tuukkanen J, Hannuniemi R, Jamsa T, Kippo K, Jalovaara P. Effects of long-term administration of clodronate on growing rat bone. Calcif Tissue Int. 2001;69:350–355. doi: 10.1007/s00223-001-2036-4. [DOI] [PubMed] [Google Scholar]
- 71.Kolosova NG, Kutorgin GD, Safina AF. Bone mineralization in senescence-accelerated OXYS rats. Bull Exp Biol Med. 2002;133:171–174. doi: 10.1023/a:1015507107763. [DOI] [PubMed] [Google Scholar]
- 72.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 73.Lanyon L, Armstrong V, Ong D, Zaman G, Price J. Is estrogen receptor alpha key to controlling bones’ resistance to fracture. J Endocrinol. 2004;182:183–191. doi: 10.1677/joe.0.1820183. [DOI] [PubMed] [Google Scholar]
- 74.Lewis EN, Kidder LH, Levin IW, Kalasinsky VF, Hanig JP, Lester DS. Applications of Fourier transform infrared imaging microscopy in neurotoxicity. Ann N Y Acad Sci. 1997;820:234–246. doi: 10.1111/j.1749-6632.1997.tb46199.x. [DOI] [PubMed] [Google Scholar]
- 75.Marcott C, Reeder RC, Paschalis EP, Tatakis DN, Boskey AL, Mendelsohn R. Infrared microspectroscopic imaging of biomineralized tissues using a mercury-cadmium-telluride focal-plane array detector. Cell Mol Biol. 1998;44:109–115. [PubMed] [Google Scholar]
- 76.McCalden RW, McGeough JA, Court-Brown CM. Age-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture. J Bone Joint Surg. 1997;79A:421–427. doi: 10.2106/00004623-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 77.Mendelsohn R, Paschalis EP, Boskey AL. Infrared spectroscopy, microscopy, and microscopic imaging of mineralizing tissues: Spectra-structure correlations from human iliac crest biopsies. J Biomed Opt. 1999;4:14–21. doi: 10.1117/1.429916. [DOI] [PubMed] [Google Scholar]
- 78.Meunier PJ, Boivin G. Bone mineral density reflects bone mass but also the degree of mineralization of bone: Therapeutic implications. Bone. 1997;21:373–377. doi: 10.1016/s8756-3282(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 79.Miller LM, Novatt JT, Hamerman D, Carlson CS. Alterations in mineral composition observed in osteoarthritic joints of cynomolgus monkeys. Bone. 2004;35:498–506. doi: 10.1016/j.bone.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 80.Mongiorgi R, Maggi G, Bertocchi G, Moroni A, Rollo G, Gnudi S, Ponziani L, Coppola G. Influence of calcitonin treatment on the bone structure and mineral content in osteoarthritis. Boll Soc Ital Biol Sper. 1992;68:85–89. [PubMed] [Google Scholar]
- 81.Monier-Faugere MC, Geng Z, Paschalis EP, Qi Q, Arnala I, Bauss F, Boskey AL, Malluche HH. Intermittent and continuous administration of the bisphosphonate ibandronate in ovariohysterectomized beagle dogs: Effects on bone morphometry and mineral properties. J Bone Miner Res. 1999;14:1768–1778. doi: 10.1359/jbmr.1999.14.10.1768. [DOI] [PubMed] [Google Scholar]
- 82.Myers ER, Sebeny EA, Hecker AT, Corcoran TA, Hipp JA, Greenspan SL, Hayes WC. Correlations between photon absorption properties and failure load of the distal radius in vitro. Calcif Tissue Int. 1991;49:292–297. doi: 10.1007/BF02556221. [DOI] [PubMed] [Google Scholar]
- 83.Nagant de Deuxchaisnes C, Devogelaer JP, Depresseux G, Malghem J, Maldague B. Treatment of the vertebral crush fracture syndrome with enteric-coated sodium fluoride tablets and calcium supplements. J Bone Miner Res. 1990;51:S5–S26. doi: 10.1002/jbmr.5650051303. [DOI] [PubMed] [Google Scholar]
- 84.Nisslein T, Freudenstein J. Effects of an isopropanolic extract of Cimicifuga racemosa on urinary crosslinks and other parameters of bone quality in an ovariectomized rat model of osteoporosis. J Bone Miner Metab. 2003;21:370–376. doi: 10.1007/s00774-003-0431-9. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen TV, Center JR, Pocock NA, Eisman JA. Limited utility of clinical indices for the prediction of symptomatic fracture risk in postmenopausal women. Osteoporos Int. 2004;15:49–55. doi: 10.1007/s00198-003-1511-3. [DOI] [PubMed] [Google Scholar]
- 86.O’Brien KO. Combined calcium and vitamin D supplementation reduces bone loss and fracture incidence in older men and women. Nutr Rev. 1998;56:148–150. doi: 10.1111/j.1753-4887.1998.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 87.Okamoto Y, Hidaka S, Yamada Y, Ouchi K, Miyazaki K, Liu SY. Thermal analysis of bones from ovariectomized rats. J Biomed Mater Res. 1998;41:221–226. doi: 10.1002/(sici)1097-4636(199808)41:2<221::aid-jbm6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 88.Ortoft G, Oxlund H. Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone. 1996;18:581–590. doi: 10.1016/8756-3282(96)00077-4. [DOI] [PubMed] [Google Scholar]
- 89.Ou-Yang H, Paschalis EP, Boskey AL, Mendelsohn R. Chemical structure-based 3D reconstruction of human cortical bone from 2D-IR Images. Appl Spectrosc. 2002;56:419–422. [Google Scholar]
- 90.Ou-Yang H, Sherman PJ, Paschalis EP, Boskey AL, Mendelsohn R. Fourier transform infrared microscopic imaging: effects of estrogen and estrogen deficiency on fracture healing in rat femurs. Appl Spectrosc. 2004;58:1–9. doi: 10.1366/000370204322729405. [DOI] [PubMed] [Google Scholar]
- 91.Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissues Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 92.Paschalis EP, Boskey AL, Kassem M, Eriksen EF. Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res. 2003;18:955–959. doi: 10.1359/jbmr.2003.18.6.955. [DOI] [PubMed] [Google Scholar]
- 93.Paschalis EP, Burr DB, Mendelsohn R, Hock JM, Boskey AL. Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH(1-34) for 18 months. J Bone Miner Res. 2003;18:769–775. doi: 10.1359/jbmr.2003.18.4.769. [DOI] [PubMed] [Google Scholar]
- 94.Paschalis EP, Recker R, DiCarlo E, Doty SB, Atti E, Boskey AL. Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res. 2003;18:1942–1946. doi: 10.1359/jbmr.2003.18.11.1942. [DOI] [PubMed] [Google Scholar]
- 95.Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL. Bone Fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 97.Patlas N, Zadik Y, Yaffe P, Schwartz Z, Ornoy A. Oophorectomy-induced osteopenia in rats in relation to age and time postoophorectomy. Cells Tissues Organs. 2000;166:267–274. doi: 10.1159/000016740. [DOI] [PubMed] [Google Scholar]
- 98.Penel G, Leroy G, Rey C, Bres E. MicroRaman spectral study of the PO4 and CO3 vibrational modes in synthetic and biological apatites. Calcif Tissue Int. 1998;63:475–481. doi: 10.1007/s002239900561. [DOI] [PubMed] [Google Scholar]
- 99.Peng Z, Tuukkanen J, Zhang H, Jamsa T, Vaananeo HK. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994;15:523–532. doi: 10.1016/8756-3282(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 100.Picherit C, Coxam V, Bennetau-Pelissero C, Kati-Coulibaly S, Davicco MJ, Lebecque P, Barlet JP. Daidzein is more efficient than genistein in preventing ovariectomy-induced bone loss in rats. J Nutr. 2000;130:1675–1681. doi: 10.1093/jn/130.7.1675. [DOI] [PubMed] [Google Scholar]
- 101.Pienkowski D, Doers TM, Monier-Faugere MC, Geng Z, Camacho NP, Boskey AL, Malluche HH. Calcitonin alters bone quality in beagle dogs. J Bone Miner Res. 1997;12:1936–1943. doi: 10.1359/jbmr.1997.12.11.1936. [DOI] [PubMed] [Google Scholar]
- 102.Pitkin J, Rees MC, Gray S, Lumsden MA, Stevenson J, Williamson J, Writing Group for the British Menopause Society Council Managing the menopause - British Menopause Society Council consensus statement on hormone replacement therapy. J Br Meno-pause Soc. 2003;9:129–131. doi: 10.1258/136218003100322260. [DOI] [PubMed] [Google Scholar]
- 103.Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362–4370. doi: 10.1210/jc.2003-030493. [DOI] [PubMed] [Google Scholar]
- 104.Rey C, Collins B, Goehl T, Dickson IR, Glimeher MJ. The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int. 1989;45:157–164. doi: 10.1007/BF02556059. [DOI] [PubMed] [Google Scholar]
- 105.Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the nu3PO4 domain. Calcif Tissue Int. 1991;49:383–388. doi: 10.1007/BF02555847. [DOI] [PubMed] [Google Scholar]
- 106.Riggs BL, Baylink DJ, Kleerekoper M, Lane JM, Melton LJ, 3rd, Meunier PJ. Incidence of hip fractures in osteoporotic women treated with sodium fluoride. J Bone Miner Res. 1987;2:123–126. doi: 10.1002/jbmr.5650020207. [DOI] [PubMed] [Google Scholar]
- 107.Rohanizadeh R, LeGeros RZ, Bohic S, Pilet P, Barbier A, Daculsi G. Ultrastructural properties of bone mineral of control and tiludronate-treated osteoporotic rat. Calcif Tissue Int. 2000;67:330–336. doi: 10.1007/s002230001141. [DOI] [PubMed] [Google Scholar]
- 108.Roschger P, Fratzl P, Klaushofer K, Rodan G. Mineralization of cancellous bone after alendronate and sodium fluoride treatment: A quantitative backscattered electron imaging study on minipig ribs. Bone. 1997;20:393–397. doi: 10.1016/s8756-3282(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 109.Roschger P, Matsuo K, Misof BM, Tesch W, Jochum W, Wagner EF, Fratzl P, Klaushofer K. Normal mineralization and nanostructure of sclerotic bone in mice overexpressing Fra-1. Bone. 1997;34:776–782. doi: 10.1016/j.bone.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaus-hofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 111.Rosen HN, Chen V, Cittadini A, Greenspan SL, Douglas PS, Moses AC, Beamer WG. Treatment with growth hormone and IGF-I in growing rats increases bone mineral content but not bone mineral density. J Bone Miner Res. 1995;10:1352–1358. doi: 10.1002/jbmr.5650100912. [DOI] [PubMed] [Google Scholar]
- 112.Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone. 1999;25:97–106. doi: 10.1016/s8756-3282(99)00116-7. [DOI] [PubMed] [Google Scholar]
- 113.Sato M, Westmore M, Clendenon J, Smith S, Hannum B, Zeng GQ, Brommage R, Turner CH. Three-dimensional modeling of the effects of parathyroid hormone on bone distribution in lumbar vertebrae of ovariectomized cynomolgus macaques. Osteoporos Int. 2000;11:871–880. doi: 10.1007/s001980070047. [DOI] [PubMed] [Google Scholar]
- 114.Shapiro R, Heaney RP. Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone. 2003;32:532–540. doi: 10.1016/s8756-3282(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 115.Staren ED, Omer S. Hormone replacement therapy in postmenopausal women. Am J Surg. 2004;188:136–149. doi: 10.1016/j.amjsurg.2003.12.063. [DOI] [PubMed] [Google Scholar]
- 116.Tannenbaum PJ, Schraer H, Posner AS. Crystalline changes in avian bone related to the reproductive cycle. II. Percent crystallinity changes. Calcif Tissue Res. 1974;14:83–86. doi: 10.1007/BF02060285. [DOI] [PubMed] [Google Scholar]
- 117.Tarnowski CP, Ignelzi MA, Jr, Morris MD. Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy. J Bone Miner Res. 2002;17:1118–1126. doi: 10.1359/jbmr.2002.17.6.1118. [DOI] [PubMed] [Google Scholar]
- 118.Thompson DD, Posner AS, Laughlin WS, Blumenthal NC. Comparison of bone apatite in osteoporotic and normal Eskimos. Calcif Tissue Int. 1983;35:392–393. doi: 10.1007/BF02405064. [DOI] [PubMed] [Google Scholar]
- 119.Tong W, Glimcher MJ, Katz JL, Kuhn L, Eppell SJ. Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif Tissue Int. 2003;72:592–598. doi: 10.1007/s00223-002-1077-7. [DOI] [PubMed] [Google Scholar]
- 120.Tsaih SW, Korrick S, Schwartz J, Lee ML, Amarasiriwardena C, Aro A, Sparrow D, Hu H. Influence of bone resorption on the mobilization of lead from bone among middle-aged and elderly men: The Normative Aging Study. Environ Health Perspect. 2001;109:995–999. doi: 10.1289/ehp.01109995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turner CH, Garetto LP, Dunipace AJ, Zhang W, Wilson ME, Grynpas MD, Chachra D, McClintock R, Peacock M, Stookey GK. Fluoride treatment increased serum IGF-1, bone turnover, and bone mass, but not bone strength, in rabbits. Calcif Tissue Int. 1997;61:77–83. doi: 10.1007/s002239900299. [DOI] [PubMed] [Google Scholar]
- 122.Turner IG, Jenkins GM. The spatial arrangement of bone mineral as revealed by ion bombardment. Biomaterials. 1981;2:234–238. doi: 10.1016/0142-9612(81)90063-6. [DOI] [PubMed] [Google Scholar]
- 123.U.S. Department of Health and Human Services . Bone Health and Osteoporosis. A report of the US Surgeon General; Rockville MD: 2004. [Google Scholar]
- 124.van der Linden JC, Day JS, Verhaar JA, Weinans H. Altered tissue properties induce changes in cancellous bone architecture in aging and diseases. J Biomech. 2004;37:367–374. doi: 10.1016/s0021-9290(03)00266-5. [DOI] [PubMed] [Google Scholar]
- 125.Vanderschueren D, Van Herck E, Schot P, Rush E, Einhorn T, Geusens P, Bouillon R. The aged male rat as a model for human osteoporosis: Evaluation by nondestructive measurements and bio-mechanical testing. Calcif Tissue Int. 1993;53:342–347. doi: 10.1007/BF01351841. [DOI] [PubMed] [Google Scholar]
- 126.Wachter NJ, Augat P, Krischak GD, Mentzel M, Kinzl L, Claes L. Prediction of cortical bone porosity in vitro by microcomputed tomography. Calcif Tissue Int. 2001;68:38–42. doi: 10.1007/s002230001182. [DOI] [PubMed] [Google Scholar]
- 127.Wallach S, Rousseau G, Martin L, Azria M. Effects of calcitonin on animal and in vitro models of skeletal metabolism. Bone. 1999;25:509–516. doi: 10.1016/s8756-3282(99)00200-8. [DOI] [PubMed] [Google Scholar]
- 128.Wu Y, Ackerman JL, Strawich ES, Rey C, Kim HM, Glimcher MJ. Phosphate ions in bone: Identification of a calcium-organic phosphate complex by 31P solid-state NMR spectroscopy at early stages of mineralization. Calcif Tissue Int. 2003;72:610–626. doi: 10.1007/s00223-002-1068-8. [DOI] [PubMed] [Google Scholar]
- 129.Yudoh K, Nishioka K. Telomerized presenescent osteoblasts prevent bone mass loss in vivo. Gene Ther. 2004;11:909–915. doi: 10.1038/sj.gt.3302234. [DOI] [PubMed] [Google Scholar]
- 130.Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF. Targeted disruption of the biglycan gene leads to osteoporosis in mice. Nat Genet. 1998;20:78–86. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]