Abstract
Pre-existing immunity against adenoviruses may compromise the efficacy of adenoviral vectors for vaccination and gene therapy. The purpose of this study was to determine whether encapsulation of adenovirus recombinants into biodegradable alginate microparticles could circumvent the vector-specific immune response. Mice were immunized either intranasally (i.n.) or intraperitoneally (i.p.) with human adenovirus type 5 (HAd5), resulting in the development of virus-specific antibodies. Immunized and naï mice were inoculated with AdCA36lacZ (an E1-deleted HAd5 recombinant containing the bacterial β-galactosidase (LacZ) gene), encapsulated (E) into alginate microparticles, or nonencapsulated (NE) i.e., as a virus suspension. LacZ expression in animals immunized once (1x) or twice (2x) with HAd5 and subsequently inoculated with NE-AdCA36lacZ (NE-Z) was significantly (p<0.001) reduced compared to those levels observed in NE-Z inoculated naï mice, suggesting that the immune response against the vector adversely affected transgene expression. In contrast, there was only slight reduction (p>0.05) in LacZ expression in mice immunized 1x or 2x with HAd5 that were subsequently inoculated with E-AdCA36lacZ (E-Z) compared to those levels obtained in E-Z inoculated naï animals. Similar results were obtained with i.n. or i.p. inoculated animals. These results indicate that microencapsulation of recombinant adenovirus effectively circumvented the vector-specific immune response.
Keywords: Adenovirus encapsulation, alginate microspheres, delivery vehicle, vector specific immune response, adenovirus recombinant
Introduction
Adenovirus-based vectors have considerable potential as delivery vehicles for both recombinant vaccines and somatic gene therapy [1-3]. However, a major disadvantage is the development of immunity to adenovirus. All human adenovirus (HAd) vectors (replication-competent, replication-defective, and helper-dependent) result in vector-specific immune responses that significantly reduce vector uptake following a second administration of the same vector [4-6]. The cellular immune response against the vector may kill the transduced cells expressing viral and transgene products [7-10], whereas the humoral response, especially neutralizing antibody, reduces virus uptake by the target cells on readministration of the same vector [11-13]. Moreover, pre-existing HAd-neutralizing antibodies are common in the human population due to the ubiquitous nature of HAd serotypes [14, 15], and therefore, may interfere with transgene expression even upon the first inoculation of the vector.
Immunosuppressive agents, such as cyclosporin, cyclophosphamide [16], deoxyspergualin [17], FK506 [18], and CTLA4Ig [19, 20] enhanced the duration of transgene survival. Another strategy to overcome vector-specific neutralizing immune responses is to switch the adenovirus serotype for vector construction [5]. Sequential administration of HAd, bovine adenovirus (BAd) and porcine adenovirus (PAd) could also circumvent vector-specific neutralizing immune response [13]. Masking of the capsid components of the virus by covalently linking polyethylene glycol (PEG) [21, 22] or N-(2-hydroxypropyl)methacrylamide (HPMA) [23] is another approach to curtail antibody-mediated virus neutralization. Retargeting of adenoviral vectors to specific cell receptors other than coxsackie/adenoviral receptor (CAR) will not only provide a mean to target specific cells but also elude immunity to the vector [24-28].
Purified protein, DNA, bacteria, or viruses can be encapsulated in sodium alginate-based biodegradable microparticles, and can be delivered by various routes [29-34]. Since the vector immunity is still considered a major hurdle in obtaining persistent transgene expression on repeated inoculations with HAd5 vectors, we attempted to extend the available options for combating the vector-specific immune response to microencapsulation of HAd5 vectors. This paper demonstrates that encapsulation of a HAd5 recombinant into alginate microparticles could evade the vector-specific immune response.
Results
Development of HAd5-specific Immune response in mice
In order to study the role of microencapsulation in eluding vector-specific immune response, mice were immunized either intranasally (i.n.) or intraperitoneally (i.p.) with HAd5 to develop HAd5-specific immune response. The purpose of selecting two routes of inoculation was to determine whether microencapsulation would be effective in circumventing both systemic as well as mucosal immunity to the vector. We also wanted to know whether the level of immune response affects the success of overcoming vector-specific immunity. Therefore, mice were inoculated once (1x) or twice (2x) with HAd5, serum samples were collected on days 21 and 35, respectively, and analyzed for the presence of HAd-specific IgG or IgA antibodies by ELISA.
The HAd5-specific IgG antibody titers were approximately 2.5 times higher in the 1x or 2x HAd5-immunized groups of the i.p. inoculated mice compared to those of i.n. inoculated animals. (Fig.1A and 1B). In contrast, the virus-specific IgA antibody titers were higher in the 1x or 2x HAd5-immunized groups inoculated i.n. compared to those of i.p. inoculated mice. (Fig. 2A and 2B). The development of systemic IgA antibody titers was used as an indirect measure of mucosal immunity. HAd5-specific IgG or IgA antibody titers in naï animals (PBS-inoculated) were similar to the background levels (data not shown).
Fig. 1.
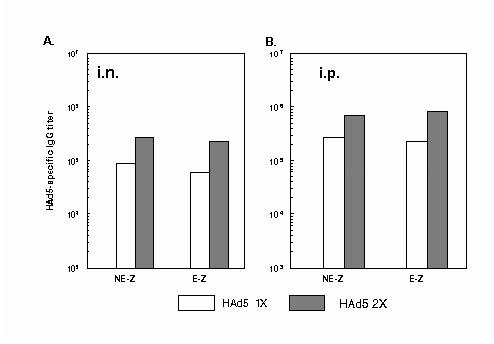
. HAd5-specific IgG ELISA antibodies in mice inoculated with HAd5. 6-8 week-old BALB/c mice (5 animals/group) were immunized A) intranasally (i.n.) or B) intraperitoneally (i.p.) either with PBS or once (1x)/twice (2x) with 1 x 108 p.f.u. of HAd5. There was no difference between NE-Z and E-Z groups at this stage. Serum samples were collected on 3 and 5 weeks post-inoculation and analyzed to determine levels of virus-specific IgG antibodies by ELISA. Each point represents the geometric mean value for five animals. NE-Z, nonencapsulated AdCA36lacZ; E-Z, AdCA36lacZ encapsulated into alginate microparticles.
Fig. 2.
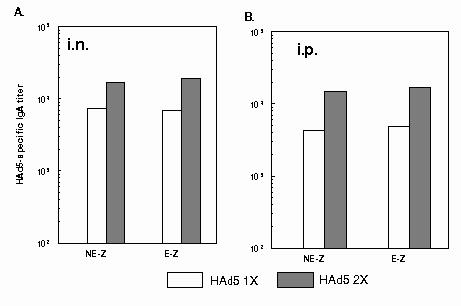
HAd5-specific IgA ELISA antibodies in mice inoculated with HAd5. 6-8 week-old BALB/c mice (5 animals/group) were immunized A) intranasally (i.n.) or B) intraperitoneally (i.p.) either with PBS or once (1x)/twice (2x) with 1 x 108 p.f.u. of HAd5. There was no difference between NE-Z and E-Z groups at this stage. Serum samples were collected on 3 and 5 weeks post-inoculation and analyzed to determine levels of virus-specific IgA antibodies by ELISA. Each point represents the geometric mean value for five animals. NE-Z, nonencapsulated AdCA36lacZ; E-Z, AdCA36lacZ encapsulated into alginate microoparticles.
Since vector-specific neutralizing antibody response is mainly responsible for virus neutralization before adsorption to the cells, the serum samples collected from mice immunized 1x or 2x with HAd5 were also analyzed for HAd5-specific neutralizing antibody by virus-neutralization assay. In 1x HAd5-immunized groups, the virus neutralizing antibody titers were 400 and 800 in i.n. or i.p. inoculated mice, respectively (Table 1), and were 800 and 1600 in i.n. or i.p. 2x HAd5-immunized groups, respectively (Table 1). Virus-neutralizing antibody titers in naï animals (PBS-inoculated) were close to the background levels.
Table 1.
HAd5-specifi c neutralizing antibody response in mice inoculated with HAd5a
| HAd5-specific neutralizing serum antibody titers | |||
|---|---|---|---|
| Group | Route of Inoculation | Mice primed once with HAd5 | Mice primed twice with HAd5 |
| Naive/NE-Z/i.n. | i.n. | <25 | |
| Naive/E-Z/i.n. | i.n. | <25 | |
| HAd5/NE-Z/i.n. | i.n. | 400 | |
| HAd5/E-Z/i.n. | i.n. | 400 | |
| HAd5-HAd5/NE-Z/i.n. | i.n. | 800 | |
| HAd5-HAd5/E-Z/i.n. | i.n. | 800 | |
| Naive/NE-Z/i.p. | i.p. | <25 | |
| Naive/E-Z/i.p. | i.p. | <25 | |
| HAd5/NE-Z/i.p. | i.p. | 800 | |
| HAd5/E-Z/i.p. | i.p. | 800 | |
| HAd5-HAd5/NE-Z/i.p. | i.p. | 1600 | |
| HAd5-HAd5/E-Z/i.p. | i.p. | 1600 | |
Serum samples obtained from mice inoculated once (1X) or twice (2X) with HAd5 were used to detect levels of HAd5-specifi c neutralizing antibodies by virus-neutralizing assay. Neutralizing antibody titers are expressed as the highest reciprocal serum dilution resulting in reduction of virus plaque formation by at least 50%. Titers are expressed as mean value from 5 mice per group.
Levels of LacZ expression by AdCA36lacZ in mice having pre-existing adenovirus-specific immune response
We previously demonstrated that in mice inoculated i.n. or i.p. with NE-Z, the maximum LacZ expression in various tissues was observed on day 1 post-inoculation [13]. In a preliminary study, the highest level of LacZ expression in different tissues of mice inoculated i.n. or i.p. with E-Z was observed on day 3 post-inoculation (data not shown). Therefore, mice inoculated with NE-Z or E-Z were sacrificed on day 1 or 3 post-inoculation, respectively.
To address the issue of circumvention of pre-existing immunity to the vector by microencapsulation, naï, 1x HAd5-immunized or 2x HAd5-immunized mice were inoculated with NE-Z or E-Z. The trachea and lungs were collected from i.n. inoculated mice, whereas the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes were collected from i.p. inoculated mice to monitor LacZ expression by LacZ assay. The same route of inoculation was used to inoculate mice with NE-Z or E-Z that was initially used for inoculating with PBS (naï) or HAd5.
Animal inoculated with i.n. route. In naï mice inoculated i.n. with NE-Z (naï/NE-Z/i.n.), expression of LacZ in the trachea and lungs was approximately 38 and 43 ng/g tissue, respectively (Figs. 3A and 3B). However, in mice 1x immunized with HAd5 and then inoculated i.n. with NE-Z (HAd5/NE-Z/i.n.), expression of LacZ in the trachea and lungs were reduced to approximately 33 and 36%, respectively, of those levels obtained in naï/NE-Z/i.n. group. The expression of LacZ was reduced to approximately 16 and 15% in the trachea and lungs, respectively, in HAd5-HAd5/NE-Z/i.n. compared to naï/NE-Z/i.n. animals suggesting that there is significant (p<0.001) inhibition of transgene expression in the presence of immune response to the vector.
Fig. 3.
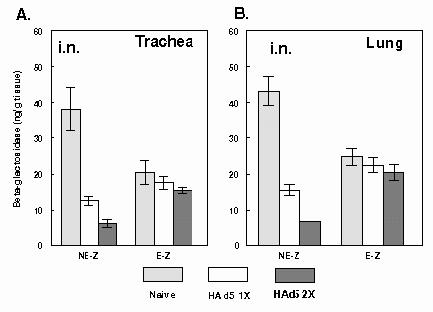
Circumvention of adenovirus-specific immune response by microencapsulation of AdCA36lacZ. 6-8 week old BALB/c mice (5 animals/group) were immunized intranasally (i.n.) with PBS or once (1x)/twice (2x) with 1 X 108 p.f.u. of HAd5, and following development of HAd5-specific antibodies animals were inoculated i.n. with a nonencapsulated (NE-Z) or encapsulated (E-Z) in alginate microspheres preparation containing 2.5 x 108 p.f.u. of AdCA36lacZ. The A) lungs and B) tracheae were collected and analyzed for LacZ expression. Each point represents the mean value for five animals ± SD.
In naï mice inoculated i.n. with E-Z (naï/E-Z/i.n.), expression of LacZ in the trachea and lungs was approximately 20 and 25 ng/g tissue, respectively (Figs. 3A and 3B). In mice 1ximmunized with HAd5 and inoculated i.n. with E-Z (HAd5/E-Z/i.n.), the expression of LacZ in the trachea and lungs was approximately 86 and 90%, respectively, of those levels obtained in naï/E-Z/i.n. group. The expression of LacZ was only reduced to 75 and 80% in the trachea and lungs, respectively in HAd5-HAd5/E-Z/i.n. compared to naï/E-Z/i.n. mice. These results indicate that high levels of transgene expression were observed and were not significantly (p > 0.05) inhibited even in the presence of HAd5-specific neutralizing antibody response.
Animal inoculated with i.p. route. In naï mice that were inoculated i.p. with NE-Z (naï/NE-Z/i.p.), transgene expression was approximately 57, 45, 19, 9.5 and 7 ng/g tissue in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes, respectively (Figs. 4A-4E). Whereas, in mice 1x-immunized with HAd5 when inoculated i.p. with NE-Z (HAd5/NEZ/i.p.), LacZ expression in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes were reduced to approximately 22, 28, 27, 30 and 23%, respectively, of the levels obtained in naï/NE-Z/i.p. mice. Transgene expression was reduced to 13, 14, 5, 8 and 7% in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes, respectively, in HAd5-HAd5/NE-Z/i.p compared to those levels observed in naï/NE-Z/i.p. animals. These results further confirm that immunity to vector has a significant (p<0.001) adverse affect on transgene expression by an adenoviral vector.
Fig. 4.

Circumvention of adenovirus-specific immune response by microencapsulation of AdCA36lacZ. 6-8 week old BALB/c mice (5 animals/group) were immunized intraperitoneally (i.p.) with PBS or once (1x)/twice (2x) with 1 X 108 p.f.u. of HAd5, and following development of HAd5-specific antibodies animals were inoculated i.p. with a nonencapsulated (NE-Z) or encapsulated (E-Z) in alginate microspheres preparation containing 2.5 x 108 p.f.u. of AdCA36lacZ. The A) spleen, B) liver, C) peritoneal cells, D) kidneys, and E) mesenteric lymph nodes were collected and analyzed for LacZ expression. Each point represents the mean value for five animals ± SD.
On the other hand, in naï animals inoculated i.p. with E-Z (naï/E-Z/i.p.), expression of LacZ was approximately 28, 22, 12, 5 and 3.5 ng/g tissue in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes, respectively (Figs. 4A-4E). In HAd5/E-Z/i.p. mice, LacZ expression in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes were only reduced to approximately 90, 92, 83, 82 and 80%, respectively, of those levels obtained with naï/E-Z/i.p. Furthermore, in HAd5-HAd5/E-Z/i.p. animals, transgene expression in the spleen, liver, peritoneal cells, kidneys and mesenteric lymph nodes was observed to be approximately 83, 79, 70, 65 and 66% of those levels seen in naï/E-Z/i.p. group. The inhibition in LacZ expression was not significant (p > 0.05) suggesting that microencapsulation can circumvent the immune response to the vector.
Discussion
Induction of an immune response to the vector significantly inhibits the levels and duration of transgene expression. Some of the approaches to elude immunity to HAd vectors include immunosuppression [25-38], retargeting HAd vectors after masking the binding site for CAR [25, 26], alteration of the immunodominant capsid epitopes [39], use of different HAd serotypes [40-42] and use of human and non-human adenovirus vectors in sequential administration [13]. However, it has been suggested that repeated intramuscular (i.m.) inoculation with helper-dependent HAd vectors could be done even in the presence of preexisting immunity [43]. The focus of this study was to develop a simple method to evade preexisting immunity so that repeated inoculations with the same vector (replication-competent, replication-defective, conditional replication-competent or helper-dependent) provide the required levels of transgene expression at each inoculation. We used biodegradable alginate microparticles to encapsulate a HAd5 recombinant (AdCA36lacZ) in an attempt to overcome HAd5-specific immune response.
In order to mimic the conditions of pre-existing immunity to the vector, mice were first immunized with HAd5 to test the hypothesis that microencapsulation of HAd5 vector could escape the vector-specific immune response. We tested the effect of levels and types (systemic or mucosal) of immunity to HAd5 on transgene expression from the vector on subsequent inoculation. In HAd5-immunized mice, LacZ expression by NE-Z, i.e. AdCA36lacZ as a virus suspension, was significantly (p<0.001) inhibited compared to expression in naï animals and its level was dependent on the level of vector-specific immune response. This concept has been demonstrated previously [13, 44]. However, in HAd5-immunized mice, transgene expression following inoculation with E-Z, i.e. AdCA36lacZ encapsulated into alginate microspheres, was slightly reduced (10% in 1x HAd5-immunized, and 20-25% in 2x HAd5-immunized) compared to levels obtained in naï animals. Similar results were obtained with i.n. and i.p. routes of inoculation indicating that microencapsulation could effectively elude both systemic and mucosal immunity to the vector. Use of polyethylene glycol and cationic lipid to coat HAd vectors [45] and poly(lactic-glycolic) acid (PLGA) copolymer encapsulation [46] have been also shown to elude virus-neutralizing antibodies.
It was not surprising to observe that in naï animals LacZ expression with E-Z was approximately 50% of the levels obtained with NE-Z inoculated mice. It has been shown that the maximum levels of LacZ expression in mice inoculated with NE-Z was on day 1 and declined rapidly thereafter [13]. Whereas, transgene expression in animals inoculated with E-Z peaked at day 3 and then declined slowly (data not shown). Therefore, transgene expression with two types of preparations (NE-Z and E-Z) that reach the maximum levels at different times post-inoculation (day 1 for NE-Z and day 3 for E-Z) should be compared with a caution. It is interesting to note that in HAd5-immunized mice there was at least 10-25% decline in LacZ expression on inoculation with E-Z. This decline may be due to 1) a limited accessibility of the virus onto or into the microspheres to the virus-neutralizing antibody. 2) A small percentage of microspheres may be degraded in the intracellular spaces releasing HAd5 recombinant that could be neutralized by the vector-specific antibody. 3) The cell-mediated immune response against the viral antigens may be responsible in removing cells that are also expressing the transgene.
More than 70% of the microspheres described in this study were approximately 5-10 μm in size, and therefore, it is expected that the majority of them will be taken up by macrophages and dendritic cells [47]. It appears that alginate microspheres may be an attractive delivery system to target macrophages and dendritic cells. There is a need to study the role of these microparticles in modulating immune response through macrophages and dendritic cells. Development of microspheres having a size range of 0.5-2 μm that could be taken up by a number of other cell types would increase the versatility of this delivery system. Furthermore, targeted delivery of microspheres to specific cells is another attractive possibility.
Materials and Methods
Cell lines and Viruses
Human cell lines, HeLa and 293, were grown as monolayer cultures in Eagle’s minimum essential medium (MEM) (Life Technologies, Inc.) supplemented with 5 or 10% reconstituted fetal bovine serum (FetalClone III; Hyclone, Inc), respectively and 50 μg/ml gentamicin. HAd5 was grown on HeLa cells and AdCA36LacZ recombinant [48], a replication-defective HAd5 recombinant containing the bacterial β-galactosidase (LacZ) gene in the E1 region under the control of mouse cytomegalo virus promoter (a kind gift from Dr. Frank Graham, Departments of Biology and Pathology, McMaster University, Hamilton, Ontario, Canada) was grown in 293 cells. The virus purification by cesium chloride-density gradient centrifugation was done as previously described [49]. Viruses were titrated in 293 cells by plaque assay.
Microencapsulation of HAd5 recombinant
A purified preparation of AdCA36LacZ recombinant was encapsulated into sodium alginate microspheres as described previously [34]. One ml suspension of microspheres was expected to contain a maximum of 5 x 109 p.f.u. of AdCA36LacZ. The size of microspheres was measured by Microtrak Particle Analyzer and the majority of them were 5-10 μm in diameter.
Experimental Design
8-10 weeks old female BALB/c mice were purchased from Harlan Sprague Dawley, Indianapolis, IN. Animals were fed and watered normally until the end of the experiment. Mice were randomly grouped into 12 groups (5 animals/group). Six groups were inoculated intranasally (i.n.) and the remaining groups were inoculated intraperitoneally (i.p.).
The experimental design is presented in Table 2. For i.n. inoculation, animals were anesthetized with sodium pentobarbital. On day 0, two naï groups were inoculated with 50 μ of PBS and four groups were inoculated with a purified preparation containing 1 x 108 l p.f.u. of HAd5. Subsequently, on day 21, one naï and one HAd5-immunized groups were inoculated i.n. with 2.5 x 108 p.f.u. of nonencapsulated (NE) AdCA36lacZ (for simplicity the term “Z” is used for this recombinant), and the other naï and HAd5 groups were inoculated i.n. with 2.5 x 108 p.f.u. of AdCA36lacZ encapsulated (E) into alginate microspheres. The remaining two HAd5-immunized groups were inoculated a second time with 1 x 108 p.f.u. of HAd5 on day 21. On day 35, one group immunized twice with HAd5 was inoculated i.n. with 2.5 x 108 p.f.u. of NE-Z (nonencapsulated AdCA36lacZ) and the other group was inoculated i.n. with 2.5 x 108 p.f.u. of E-Z (AdCA36lacZ encapsulated into alginate microspheres). The lungs and trachea were collected on day 1 or 3 following NE-Z or E-Z inoculation, respectively. Blood samples were collected on days 21 and/or 35.
Table 2.
Experimental Design
| No. | Group | Route of | Mice Inoculated With | Blood Collection | Tissue Collection | |||
|---|---|---|---|---|---|---|---|---|
| Inoculation | Day 0 | Day 21 | Day 35 | Day 21 | Day 35 | on Daya | ||
| 1 | Naive/NE-Z/i.n. | i.n. | PBS | AdCA36LacZ | Yes | 22 | ||
| 2 | Naive/E-Z/i.p. | i.n. | PBS | AdCA36LacZ in microspheres | Yes | 24 | ||
| 3 | HAd5/NE-Z/i.n. | i.n. | HAd5 | AdCA36LacZ | yes | 22 | ||
| 4 | HAd5/E-Z/i.n. | i.n. | HAd5 | AdCA36LacZ in microspheres | yes | 24 | ||
| 5 | HAd5-HAd5/NE-Z/i.n. | i.n. | HAd5 | HAd5 | AdCA36LacZ | yes | yes | 36 |
| 6 | HAd5-HAd5/E-Z/i.n. | i.n. | HAd5 | HAd5 | AdCA36LacZ in microspheres | yes | yes | 38 |
| 7 | Naive/NE-Z/i.p. | i.p. | PBS | AdCA36LacZ | yes | 22 | ||
| 8 | Naive/E-Z/i.p. | i.p. | PBS | AdCA36LacZ in microspheres | yes | 24 | ||
| 9 | HAd5/NE-Z/i.p. | i.p. | HAd5 | AdCA36LacZ | yes | 22 | ||
| 10 | HAd5/E-Z/i.p. | i.p. | HAd5 | AdCA36LacZ in microspheres | yes | 24 | ||
| 11 | HAd5-HAd5/NE-Z/i.p. | i.p. | HAd5 | HAd5 | AdCA36LacZ | yes | yes | 36 |
| 12 | HAd5-HAd5/E-Z/i.p. | i.p. | HAd5 | HAd5 | AdCA36LacZ in microspheres | yes | yes | 38 |
The lungs and trachea were collected from i.n. inoculated mice, whereas, the liver, spleen, kidneys, peritoneal cells and mesenteric lymph nodes were collected from i.p. inoculated animals.
For i.p. inoculation, two naï groups were inoculated with 100 μl of PBS, and four groups were inoculated with a purified preparation containing 1 x 108 p.f.u. of HAd5 on day 0. Subsequently, on day 21, one naï and one HAd5-immunized group were inoculated i.p. with 2.5 x 108 2.5 x 108 p.f.u. of NE-Z, and the other naï and HAd5-immunized groups were inoculated with 2.5 x 108 2.5 x 108 p.f.u. of E-Z. The remaining two HAd5 groups were inoculated a second time with 1 x 10 p.f.u. of HAd5 on day 21. On day 35, one of each group was inoculated i.p. with 2.5 x 108 p.f.u. of NE-Z or E-Z. The liver, spleen, peritoneal cells, kidneys and mesenteric lymph nodes were collected one or three days following NE-Z or E-Z inoculation, respectively. Blood samples were collected on days 21 and/or 35.
The serum samples were used to examine the development of virus-specific antibodies by enzyme-linked immunosorbent assay (ELISA) and virus neutralization assay. The tissue samples were processed to measure the expression of LacZ by β-galacatosidase assay.
ELISA
The serum samples obtained from mice were used to detect HAd5 specific IgG and IgA antibodies by ELISA as previously described [50]. Briefly, 96 well ELISA plates were coated with a purified preparation of HAd5 (10 μg/ml) and then reacted with serial dilutions of each of the test sample. For secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Bio-Rad) or IgA (Southern Biotechnology Associates, AL) was used to detect IgG or IgA antibody, respectively. The OD was measured at dual wavelength of 490-650 nm using an ELISA reader (Molecular Devices, Inc.). The reciprocal of the highest serum dilution with an OD reading of at least the mean ± 2 SD above negative control sera was taken as the ELISA antibody titer. The data represent the geometric means from five animals.
Virus neutralization assay
Serum Samples from mice were tested for the presence of HAd5-neutralizing antibodies as described previously [13]. Briefly, serial dilutions of each of the serum sample were reacted with 100 p.f.u. of HAd5 for 1 h at 37°C. The numbers of viable HAd5 remaining after the antibody treatment were identified by plaque assay on 293 cells. The virus neutralization antibody titer was the reciprocal of the highest serum dilution that leads to reduction of number of virus plaque by at least 50%.
β- Galactosidase assay
β-Galactosidase assay was performed as previously described [33]. LacZ activity is expressed as nanograms per gram of tissue using purified β-galactosidase as a control.
Statistical analysis
Statistical analysis of the data collected from the animal inoculation study was done using Student's t-distribution. Significance was set at P < 0.05.
Acknowledgements
We thank Dr. F. L. Graham, Departments of Biology and Pathology, McMaster University, Hamilton, Ontario, Canada for providing AdCA36lacZ and Jane Kovach for excellent secretary assistance. This work was supported by Public Health Service grant GM5516 from NIH/NIGMS to S.K.M.
REFERENCES
- 1.Bramson JL, Graham FL, Gauldie J. The use of adenoviral vectors for gene therapy and gene transfer in vivo. Curr. Opin. Biotechnol. 1995;6:590–595. doi: 10.1016/0958-1669(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 2.Imler J-L. Adenovirus vectors as recombinant viral vaccines. Vaccine. 1995;13:1143–1151. doi: 10.1016/0264-410x(95)00032-v. [DOI] [PubMed] [Google Scholar]
- 3.Alemany R, Balague C, Curiel DT. Replicative adenoviruses for cancer therapy. Nat. Biotechnol. 2000;18:723–727. doi: 10.1038/77283. [DOI] [PubMed] [Google Scholar]
- 4.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 5.Kass-Eisler A, Leinwand L, Gall J, Bloom B, Falck-Pederson E. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 1996;3:154–162. [PubMed] [Google Scholar]
- 6.Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr. Opin. Mol. Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- 7.Yang Y, et al. Cellular immunity to viral antigens limits E1-deleted adenovirus for gene therapy. Proc. Natl. Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Li Q, Ertl HC, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinat adenoviruses. J. Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai Y, et al. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: Tolerization of factor IX and vector antigensallows for long term expression. Proc. Natl. Acad. Sci. USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kafri T, et al. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc. Natl. Acad. Sci. USA. 1998;95:11377–11382. doi: 10.1073/pnas.95.19.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong JY, Wang DH, Vanginkel FW, Pascual DW, Frizzell RA. Systemic analysis of repeated gene delivery into animal lungs with a recombinant adenovirus vector. Hum. Gene Ther. 1996;7:319–331. doi: 10.1089/hum.1996.7.3-319. [DOI] [PubMed] [Google Scholar]
- 12.Walter J, You QM, Hagstrom JN, Sands M, High KA. Successful expression of human factor IX following repeat administration of an adenoviral vector in mice. Proc. Natl. Acad. Sci. USA. 1996;93:3056–3061. doi: 10.1073/pnas.93.7.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffatt S, Hays J, HogenEsch H, Mittal SK. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology. 2000;272:159–167. doi: 10.1006/viro.2000.0350. [DOI] [PubMed] [Google Scholar]
- 14.Chirmule N, et al. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 15.Harvey BG, et al. Variability of human systemic humoral immune responses to adenovirus gene transfer vectors administered to different organs. J. Virol. 1999;73:6729–6742. doi: 10.1128/jvi.73.8.6729-6742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- 17.Kaplan JM, Smith AE. Transient immunosuppression with deoxyspergualin improves longevity of transgene expression and ability to readminister adenoviral vector to the mouse lung. Hum. Gene Ther. 1997;8:1095–1104. doi: 10.1089/hum.1997.8.9-1095. [DOI] [PubMed] [Google Scholar]
- 18.Ilan Y, et al. Transient immunosuppression with FK506 permits long-term expression of therapeutic genes introduced into the liver using recombinant adenoviruses in the rat. Hepatology. 1997;26:949–956. doi: 10.1002/hep.510260422. [DOI] [PubMed] [Google Scholar]
- 19.Guerette B, et al. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum. Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- 20.Jooss K, Turka LA, Wilson JM. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998;5:309–319. doi: 10.1038/sj.gt.3300595. [DOI] [PubMed] [Google Scholar]
- 21.O’Riordan CR, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999;10:1349–1358. doi: 10.1089/10430349950018021. [DOI] [PubMed] [Google Scholar]
- 22.Croyle MA, Yu QC, Wilson JM. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 2000;11:1713–1722. doi: 10.1089/10430340050111368. [DOI] [PubMed] [Google Scholar]
- 23.Fisher K, Stallwood Y, Ulbrich K, Mautner V, Seymour L. Protection and retargeting of adenovirus using a multifunctional hydrophilic polymer. Mol. Ther. 2000;1:S57. [Google Scholar]
- 24.Douglas JT, et al. Targeted gene delivery by tropism-modified adenoviral vectors. Nat. Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 25.Bilbao G, Gomez-Navarro J, Curiel DT. Targeted adenoviral vectors for cancer gene therapy. Adv. Exp. Med Biol. 1998;451:365–374. doi: 10.1007/978-1-4615-5357-1_57. [DOI] [PubMed] [Google Scholar]
- 26.Douglas JT, et al. A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nat. Biotechnol. 1999;17:470–475. doi: 10.1038/8647. [DOI] [PubMed] [Google Scholar]
- 27.Nicklin SA, et al. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol. Ther. 2001;4:534–542. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, et al. Targeting adenoviral vectors by using the extracellular domain of the coxsackie-adenovirus receptor: improved potency via trimerization. J. Virol. 2002;76:1892–1903. doi: 10.1128/JVI.76.4.1892-1903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowersock TL, Hogenesch H. Oral immunization using microparticles. In: Park K, editor. Controlled Drug Delivery Challenges and Strategies. American Chemical Society; Washington: 1997. pp. 269–288. [Google Scholar]
- 30.Bowersock TL, et al. Oral vaccination of animals with antigens encapsulated in alginate microspheres. Vaccine. 1999;17:1804–1811. doi: 10.1016/s0264-410x(98)00437-x. [DOI] [PubMed] [Google Scholar]
- 31.Periwal SB, Speaker TJ, Cebra JJ. Orally administered microencapsulated reovirus can bypass suckled, neutralizing maternal antibody that inhibits active immunization of neonates. J. Virol. 1997;71:2844–2850. doi: 10.1128/jvi.71.4.2844-2850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilbert AK, Fritzsche U, Kissel T. Biodegradable microspheres containing influenza A vaccine: immune response in mice. Vaccine. 1999;17:1065–2073. doi: 10.1016/s0264-410x(98)00323-5. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal N, et al. Biodegrable alginate microspheres as a delivery system for naked DNA. Can. J. Vet. Sci. 1999;63:148–152. [PMC free article] [PubMed] [Google Scholar]
- 34.Mittal SK, et al. Immunization with DNA, adenovirus or both in biodegradable alginate microspheres: effect of route of inoculation on immune response. Vaccine. 2000;19:253–263. doi: 10.1016/s0264-410x(00)00170-5. [DOI] [PubMed] [Google Scholar]
- 35.Kay MA, et al. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat. Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 36.Guibinga G-H, et al. Combinatorial blockade of calcineurin and CD28 signaling facilitates primary and secondary therapeutic gene transfer by adenovirus vectors in dystrophic (mdx) mouse muscles. J. Virol. 1998;72:4601–4609. doi: 10.1128/jvi.72.6.4601-4609.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilan Y, et al. Oral tolerization to adenoviral proteins permits repeated adenovirus mediated gene therapy in rats with pre-existing immunity to adenoviruses. Hepatology. 1998;27:1368–1376. doi: 10.1002/hep.510270525. [DOI] [PubMed] [Google Scholar]
- 38.Ye X, Robinson MB, Pabin C, Batshaw ML, Wilson JM. Transient depletion of CD4 lymphocyte improves efficacy of repeated administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mouse. Gene Ther. 2000;7:1761–1767. doi: 10.1038/sj.gt.3301299. [DOI] [PubMed] [Google Scholar]
- 39.Roy S, Shirley PS, McClelland A, Kaleko M. Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 1998;72:6875–6879. doi: 10.1128/jvi.72.8.6875-6879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mack CA, et al. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 1997;8:99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 41.Morral N, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parks R, Evelegh C, Graham FL. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 43.Maione D, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittal SK, McDermott MR, Johnson DC, Prevec L, Graham FL. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 1993;28:67–90. doi: 10.1016/0168-1702(93)90090-a. [DOI] [PubMed] [Google Scholar]
- 45.Chillon M, Lee JH, Fasbender A, Welsh MJ. Adenovirus complexed with polyethylene glycol and cationic lipid is shielded from neutralizing antibodies in vitro. Gene Ther. 1998;5:995–1002. doi: 10.1038/sj.gt.3300665. [DOI] [PubMed] [Google Scholar]
- 46.Beer SJ, et al. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 47.Lomotan EA, Brown KA, Speaker TJ, Offita PA. Aqueous-based microcapsules are detected primarily in gut-associated dendritic cells after oral inoculation of mice. Vaccine. 1997;15:1959–1962. doi: 10.1016/s0264-410x(97)00108-4. [DOI] [PubMed] [Google Scholar]
- 48.Addison CL, Hitt M, Kunsken D, Graham FL. Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 1997;78:1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- 49.Graham FL, Prevec L. Adenovirus expression vectors and recombinant vaccines. In: Ellis RW, editor. Vaccines: New Approaches to Immunological Problems. Butterworth-Heineman; Boston: 1992. pp. 363–390. [Google Scholar]
- 50.Mittal SK, Middleton DM, Tikoo SK, Babiuk LA. Pathogenesis and immunogenicity of bovine adenovirus type 3 in cotton rats (Sigmodon hispidus) Virology. 1995;213:131–139. doi: 10.1006/viro.1995.1553. [DOI] [PubMed] [Google Scholar]


