Abstract
The formation of distant metastases is the deadliest phase of cancer progression. Although numerous studies have identified genes and mechanisms that affect metastasis after tumors have reached secondary sites, our knowledge about how cancer cells initially gain access to systemic circulation is limited. Since tumors can enter the blood directly by intravasating into venous capillaries or indirectly via lymphatics, it is important to evaluate the relative contributions of both pathways as routes of egress from the primary site. Insights into tumor and stromal factors governing the intravasation process may help explain why certain tumors exhibit “preferred” pathways for metastatic dissemination, both clinically and in experimental animal models.
Keywords: metastasis, intravasation, angiogenesis, lymphangiogenesis
WHICH TUMORS METASTASIZE?
What makes a tumor cell metastatic? Certainly, proliferative ability at a distant site is essential for metastasis (Paget’s “seed and soil” hypothesis), and difficulties in establishing secondary growth might explain why fewer than 0.01% of circulating tumor cells actually form metastases.1–3 However, exactly what enables a cancer cell to complete the metastatic process is not entirely clear. While recent gene expression studies have suggested that distant metastases resemble their primary tumors of origin,4,5 other studies have indicated that the expression of specific genes is altered in metastatic cells.6–8 A model combining both these observations has speculated that cells derived from metastases and from their corresponding primary tumors share an overall gene expression signature that confers the ability to complete some, but not all, of the steps required for metastasis.7,9 On top of this, the altered expression of a limited number of additional genes may render a sub-population of cells fully competent for metastasis, without changing its overall similarity with the primary tumor.
Although metastasis is widely regarded as an inefficient process, most cancer patients die from metastases rather than from their primary tumors. Metastatic inefficiency is likely overcome by the sheer number of tumor cells that enter the systemic circulation daily, estimated in one study to be up to ~4 × 106 tumor cells released per gram of primary tumor.10 Consequently, it is important that we gain a detailed understanding of how tumors complete the earliest steps of metastasis, including intravasation into vasculature.
In order to metastasize, cancer cells must first detach from the primary tumor and invade blood vessels or lymphatics. This may be a passive process where cells are simply sloughed off from the primary tumor or an active one involving directed migration.11,12 Almost certainly, a tumor’s cell of origin and its accompanying differentiation program will affect its metastatic proclivity.13 Cells from connective tissue tumors such as fibrosarcomas and gliomas tend to migrate individually, for instance, whereas those from melanomas and carcinomas often migrate collectively.14 In addition, highly differentiated epithelial tumors may initially display collective migration, only to de-differentiate and exhibit single cell invasion, a process termed epithelial-to-mesenchymal-transition (EMT).15 Indeed, genes that promote EMT—including Twist;8 Slug and Snail transcription factors;16 and components of the TGF-β signaling pathway17,18—have all been reported to enhance the earliest stages of metastasis. E-cadherin, which is often lost during EMT, is thought to suppress cell migration and tumor progression.19 Finally, stromal cells such as fibroblasts and macrophages have also been reported to affect metastasis by contributing growth factors (e.g., EGF, FGF-1), matrix metalloproteinases and chemotactic/pro-migratory factors (e.g., SF/HGF, chemokines).12,14
BLOOD VESSEL OR LYMPHATIC DISSEMINATION?
Once a migratory cell(s) has detached from the primary tumor, it may intravasate into blood vessels or lymphatics. Either route of dissemination can lead to venous circulation, as lymphatics drain into blood, most commonly through the left lymphatic duct (thoracic duct) or the right lymphatic duct, and then subsequently into the subclavian veins. Along the way, lymphatic fluid is filtered by lymph nodes.
In the absence of overt metastases, hematogenous dissemination of tumors is assayed by detecting cancer cells in the peripheral blood of patients or from bone marrow aspirates.20 The presence of circulating tumor cells and micrometastases can be determined by RT-PCR or immunohistochemistry (IHC), particularly for cytokeratins in the case of epithelial tumors. Lymphatic spread is also assayed by IHC and/or RT-PCR following surgical removal of regional lymph nodes. Tumors almost invariably invade lymph nodes in sequence, starting with the nearest (sentinel or draining) node, followed by increasingly distal ones.21 If the draining lymph node is uninvaded, other lymph nodes are also likely free of metastases.22
Metastatic bias is illustrated by the fact that carcinomas and melanomas tend to develop lymph node metastases more frequently than sarcomas,14 although it is unclear whether this disparity is due to differences in intravasation and/or growth. Lymph nodes are often the first site of metastasis in a variety of cancers, and are critical for tumor staging and prognosis.22 In prostate cancer, for instance, 75% of patients bearing lymph node metastases at the time of diagnosis will possess bone metastases within 5 years, regardless of treatment.23 The presence of tumor cells in the bone-marrow is also predictive of distant metastases in a variety of tumors, particularly carcinomas.20 On the other hand, the prognostic value of circulating tumor cells in the blood is debated, as current techniques for detection suffer from problems such as low sensitivity and high rates of false positives.24,25 However, recent studies using an automated platform for detecting tumor cells in the blood, called CellSearch, have reported significant correlations between the presence of circulating tumor cells and poor clinical outcome for breast cancer patients.26
The decision to intravasate into either blood or lymphatic vessels may rest largely on physical restrictions imposed on invasive tumors, although active mechanisms for attracting cells to specific types of vasculature have also recently been proposed (see below). Lymphatic capillaries lack the tight interendothelial junctions typically seen in blood vessels, as well as the surrounding layers of pericytes/smooth muscle cells and basement membranes.27 This inevitably renders lymphatics “leaky” relative to blood vessels, thus lowering the barriers for tumor intravasation. In addition, tumor cell survival may benefit from the passive, low-shear system of fluid transport characteristic of lymphatics.
Accessibility of blood and lymphatic vasculature may also influence the pathway taken for metastasis. Induction of angiogenesis, the growth of blood vessels, has been shown to be necessary for tumors growing beyond 0.4 mm in diameter.28,29 Lymphangiogenesis, the growth of lymphatic vessels, has been inhibited by us30 and others31–37 in experimental mouse cancer models without affecting primary tumor growth. Because blood and lymphatic vessels share a common embryonic origin, and respond to many similar growth factors—VEGF-A, VEGF-C, VEGF-D, FGF2, PDGF-B, HGF and others38—tumors might be expected to induce lymphangiogenesis concomitant with angiogenesis. But for reasons unclear, this is often not the case. While proliferating intratumoral lymphatics have been detected in human melanomas,39 as well as in head and neck squamous cell carcinomas,40 evidence for lymphangiogenesis in other cancers has been less well documented. The presence of anti-lymphangiogenic factors may be one reason why proliferating intratumoral lymphatics are not more commonly found in human clinical tumors,41 though the identity of these proposed factors is currently unknown.
Nonetheless, intratumoral lymphatics may provide a possible escape route from the primary tumor to draining lymph nodes, and indeed, several studies have reported that inhibition of lymphangiogenesis in xenograft tumor models can significantly reduce lymph node metastasis.31–33,36 However, other studies have suggested that intratumoral lymphatics are compressed and nonfunctional.42–45 This apparent absence of functional intratumoral lymphatics would imply that tumor cells intravasated into these vessels will encounter blockages and dead ends that actually impede metastasis. The fact that many tumors metastasize to local lymph nodes despite absence of lymphangiogenesis or functional intratumoral lymphatics, has led some to propose that it is the peripheral, peritumoral lymphatics that mediate tumor cell dissemination.37,46 We recently obtained results consistent with this hypothesis by selectively ablating intratumoral, but not peritumoral, lymphatics in a prostate cancer orthotopic model and showing that lymph node metastasis was not significantly altered.30 Other studies that ablated peritumoral lymphatics or inhibited their “activation”—local vessel sprouting, dilation and permeability—were successful at reducing metastasis (Fig. 1).32,37,47 It is possible that the studies reporting metastatic inhibition associated with ablation of intratumoral lymphatics31–33,36 may actually reflect interference with tumor cell intravasation into peritumoral lymphatics. Recent clinical and spontaneous animal tumor studies have also reported that prostate,48 breast49,50 and pancreatic tumors51 develop lymphatic metastases in the absence of intratumoral lymphangiogenesis.
Figure 1.
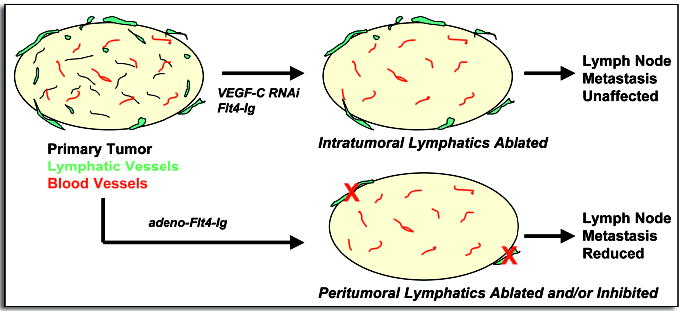
Tumors possess blood vessels (red) and, in some cases, lymphatics (green). Experimental ablation of intratumoral lymphatics does not inhibit lymph node metastasis (top).30 Eliminating or inhibiting the activation of peritumoral lymphatics has been shown to reduce lymphatic spread (bottom).32,37,47 In addition, intratumoral lymphatics are absent in many tumors that nevertheless metastasize to lymph nodes.51 These observations imply that peritumoral lymphatics mediate the majority of tumor cell dissemination. (Flt4-Ig, soluble Flt4 receptor/VEGFR3; adeno, adenoviral delivery).
Perhaps the best approach for studying blood and lymphatic vessel intravasation is to observe the process in real time, using in vivo intravital microscopy. Wyckoff et al imaged rat mammary adenocarcinomas and discovered that metastatic cells were more likely to polarize towards blood vessels than were nonmetastatic cells.11,52 Interestingly, polarization of metastatic cells was explained by increased expression of EGF receptor, which made the cells chemotactic to EGF released by macrophages lining blood vessels. Furthermore, individual metastatic cells were seen intravasating into blood vessels using an amoeboid form of movement. Nonmetastatic cells, however, often fragmented upon crossing endothelial junctions. Consequently, the authors speculated that intravasation was a rate-limiting step for metastasis.
Although this work dealt with a limited number of established cell lines and did not examine lymphatics, it does raise several important considerations about the intravasation process. These considerations include the mode of cell migration utilized (individual amoeboid or fibroblastic movement, versus collective sheet/nest migration); the role of stromal cells in promoting polarized movement; and the effect of hemodynamic shear forces on cell viability—all of which may influence a tumor’s preference for disseminating via blood vessels or lymphatics.
The type of cell movement undertaken is affected, in large part, by the surrounding extracellular matrix (ECM) and by the integrity of cell-cell junctions.14 Mesenchymal, or fibroblast-like, single cell migration tends to occur when mature, integrin-containing focal contacts develop in the presence of dense matrix networks. Amoeboid migration is favored under less adhesive conditions, as is often seen in vivo or in three-dimensional cultures, when focal contacts are lacking.53 The speed of amoeboid migration is about 10–30 times faster than mesenchymal migration and is protease-independent.14,53 Given that lymphatic vessels lack basement membranes, and that ECM networks are likely less dense around peritumoral lymphatics than around intratumoral blood vessels, this would seem to favor rapid and efficient amoeboid-type intravasation into lymphatic circulation. Lymphatic permeability may also allow passage of cell aggregates that have retained expression of homotypic cell-cell adhesion receptors such as cadherins.54
In addition, active recruitment of tumor cells towards lymphatics may occur via EGF-EGFR-mediated chemotaxis, since macrophages have been found in proximity to lymphatic vessels.55,56 In one study, macrophages were even reported to transdifferentiate into lymphatics in response to inflammation in an eye cornea model,57 though the generality of this finding remains to be determined. Lymphatic stromal cells have also been reported to be a source of EGF and IGF-I.58 In addition, lymph node secretion of chemokines such as SCL/CCL21 and CCL1 may attract tumor cells that express the receptors CCR7 and CCR8, respectively.59 Overexpression of CCR7 in B16 melanoma cells has been shown to increase lymph node metastasis,60 and others have reported that breast cancer cells or melanomas expressing CXCR4 may actively home to lymph nodes containing CXCL12/SDF-1 ligand.61 Activated cancer-associated fibroblasts may also secrete chemokines that enhance tumor growth and invasion.62
Lastly, although intravasation into lymphatics may seem to be favored due to reduced shear stress inflicted upon the cell, increased hemodynamic flow rate may also help dislodge individual cells from the primary tumor. Disaggregation of cells under flow has been reported to be affected, at least in part, by levels of E-cadherin expression.54
HOW DO TUMOR CELLS REACH SYSTEMIC CIRCULATION?
Viable tumor cells have been isolated in the blood of patients bearing nearly all types of cancer, including the most common forms of carcinomas.63 Although the amount of time a tumor cell spends circulating throughout the body is believed to be short, the sheer number of cells potentially available for seeding distant metastases makes it imperative for us to understand how tumors gain initial access to systemic circulation.
In many clinical studies involving different human tumors, a positive association between lymphatic and hematogenous metastasis has generally been observed. For instance, Bubendorf et al reported that 84% of patients with node-positive prostate cancer bore evidence of hematogenous dissemination, as opposed to 16% of patients without local lymph node spread.64 In breast cancer, lymph node metastasis has been linked with poor prognosis and distant metastasis,65 and similar observations have also been noted in pancreatic cancer,66 ovarian cancer,67 and head and neck cancer,68 among others.
Recently, we observed a strong correlation between lymphatic and hematogenous dissemination in a mouse orthotopic xenograft model of prostate cancer (Fig. 2).30 As expected, the lymph nodes directly draining the prostate, the para-aortic/sub-lumbar lymph nodes, were invaded first by the tumors, followed by the more distant sub-renal lymph nodes. Mice that bore tumors which had not formed macroscopic metastases in the lumbar lymph nodes (~50% of mice) also did not possess renal lymph node macro-metastases. Not surprisingly, the appearance of lung micrometastases was well-correlated with the detection of viable circulating tumor cells in the blood. Interestingly, however, significant numbers of lung metastases and circulating cells were found only in mice that possessed both renal and lumbar lymph node metastases, regardless of primary tumor size.
Figure 2.
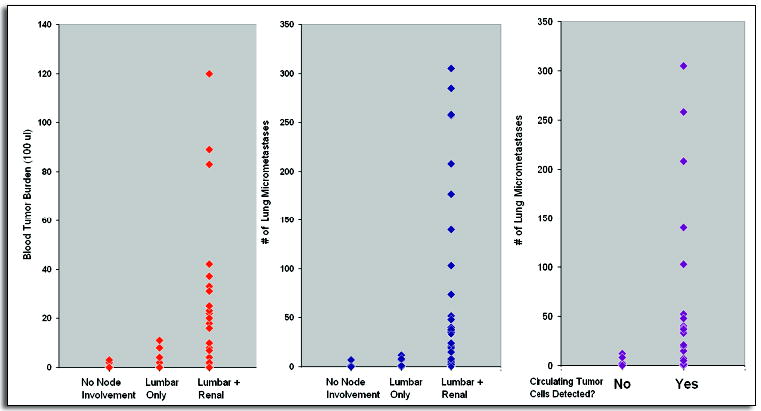
After surgical orthotopic implantation of human prostate PC-3 cells into nude mice, associations were observed among lymph node metastasis, circulating tumor cells and lung micrometastases. Left, significant numbers of circulating tumor cells in the blood were detected only in mice that bore macrometastases in both the lumbar and renal lymph nodes. Middle, similarly, most lung metastases were seen in mice with both lymph node sites invaded. Right, lung metastases were correlated with the presence of circulating tumor cells in the blood.
(Reproduced with permission from ref. 30).
These clinical and experimental correlations can be interpreted in at least a couple of ways (Fig. 3). It is possible that some tumors may be unable to intravasate directly into blood vessels; thus they must establish satellite lymph node metastases first to disperse metastatic cells via the thoracic duct. Another possibility is that the primary tumors may be completely noninvasive until somehow triggered to metastasize via both lymphatics and blood vessels simultaneously. Either possibility would potentially yield an apparent correlation between lymphatic and hematogenous spread. But in the case of human patients, those with node-positive tumors at the time of diagnosis might be free of distal metastases in the first scenario but not in the second.
Figure 3.
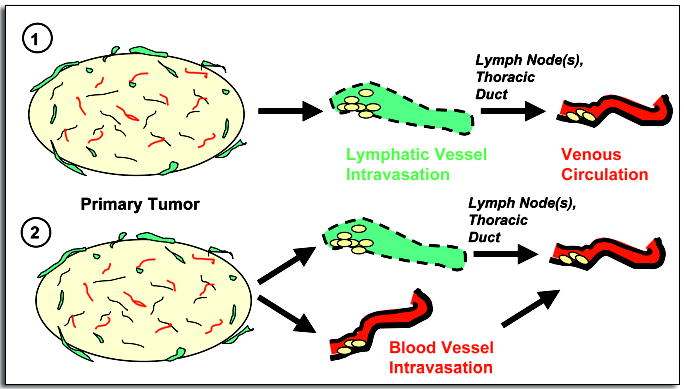
Two possible pathways for metastasis could explain why, in a mouse model of prostate cancer, hematogenous spread is observed only in the presence of significant lymphatic spread. (1) The tumors might be incapable of intravasating directly into blood vessels, so metastatic cells enter venous circulation indirectly via lymphatics (“metachronous seeding”). Or, the tumor is completely nonmetastatic until mobilized to metastasize via both lymphatic and hematogenous routes at the same time (2).
In support of the former possibility, Sleeman has noted that the physiology of lymph nodes may actually favor formation of local metastases that could serve as “bridgeheads” for further dissemination.69 The low shear flow of lymphatic fluid coupled with the filtering of cells into a confined space—the subcapsular sinuses—may increase the local concentration of tumor cell aggregates in the node. This would be in contrast to the “scatter-shot” dispersal of individual tumor cells into large capillary beds such as the lung, where metastatic progression after seeding is known to be highly inefficient.1 Indeed, increased cell aggregation has been previously found to enhance formation of experimental metastases.70 Furthermore, according to Sleeman, tumor cells that have arrived in the subcapsular sinuses would not need to extravasate.69
Others have proposed that lymph nodes may act as initial “selection” sites where tumor cells with partial metastatic competence could seed and expand, while selecting for increasingly malignant variants that could later spread to more distant sites.32 This would agree with hypotheses previously set forth that metastatic cells are similar to, but also different from, their primary tumors of origin.7,9
If entrance into systemic circulation were dependent on lymphatics, experimental inhibition of lymph node metastasis should also inhibit hematogenous spread. But, while some have indeed reported such results,32,34,36 others found that inhibiting lymph node metastasis had no effect on lung metastasis.31,37,71 These findings are likely attributable to differences in the cell lines utilized and whether the cells were implanted orthotopically or ectopically. In another study, resection of MT-100-TC mammary carcinomas along with draining lymph nodes prevented metastatic recurrence, but removal of the primary tumors alone did not.72 This would suggest that MT-100-TC cells reached systemic circulation via lymphatics, a progression the authors termed “metachronous seeding.”
In contrast, the presence of hematogenous metastases in the absence of lymphatic spread would clearly indicate direct dispersal of tumor cells into blood vessels. This is a likely scenario for patients harboring bone marrow micrometastases in the absence of other detectable signs of spread, which has been reported to occur in 20–40% of carcinomas.73 Interestingly, comparative genomic hybridization (CGH) analyses have suggested that malignant cells may disseminate through the blood very early in breast cancer.74,75 These cells were also found to be distinct from lymph node metastases by CGH, thus arguing against metachronous seeding.74
Lastly, in patients harboring both lymphatic and hematogenous metastases, assessing the order of events remains difficult. One possible experimental approach to determine whether distant metastases arise directly from the primary tumor or indirectly from lymph nodes might be to construct a detailed time course tracking the relative temporal appearance of tumor cells in the blood and lymph nodes. If hematogenous spread occurs via lymphatics, for instance, malignant cells should appear in lymph nodes before blood. Such an approach could be coupled with methods such as CGH74 or gene expression profiling76 to track dispersed tumor cells. Detailed genomic analyses comparing primary tumors with micrometastases isolated from lymph nodes and/or distant sites should be able to distinguish the pathways undertaken for metastasis.
CONCLUSIONS
A confluence of factors likely influences whether primary tumors metastasize via blood vessel or lymphatic routes and, related to that, how tumor cells reach the systemic circulation. Differentiation programs innate to the cell of origin of each tumor may predetermine the metastatic phenotype, though additional genetic or epigenetic changes may also affect a cell’s ability to intravasate. Morphological differences between blood vessels and lymphatics will almost certainly affect the initial route of spread, and in this regard, peritumoral lymphatics might be considered a default pathway for tumors incapable of crossing blood endothelial boundaries. However, active mechanisms for attracting tumor cells towards one type of vasculature versus another cannot be discounted. In addition, the roles played by inflammatory77 and host hematopoietic precursor cells78 in affecting the process will need to be further examined.
At the same time, improved imaging techniques should allow simultaneous visualization of blood vessel and lymphatic intravasation within the same tumor, allowing direct measurements of the relative frequencies of each occurrence. In addition, genomic approaches combined with clustering algorithms should be able to elucidate molecular relationships between disseminated tumor cells and cells derived from the primary tumor and/or lymph node metastases. These studies will likely yield detailed information about how and when metastatic cells leave the primary tumor. Lastly, identification and validation of genes and proteins that affect the intravasation process and perhaps specify whether a tumor invades via blood vessel or lymphatic routes, as has been recently proposed,79 will have valuable clinical implications for prognosis and treatment.
Acknowledgments
The authors thank S. Astrof and L. Xu for critical reading of this manuscript, and acknowledge support from the NIH (RO1CA17007); the Virginia and D.K. Ludwig Fund for Cancer Research; the Prostate Cancer Foundation; and the Howard Hughes Medical Institute. S.Y. Wong was further supported by an NIGMS Predoctoral Training Grant to the MIT Biology Department and by a Koch Research Fellowship from the Center for Cancer Research.
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency. Am J Pathol. 1998;153:865–73. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 5.Veer LJ, Dai H, Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, Kooy K, Marton MJ, Kitteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–5. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 6.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;6:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Hynes RO. Metastatic potential: Genetic predisposition of the primary tumor or rare, metastatic variants-or both? Cell. 2003;113:821–3. doi: 10.1016/s0092-8674(03)00468-9. [DOI] [PubMed] [Google Scholar]
- 10.Butler TP, Gullino PM. Quantification of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:3512–6. [PubMed] [Google Scholar]
- 11.Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, Graf T, Pollard JW, Segall J, Condeelis J. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 12.Condeelis J, Segall J. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3:921–30. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- 13.Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nat Genet. 2005;37:1047–54. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 15.Thiery J. Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 16.Kurrey NK,AK, Bapat SA. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol. 2005;97:155–65. doi: 10.1016/j.ygyno.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 17.Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–94. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- 18.Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci USA. 2003;100:8430–5. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 20.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 21.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413–23. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 22.Wittekind C. Diagnosis and staging of lymph node metastasis. Recent Results Cancer Res: Lymphatic Metastasis and Sentinel Lymphonodectomy. 2000;157:20–8. doi: 10.1007/978-3-642-57151-0_3. [DOI] [PubMed] [Google Scholar]
- 23.Smith JA, Seaman JP, Gleidman JB, Middleton RG. Pelvic lymph node metastasis from prostatic cancer: Influence of tumor grade and stage in 452 consecutive patients. J Urol. 1982;130:290–2. doi: 10.1016/s0022-5347(17)51112-x. [DOI] [PubMed] [Google Scholar]
- 24.Smerage JB, Hayes DF. The measurement and therapeutic implications of circulating tumour cells in breast cancer. Br J Cancer. 2005;94:1–5. doi: 10.1038/sj.bjc.6602871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su D, Yamaguchi K, Tanaka M. The characteristics of disseminated tumor cells in pancreatic cancer: A black box needs to be explored. Pancreatology. 2005;5:316–24. doi: 10.1159/000086532. [DOI] [PubMed] [Google Scholar]
- 26.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Haynes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 27.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–27. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 28.Gimbrone MA, Leapman SB, Cotran RS, Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972;136:261–76. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrara N. VEGF and the quest for tumor angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 30.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–98. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor-3 signaling. J Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–22. [PubMed] [Google Scholar]
- 33.Shimizu K, Kubo H, Yamaguchi K, Kawashima K, Ueda Y, Matsuo K, Awane K, Shimahara Y, Takabayashi A, Yamaoka Y, Satoh S. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328–33. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–11. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- 35.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–90. [PubMed] [Google Scholar]
- 36.Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- 37.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 38.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–53. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 39.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maula S, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–6. [PubMed] [Google Scholar]
- 41.Alitalo K, Mohla S, Ruoslahti E. Lymphangiogenesis and cancer: Meeting report. Cancer Res. 2004;64:9225–9. doi: 10.1158/0008-5472.CAN-04-2475. [DOI] [PubMed] [Google Scholar]
- 42.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–6. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 43.Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: Cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 44.Isaka N, Padera TP, Hagendoorn J, Fukumura D, Jain RK. Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res. 2004;64:4400–4. doi: 10.1158/0008-5472.CAN-04-0752. [DOI] [PubMed] [Google Scholar]
- 45.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain R. Absence of functional lymphatics within a murine sarcoma: A molecular and functional evaluation. Cancer Res. 2000;60:4324–7. [PubMed] [Google Scholar]
- 46.Jain RK, Padera TP. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. J Natl Cancer Inst. 2002;94:785–7. doi: 10.1093/jnci/94.11.785. [DOI] [PubMed] [Google Scholar]
- 47.Crnic I, Strittmatter K, Cavallaro U, Kopfstein L, Jussila L, Alitalo K, Christofori G. Loss of neural cell adhesion molecule induces tumor metastasis by upregulating lymphangiogenesis. Cancer Res. 2004;64:8630–8. doi: 10.1158/0008-5472.CAN-04-2523. [DOI] [PubMed] [Google Scholar]
- 48.Trojan L, Michel MS, Rensch F, Jackson DG, Alken P, Grobholz R. Lymph and blood vessel architecture in benign and malignant prostatic tissue: Lack of lymphangiogenesis in prostate carcinoma assessed with novel lymphatic marker lymphatic vessel endothelial hyaluronan receptor (LYVE-1) J Urol. 2004;172:103–7. doi: 10.1097/01.ju.0000128860.00639.9c. [DOI] [PubMed] [Google Scholar]
- 49.Vleugel MM, Bos R, van der Groep P, Greijer AE, Shvarts A, Stel HV, van der Wall E, van Diest PJ. Lack of lymphangiogenesis during breast carcinogenesis. J Clin Pathol. 2004;57:746–51. doi: 10.1136/jcp.2003.014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoral lymph vessels in human metastatic breast cancer. J Pathol. 2003;200:195–206. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 51.Sipos B, Kojima M, Tiemann K, Klapper W, Kruse ML, Kalthoff H, Schniewind B, Tepel J, Weich H, Kerjaschki D, Kloppel G. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J Pathol. 2005;207:301–12. doi: 10.1002/path.1840. [DOI] [PubMed] [Google Scholar]
- 52.Wyckoff JB, Jones JG, Condeelis JS, Segall JE. A critical step in metastasis: In vivo analysis of intravasation at the primary tumor. Cancer Res. 2000;60:2504–11. [PubMed] [Google Scholar]
- 53.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–8. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 54.Byers SW, Sommers CL, Hoxter B, Mercurio AM, Tozeren A. Role of E-cadherin in the response of tumor cell aggregates to lymphatic, venous and arterial flow: Measurement of cell-cell adhesion strength. J Cell Sci. 1995;108:2053–64. doi: 10.1242/jcs.108.5.2053. [DOI] [PubMed] [Google Scholar]
- 55.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Rooijen NV, Takenaka H, D’Amore PA, Stein-Streilen J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–72. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeBedis C, Chen K, Fallavollita L, Boutros T, Brodt P. Peripheral lymph node stromal cells can promote growth and tumorigenicity of breast carcinoma cells through the release of IGF-I and EGF. Int J Cancer. 2002;100:2–8. doi: 10.1002/ijc.10481. [DOI] [PubMed] [Google Scholar]
- 59.Homey B, Muller A, Zlotnik A. Chemokines: Agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;3:175–84. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 60.Wiley HE, Gonzalez EB, Maki W, Wu M, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–43. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 61.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 62.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 63.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 64.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–82. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 65.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early-stage breast cancer. Oncologist. 2004;9:606–16. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida T, Matsumoto T, Sasaki A, Shibata K, Aramaki M, Kitano S. Outcome of paraaortic node-positive pancreatic head and bile duct adenocarcinoma. Am J Surg. 2004;187:736–40. doi: 10.1016/j.amjsurg.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 67.Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Stoler MH, Beecham JB, Bonfiglio TA. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988;19:57–63. doi: 10.1016/s0046-8177(88)80316-2. [DOI] [PubMed] [Google Scholar]
- 68.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71:452–6. doi: 10.1002/1097-0142(19930115)71:2<452::aid-cncr2820710228>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 69.Sleeman J. The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res. 2000;157:55–81. doi: 10.1007/978-3-642-57151-0_6. [DOI] [PubMed] [Google Scholar]
- 70.Glinsky VV, Glinsky GV, Glinsky OV, Huxley VH, Turk JR, Mossine VV, Deutscher SL, Pienta KJ, Quinn TP. Intravascular metastatic cancer cell homotypic aggregation at the sites of primary attachment to the endothelium. Cancer Res. 2003;63:3805–11. [PubMed] [Google Scholar]
- 71.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–40. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 72.Ward PM, Weiss L. Metachronous seeding of lymph node metastases in rats bearing the MT-100-TC mammary carcinoma: The effect of elective lymph node dissection. Breast Cancer Res Treat. 1989;14:315–20. doi: 10.1007/BF01806303. [DOI] [PubMed] [Google Scholar]
- 73.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA. From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003;100:7737–42. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schardt JA, Meyer M, Hartmann CH, Schubert F, Schmidt-Kittler O, Fuhrmann C, Polzer B, Petronio M, Eils R, Klein CA. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–39. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 76.Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LW, O’Hara SM. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–7. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- 77.Schoppmann SF, Schindl M, Breiteneder-Geleff S, Soleiman A, Breitenecker G, Karner B, Birner P. Inflammatory stromal reaction correlates with lymphatic microvessel density in early-stage cervical cancer. Anticancer Res. 2001;21:3419–24. [PubMed] [Google Scholar]
- 78.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woelfle U, Cloos J, Sauter G, Riethdorf L, Janicke F, van Diest P, Brakenhoff R, Pantel K. Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res. 2003;63:5679–84. [PubMed] [Google Scholar]


