Abstract
Sphingosine, at 5–15 mol % total lipids, remarkably increases the permeability to aqueous solutes of liposomal and erythrocyte ghost membranes. The increased permeability cannot be interpreted in terms of leakage occurring at the early stages of a putative membrane solubilization by sphingosine, nor is it due to a sphingosine-induced generation of nonlamellar structures, or flip-flop lipid movement. Instead, sphingosine stabilizes (rigidifies) gel domains in membranes, raising their melting temperatures and increasing the transition cooperativity. Structural defects originating during the lateral phase separation of the “more rigid” and “less rigid” domains are likely sites for the leakage of aqueous solutes to the extravesicular medium. The presence of coexisting domains in the plasma membrane makes it a target for sphingosine permeabilization. The sphingosine-induced increase in rigidity and breakdown of the plasma membrane permeability barrier could be responsible for some of the physiological effects of sphingosine.
INTRODUCTION
Sphingosine ((2S, 3R, 4E)-2-amino-4-octadecen-1,3-diol) is the most common sphingoid long chain base in sphingolipids. It is the precursor of important cell signaling molecules, such as ceramides, through amide binding of a fatty acid to the C2 amino group, and sphingosine-1-phosphate (see review in Futerman and Hannun (1)). In addition, sphingosine is a “bioactive lipid” itself. It has been found to inhibit protein kinase C in human platelets (2), to inhibit phosphatidate phosphohydrolases in rat hepatocytes (3), and to activate PKH protein kinases in yeast endocytosis (4).
As with most bioactive lipids, sphingosine can, in principle, exert its effects either through specific binding to an enzyme, or indirectly, through changes in cell membrane properties. This dual possibility has been explored in detail for ceramides (5,6) but not for sphingosine, whose structural effects on the lipid bilayer have received less attention. Most of what we know of the biophysical properties of sphingosine comes from the studies by the groups of Gómez-Fernández (7–10) and Kinnunen (11–15). The former authors observed, using 31P-NMR, that sphingosine in membranes has an apparent pKa = 8.9, thus it is positively charged under physiological conditions (8). The interaction of fully protonated sphingosine (pH 6.0) with dipalmitoylphosphatidylcholine (DPPC) and dielaidoylphosphatidylethanolamine (DEPE) bilayers was described in detail (7) using differential scanning calorimetry (DSC) and 31P-NMR.
In mixtures with either diacylphosphatidylcholines (7,11,13) or phosphatidylserine (PS) (8,11), DSC, NMR, and surface pressure measurements revealed that sphingosine rigidified the membranes, forming azeotropic mixtures. Moreover, at neutral pH, being positively charged, sphingosine neutralized the electrostatic charge of PS, and prevented this phospholipid from binding cations such as Ca2+ (9). Because of its cationic nature at neutral pH, sphingosine has been used in the preparation of positively charged liposomes for DNA transfection (12). Previously, Kinnunen et al. had demonstrated that the association between DNA and sphingosine could be reverted by acidic phospholipids (14). Sphingosine has also been found to promote binding of the assembly factor P17 from bacteriophage PRD1 to lipid bilayers (13). Mustonen et al. (15) showed very intriguing, apparently electrostatically mediated effects of sphingosine on the interactions of proteins (phospholipase A2, cytochrome c) with membranes. Furthermore, a recent report (16) indicates that sphingosine, like ceramide, may increase the permeability of model and cell membranes. The membrane-permeabilizing effects of ceramide were first described by Ruiz-Argüello et al. (17) and later confirmed by further work by Siskind et al. (16) and by ourselves (18–20).
This report deals with the amphiphillic properties of sphingosine, particularly its permeabilizing effects in lipid vesicles and erythrocyte ghosts. These effects are attributed to a peculiar property of sphingosine when interacting with certain lipid mixtures, namely the stabilization of high-melting domains. The concomitant generation of interfaces between rigid and fluid domains would be at the origin of the observed increased permeability.
MATERIALS AND METHODS
Materials
Egg phosphatidylcholine (PC) and egg phosphatidylethanolamine were from Lipid Products (Redhill, UK); cholesterol (Ch) and 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO, free radical) were purchased from Sigma (St. Louis, MO); egg ceramide, D-erythro-sphingosine, egg sphingomyelin (SM), and 1,2-dielaidoyl-sn-glycero-3-phosphocholine (DEPC) were obtained from Avanti Polar Lipids (Alabaster, AL). 1,6-Diphenyl-1,3,5-hexatriene (DPH) was from Molecular Probes (Eugene, OR). Ethanol was obtained from Prolabo (Paris, France).
8-Aminonaphthalene-1,3,6-trisulfonic acid (ANTS) and p-xylenebis (pyridinium bromide) (DPX) were supplied by Molecular Probes.
Liposome preparation
Lipids were dissolved in chloroform/methanol, 2:1 (v/v), at the desired molar ratios. The lipid was deposited as a film on the wall of a glass test tube by solvent evaporation under nitrogen. Final traces of solvent were removed for 2 h in a vacuum chamber. The lipid film was suspended in the appropriate HEPES buffer (10 mM HEPES, 200 mM NaCl, 10 mM CaCl2, 2 mM Mgcl2, pH 7) by vortexing at room temperature to form multilamellar vesicles. To ensure homogeneous dispersion, the hydrated samples were extruded between two syringes through a narrow tubing (0.5 mm internal diameter) 100 times at 45°C. The lipid suspensions were further processed through 10 cycles of freezing and thawing, followed by 10 passes through two polycarbonate filters (Nuclepore, Pleasanton, CA), 0.1 μm pore diameter, in an extruder at room temperature. For assays of vesicle and erythrocyte ghost efflux, the buffer contained ANTS and DPX (21). Nonencapsulated fluorescent probes were separated from the vesicle suspension using a Sephadex G-75 gel filtration column (Pharmacia, Uppsala, Sweden). Solution osmolarities were measured using an Osmomat 030 instrument (Gonotec, Berlin, Germany). The resulting vesicles had an average diameter of 120–150 nm, depending on lipid compositions. Liposomes (large unilamellar vesicles (LUVs)) were kept on ice and used immediately after preparation.
Ghost membrane preparation
Human erythrocyte ghost membranes were obtained by a modified Steck and Kant method (22). Briefly, the membranes obtained from 20 ml of erythrocyte concentrate (as provided by the blood bank) were washed three times by centrifugation in 0.9% NaCl, and the pellet was resuspended in cold 1.3 mM acetic acid, 4 mM MgSO4, pH 3.2, buffer, left for 30 min at 4°C, and centrifuged. In the next step, the pellet was suspended in 3 volumes of 10 mM HEPES, 200 mM NaCl, 10 mM CaCl2, 2 mM MgCl2, 20 mM ANTS, and 90 mM DPX, pH 7,4. followed by 10 cycles of freezing and thawing. Finally, the membranes were stabilized and allowed to reseal by incubating overnight at 37°C. The membranes were washed three times by centrifugation (20 min, 48,000 × g, 4°C) in 10 mM HEPES, 200 mM NaCl, 10 mM Cacl2, 2mM MgCl2, pH 7.4 buffer to separate the nonencapsulated fluorescent probes from the membranes. Finally, the pellet was resuspended in 2 ml HEPES buffer. The resulting membranes had an average diameter of 300–310 nm, according to quasielastic light scattering measurements performed with a Zetasizer4 instrument (Malvern Instruments, Malvern, UK). Membranes from erythrocytes ghosts were kept on ice and used immediately after preparation.
Differential scanning calorimetry
Samples for DSC were prepared by mixing the appropriate lipids in organic solvent that was later evaporated under nitrogen current. The resulting dry lipid films were left under vacuum for at least 2 h to remove solvent traces. The lipids were dispersed in the HEPES buffer at 45°C with shaking. Both lipid suspensions and buffer were degassed before being loaded into the sample or reference cell of an MC-2 high-sensitivity scanning calorimeter (MicroCal, Northampton, MA). Three heating scans, and occasionally a cooling one, at 45°C/h were recorded for each sample. After the fist one, successive heating scans on the same sample always gave superimposable thermograms. Transitions enthalpies were determined using the software ORIGIN (MicroCal) provided with the calorimeter.
Efflux of liposomal and resealed erythrocyte ghosts contents
The efflux of encapsulated solutes was assayed as described by Ellens et al. (23), using ANTS and DPX. The probe-loaded liposomes and erythrocyte ghosts (final lipid concentration 0.3 mM) signal was recorded as 0% efflux, then sphingosine or ceramide was added and the ANTS and DPX efflux was recorded for 7200 s. The reaction was performed at 37°C. Changes in fluorescence intensity were recorded in an Aminco-Bowman (Urbana, IL,) AB-2 spectrofluorometer using 1-ml quartz cuvettes with continuous stirring. Excitation and emission wavelengths were 355 and 520 nm, respectively. An interference filter with a nominal cutoff value of 475 nm was placed in the emission light path to minimize the scattered-light contribution of the vesicles and the erythrocyte ghosts to the fluorescence signal. The percentage of efflux is calculated after all of the fluorescent probe is released by the addition of the nonionic Triton X-100.
Fluorescence polarization assays
The fluorescence polarization of DPH was measured at 37°C, using an SLM 8100 spectrofluorometer, equipped with standard polarization accessories and a circulating water bath. The excitation and emission wavelengths were 360 and 430 nm, respectively.
The fluorescence polarization was calculated as
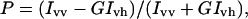 |
where Ivv and Ivh represent the intensity of vertically and horizontally polarized fluorescent light, respectively, when excitation light is vertically polarized. The correction factor is G = Ihv/Ihh. Ihv and Ihh represent the intensity of vertically and horizontally polarized fluorescent light, respectively, when excitation light is horizontally polarized. DPH was added to the phospholipids to obtain a probe/lipid molar ratio of 1/250.
Fluorescence quenching experiments
Quenching of the DPH fluorescence by TEMPO was measured as follows. Lipids, DPH, and (when required) TEMPO were mixed at a 300:1:1 ratio in organic solvent, then the solvent was evaporated, and the mixture was vacuum dried for at least 2 h in the dark. The vesicles were prepared in 20 mM PIPES, 150 mM NaCl, 1 mM EDTA, pH 7.4 as described above. Vesicles with and without TEMPO were prepared. Fluorescence quenching was recorded in an SLM 8100 spectrofluorometer equipped with thermoregulated cell holders. DPH fluorescence was excited at 360 nm; emission was recorded at 428 nm.
RESULTS AND DISCUSSION
Permeability studies
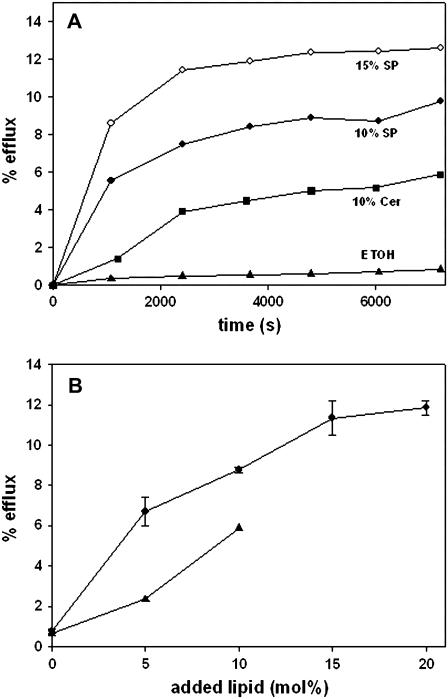
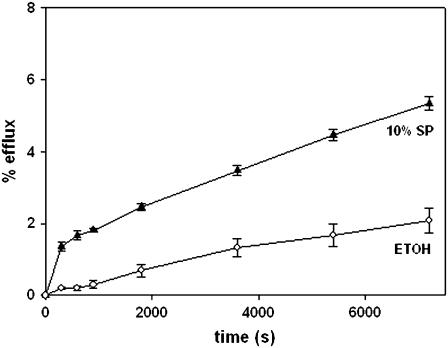
The capacity of sphingosine to increase the permeability of model and cell membranes was tested using the same procedures that had revealed in the past the permeabilizing properties of ceramides (17,20). In fact, egg ceramide was used as a positive control in these studies. Essentially, permeabilization was detected as the efflux of low-molecular weight, water-soluble fluorescent molecules entrapped within the vesicles. LUVs composed of SM/PE/Ch (2:1:1 mol ratio) and containing ANTS/DPX were treated with either sphingosine or egg ceramide dissolved in a very small volume of ethanol, and efflux of vesicle contents was followed by an increase in fluorescence as a function of time, according the procedure described by Montes et al. (20). The results in Fig. 1 A show that, under our conditions, sphingosine was even more active than ceramide in inducing release of ANTS/DPX, whereas ethanol by itself was inactive in this respect. The permeabilizing effect of sphingosine was dose-dependent (Fig. 1 B) and reached a plateau at ∼15 mol %. Qualitatively similar, but less important in magnitude, was the efflux of ANTS/DPX caused by sphingosine in erythrocyte ghosts in which the fluorophore/quencher couple had been entrapped (Fig. 2).
FIGURE 1.
Sphingosine- and ceramide-induced efflux of vesicular aqueous contents. LUVs were prepared, composed of SM/PE/Ch (2:1:1, mol ratio) containing entrapped ANTS/DPX. Sphingosine or ceramide in ethanol were added at time 0. (A) Time course of efflux. (▴) Ethanol (control); (▪) + 10 mol % ceramide; (•) + 10 mol % sphingosine; and (○) + 15 mol % sphingosine. (B) Dose-response curves. Data at time = 6000 s. (▴) Ceramide; (•) sphingosine (average values ± SD, n = 3).
FIGURE 2.
Sphingosine-induced release of ANTS/DPX from resealed erythrocyte ghosts. Sphingosine in ethanol (▴) or pure ethanol as a control (○) were added at time 0. Average values ± SD (n =3).
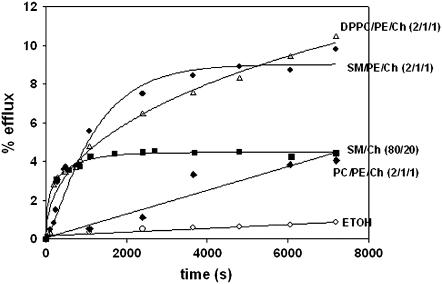
Bilayer composition of LUV was found to be an important factor in these experiments. The data in Fig. 3 show that, although SM could be substituted by DPPC in the mixture with PE and Ch without significant effects, the presence of egg PC allowed only very low rates of release. Rapid release was, however, observed with SM/Ch (80:20 mol ratio) mixtures. The relevance of these observations will become apparent below.
FIGURE 3.
The effect of bilayer lipid composition on vesicle efflux induced by 10 mol % sphingosine. Vesicle composition was: (♦) egg PC/PE/Ch (2:1:1, mol ratio), (•) SM/PE/Ch (2:1:1, mol ratio), (Δ) DPPC/PE/Ch (2:1:1, mol ratio), and (▪) SM/Ch (80:20 mol ratio). (○) Control: effect of ethanol alone on SM/PE/Ch vesicles.
The cause of the increased permeability
The phenomenon under consideration could be explained in terms of i), detergent solubilization effects (24), ii), induction of nonlamellar structures (16,20), or iii), generation of structural defects between rigid and fluid lamellar phases (20,25). These three hypotheses were tested for the case of sphingosine. Detergents are soluble amphiphiles with the capacity to form mixed micelles with membrane lipids and proteins (26,27). The initial stages of membrane solubilization (i.e., micellization) often include the breaking down of the permeability barrier (28). Sphingosine is amphiphilic in nature, and moderately soluble in water. Its critical micellar concentration (cmc) was measured with the 1-anilinonapthaline-8-sulphonate method (29) under our permeability assay conditions (buffer, pH, temperature), and a value of 18 μM was found. Similar cmc have been observed for structurally related molecules, such as palmitoylcarnitine (10 μM) (30) and N-acetylsphingosine (6 μM) (31). However, against what is usually found for detergents (26,28), when permeability was measured as a function of sphingosine concentration, no increase in efflux was observed at the cmc (data not shown). Also, the fact that our systems reached an apparent equilibrium when <20% of the vesicle contents had been released, and that increasing sphingosine concentration beyond 15 mol % had no further effect, speaks against sphingosine-induced vesicle solubilization as the reason for the observed release. Moreover, formation of phospholipid/sphingosine mixed micelles has never been reported, to the authors' knowledge. We conclude that the observed increase in permeability is not due to putative membrane solubilizing properties of sphingosine.
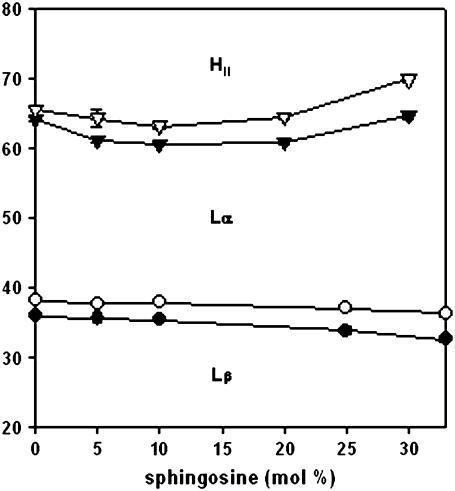
The transient formation of nonlamellar inverted structures in the bilayer has been evoked as an explanation for certain phenomena of efflux. The fact that long-chain ceramides, for instance, facilitate the formation of the inverted hexagonal phase (32) supports this mechanism as the basis for the ceramide-induced increase in permeability. To test this hypothesis for sphingosine, a systematic study of the thermotropic phase transitions of DEPE/sphingosine mixtures at pH 7.4 was performed, using DSC. As a result, a temperature-composition diagram (or partial phase diagram) was obtained, as shown in Fig. 4. The data were very similar to the corresponding region of the phase diagram obtained by López-García et al. (7) at pH 6.0. The main conclusion that was pertinent to our study was that, unlike ceramides, sphingosine hardly displaced the boundaries of the fluid-lamellar-inverted hexagonal phases; in other words, it did not facilitate hexagonal phase formation. In agreement with this, the ability of sphingosine to induce transbilayer (“flip-flop”) lipid motion was tested according to the method of Contreras et al. (33,34), using pyrene-PC and pyrene-PS. In neither case was transbilayer motion detected (data not shown). A degree of flip-flop would have been expected to accompany the formation of nonlamellar structures. Consequently, the experimental evidence did not favor the hypothesis of the induction of nonlamellar structures as the basis for the observed sphingosine-induced increase in permeability. The lack of a major effect on the lamellar-hexagonal phase transition was in agreement with the idea that, as discussed above, sphingosine did not act as a detergent. Detergents usually increase the lamellar-to-inverted hexagonal transition temperature (30).
FIGURE 4.
Temperature-composition diagram for DEPE/sphingosine in excess water, pH 7.4. (•, ○) Onset and completion temperatures of the gel-fluid lamellar phase transition, derived from DSC thermograms. (▾, ∇) Onset and completion temperatures of the lamellar-to-inverted hexagonal phase transition, also derived from DSC thermograms. Lβ, lamellar gel phase Lα, lamellar fluid phase HII, and inverted hexagonal phase. Average of three measurements. The SD are about the size of the symbols.
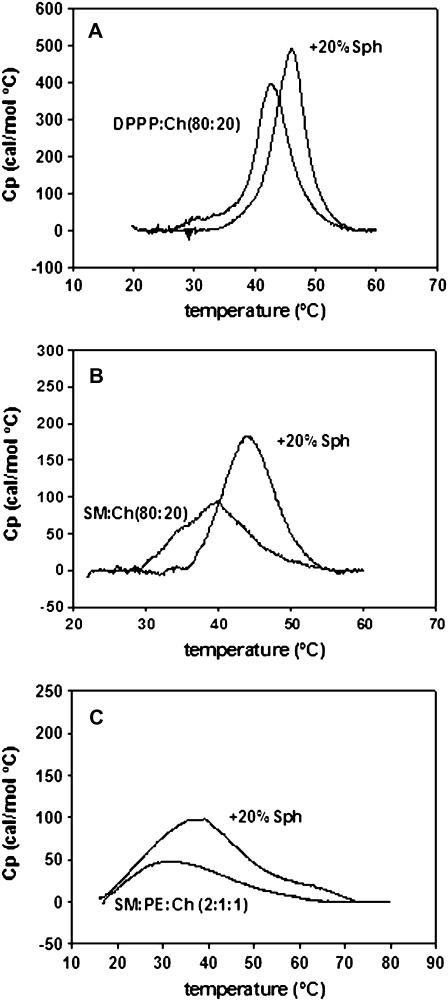
The structural defects originating at the interface between lamellar gel and fluid domains during lateral phase separation were proposed long ago (25) to be responsible for the increased permeability of phospholipid bilayers at the transition temperature. We have proposed a similar explanation, at least as a possibility among others, for ceramide-induced efflux (20,35), particularly in view of the tendency of long-chain ceramides to separate into ceramide-rich, rigid domains in the plane of the membrane. The hypothesis of the sphingosine involvement in the rigidification of membrane domains was tested by DSC. Different systems were tested that had in common a relatively broad gel-fluid transition temperature in the absence of sphingosine, namely DPPC/Ch (80:20, mol ratio), SM/Ch (80:20, mole ratio), and SM/PE/Ch (2:1:1, mole ratio). Note that the same mixtures were used in permeability experiments in Figs. 1 and 3. Typical thermograms for these mixtures are shown in Fig. 5. The same DSC analysis was performed on samples that contained, in addition, 20 mol % sphingosine. The results are also shown in Fig. 5 and the corresponding thermodynamic parameters are given in Table 1. In all cases, sphingosine had the effect of increasing the midpoint transition temperature, increasing the transition enthalpy, and narrowing the transition width. The broad and (particularly for SM-containing mixtures) asymmetric thermograms reveal the presence of heterogeneous microdomains in the bilayers. The interpretation of these data is that, at least for certain lipid compositions, sphingosine mixes with the lipids in the gel phase and gives rise to more rigid, more cooperative domains. During this essentially isothermal, sphingosine-driven transition, more and less fluid domains will coexist. Structural defects will appear at the interfaces between the “more fluid” and the “less fluid” domains, and permeability will be enhanced. Efflux appears to reach an equilibrium after ∼2 h (Figs. 1 and 3). This corresponds probably to the time required for the structural defects in the sphingosine-containing bilayer to anneal, i.e., to reach an equilibrium at constant temperature. There are abundant examples in the literature of molecules that produce a transient perturbation of the membrane bilayer, so that leakage occurs for a while, then it stops when equilibrium is restored. This behavior is found for leakage induced by certain lipids, e.g., the above-mentioned ceramides (33), bacterial protein toxins, e.g., Escherichia coli α-haemolysin (36), or even detergents at subsolubilizing concentrations (24). In contrast, other molecules give rise to stable pores or channels, so that leakage proceeds until the inside and outside solute concentrations reach an equilibrium. This would be the case of, e.g., valinomycin with respect to K+ gradients (37).
FIGURE 5.
Effect of sphingosine on the gel-fluid transition of different lipid mixtures. (A) DPPC/Ch (80:20, mol ratio) ± 20 mol % sphingosine. (B) SM/Ch (80:20, mol ratio) ± 20 mol % sphingosine. (C) SM/PE/Ch (2:1:1, mol ratio) ± 20 mol %sphingosine. Representative thermograms of the second or third scans.
TABLE 1.
Thermodynamic parameters for the gel-fluid phase transitions shown in Fig. 5; average values ± SD (n = 3)
| Sample (mol ratio) | Midpoint transition temperature (°C) | ΔH (kcal/mol) | ΔT1/2 (°C) |
|---|---|---|---|
| DPPC/Ch (80:20) | 42.3 ± 0.61 | 2.8 ± 0.39 | 6.1 ± 0.05 |
| As above + 20 mol % sphingosine | 46.1 ± 0.31 | 3.3 ± 0.40 | 5.7 ± 0.05 |
| SM/Ch (80:20) | 38.9 ± 0.91 | 1.0 ± 0.12 | 10.8 ± 0.32 |
| As above + 20 mol % sphingosine | 43.9 ± 0.34 | 1.3 ± 0.44 | 8.5 ± 0.53 |
| SM/PE/Ch (2:1:1) | 33.0 ± 1.56 | 1.3 ± 0.12 | 27.3 ± 0.30 |
| As above + 20 mol % sphingosine | 37.4 ± 1.60 | 2.3 ± 0.30 | 23.5 ± 0.88 |
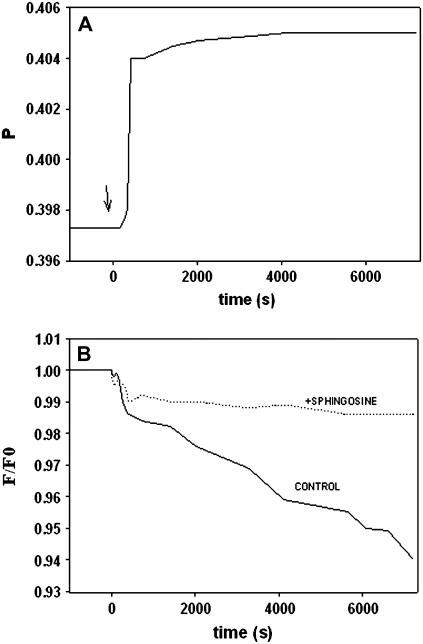
The rigidifying effect of sphingosine was further explored using fluorescence spectroscopic techniques, namely DPH fluorescence polarization and TEMPO-quenching of DPH fluorescence. DPH fluorescence polarization is a well-known method for detecting changes in bilayer order/microviscosity (25). When DPH is incorporated into SM/PE/Ch vesicle bilayers, and its fluorescence polarization is continuously monitored, addition of 10 mol % sphingosine causes an immediate increase in polarization (Fig. 6 A). The increase is small in magnitude (typically of ≈0.01–0.02 polarization units), but highly reproducible. This is interpreted as the bilayer becoming more rigid as a result of sphingosine incorporation. Qualitatively similar results are obtained when vesicles consisting of DPPC/PE/Ch (2:1:1) or of SM/Ch (80:20) are used (data not shown). DPH fluorescence emission intensity can be quenched by the stable radical TEMPO (38). TEMPO partitions into fluid, but not rigid bilayers, and quenches DPH fluorescence (38). With vesicles composed of SM/PE/Ch (2:1:1) that contain DPH in their bilayers, addition of TEMPO causes a slow and steady quenching, i.e., a decrease in fluorescence emission intensity (Fig. 6 B). This slow effect of TEMPO is an indication that the bilayers already have an average low fluidity in the absence of sphingosine (38). However, when sphingosine (10 mol %) is added to the same bilayers, TEMPO is unable to quench DPH fluorescence (Fig. 6 B, dotted line), confirming again the rigidifying effect of sphingosine. Qualitatively similar effects are found with DPPC/PE/Ch (2:1:1), or SM/Ch (80:20) vesicles (results not shown).
FIGURE 6.
Rigidifying effect of sphingosine on vesicle membranes. (A) DPH fluorescence polarization. Vesicles composed of SM/PE/Ch (2:1:1, mol ratio), containing DPH at a 1:250 DPH/lipid mol ratio, were incubated while DPH fluorescence polarization was continuously recorded. Sphingosine (10 mol %) was added at the point indicated by the arrow (time 0). (B) TEMPO quenching of DPH fluorescence emission. Vesicles composed of SM/PE/Ch (2:1:1, mol ratio), containing DPH as above, were incubated while DPH fluorescence emission intensity was recorded. The change in fluorescence is plotted as F/Fo (fluorescence measured in the presence/in the absence of TEMPO). (Solid line) Experiment in the absence of sphingosine. (Dotted line) Experiment in the presence of 10 mol % sphingosine.
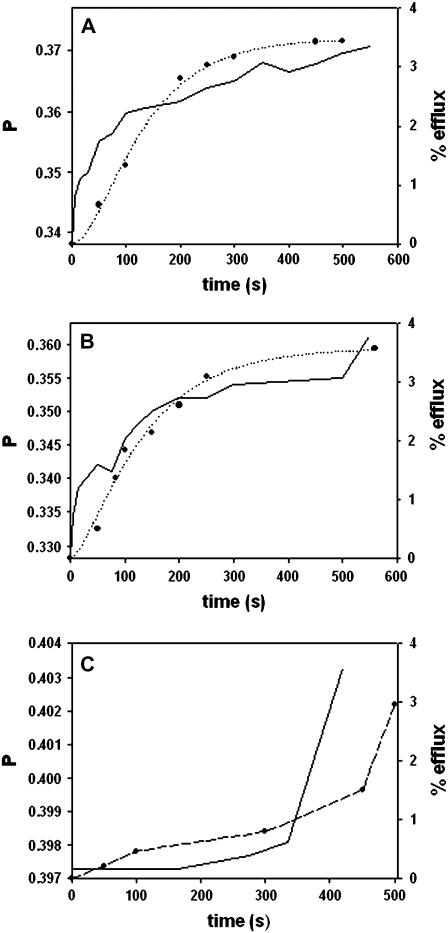
The relationship between the rigidifying effect of sphingosine and sphingosine-induced release of vesicular contents is put forward in Fig. 7, in which the DPH polarization change is plotted in parallel with the initial stages of efflux for three different membrane compositions. The remarkable parallelism of both events, recorded in different experiments, and with different vesicle preparations (DPH-doped LUV for polarization measurements, ANTS/DPX-loaded LUV for efflux assays) supports the idea that both are mechanistically linked. The reason for the delayed onset of the observed phenomena in SM/PE/Ch bilayers (Fig. 7 C) remains to be elucidated. Note that in the fluorescence spectroscopic observations in Figs. 6 and 7, the retrieved data correspond to the summation of signals arising from the many individual probe molecules that may be distributed into different microdomains, each with its own physical properties. Thus our fluorescence data point to an overall rigidification of the bilayers caused by sphingosine, but our inference of coexisting domains in the LUV rely on the calorimetric, rather than on the spectroscopic data.
FIGURE 7.
Parallel behavior of sphingosine-induced rigidification and vesicle efflux. (Solid line) DPH fluorescence polarization. (•) Vesicle efflux. The dotted lines are only meant to guide the eye. Vesicle composition was as follows: (A) DPPC/PE/Ch (2:1:1, mol ratio). (B) SM/Ch (80:20, mol ratio). (C) SM/PE/Ch (2:1:1, mol ratio).
It is important in this context that DPPC, but not egg PC, could substitute SM in bilayers supporting a sphingosine-dependent increase in permeability (Fig. 3). PC/PE/Ch mixtures do not give rise to visible DSC endotherms, under our conditions. This means that no significant gel domains exist in these mixtures. Whether the small degree of efflux induced by sphingosine in PC/PE/Ch vesicles (Fig. 3) is due to formation of (low-cooperativity) domains, or to other causes, remains to be explored.
The tendency of sphingosine to mix with lipids in the gel state has been observed for similar molecules, such as saturated fatty acids (39,40). Sphingosine had been found to rigidify PC and PS membranes (7,8,11,13), and, very recently, also sphingomyelin-rich domains (41). What a well-known detergent such as Triton X-100 has in common with sphingosine are that it mixes well with lipids in the gel state (42) and that it helps create ordered domains in membranes (43), although, as mentioned above, sphingosine cannot solubilize membrane bilayers. Saily et al. (44) performed a Langmuir film balance study in which sphingosine was seen to increase lateral packing of PC monolayers. In our systems, all of which contain cholesterol, sphingosine may exert its gel-stabilizing effect by counteracting the propensity of cholesterol to increase the “negative curvature” (45) of the bilayer. Sphingosine and cholesterol have, in fact, opposed molecular geometries (46) that give them a tendency to favor positive and negative curvatures respectively. The coexistence of both lipids at roughly similar mole ratios may compensate both effects. Siskind et al. (16) noted that ceramides and sphingosine increased membrane permeability through different mechanisms. Although the electrophysiological techniques used by these authors provide data at a very different scale (in time and space) from ours, we concur in suggesting that nonlamellar phase formation is probably an important factor in ceramide-induced efflux of vesicle contents, whereas it is probably irrelevant for sphingosine. Conversely, the reinforcement of rigid domains appears to be the key phenomenon for sphingosine action. The current acceptance of rigid, or at least liquid-ordered, domains in the plasma membrane of cells makes such membranes a target for sphingosine-induced permeabilization, as shown in Fig. 2 for the simple case of the erythrocyte membrane.
Sphingosine is known to have apoptotic and antiproliferative effects on cells (see review in Birbes et al. (47)). These effects are the overall result of a large number of direct and indirect inhibitory and stimulatory actions on individual enzymes, e.g., inhibition of conventional and novel isoforms of protein kinase C (48), stimulation of diacylglycerol kinase (49), or stimulation of phospholipase C (50). The observations in our study open a number of novel possibilities for explaining sphingosine effects at a molecular level. In general, the above enzymes are not known to possess sphingosine-binding sites, but most of them are known to be membrane-bound at some stage in the catalytic cycle (47,50), thus sphingosine effects on fluidity could modify the enzyme activities. In addition, sphingosine-induced permeabilization of the plasma membrane should lead to (localized and transient) alterations of the ion gradients, e.g., Ca2+ entrance into the cytoplasm. These changes in ion concentrations could in turn modify enzyme activities, since many protein kinases C, diacylglycerol kinases, and phospholipases C, among other enzymes, are dependent on Ca2+ for catalysis (51–53). Thus our results may be used as a guide in future studies at the cellular level.
Acknowledgments
The authors are grateful to Dr. M. P. Veiga for recording part of the DSC thermograms, and to Dr. L. R. Montes for making available to them some of her ceramide permeability results.
This work was supported in part by grants from the Spanish Ministerio de Educación y Ciencia (BMC 2002-00784) and the University of the Basque Country (00042.310-13552/2001). F.X.C. and J.S. were predoctoral students supported, respectively, by the Ministerio de Educación y Ciencia and by the Basque government.
References
- 1.Futerman, A. H., and Y. A. Hannun. 2004. The complex life of simple sphingolipids. EMBO Rep. 5:777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannun, Y. A., C. R. Loomis, A. H. Merrill Jr., and R. M. Bell. 1986. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J. Biol. Chem. 261:12604–12609. [PubMed] [Google Scholar]
- 3.Gomez-Munoz, A., E. H. Hamza, and D. N. Brindley. 1992. Effects of sphingosine, albumin and unsaturated fatty acids on the activation and translocation of phosphatidate phosphohydrolases in rat hepatocytes. Biochim. Biophys. Acta. 1127:49–56. [DOI] [PubMed] [Google Scholar]
- 4.Friant, S., R. Lombardi, T. Schmelzle, M. N. Hall, and H. Riezman. 2001. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. EMBO J. 20:6783–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolesnick, R. N., F. M. Goni, and A. Alonso. 2000. Compartmentalization of ceramide signaling: physical foundations and biological effects. J. Cell. Physiol. 184:285–300. [DOI] [PubMed] [Google Scholar]
- 6.van Blitterswijk, W. J., A. H. van der Luit, R. J. Veldman, M. Verheij, and J. Borst. 2003. Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem. J. 369:199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Fernandez, J. C., and J. Villalain. 1998. The use of FT-IR for quantitative studies of the apparent pKa of lipid carboxyl groups and the dehydration degree of the phosphate group of phospholipids. Chem. Phys. Lipids. 96:41–52. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Garcia, F., J. Villalain, and J. C. Gomez-Fernandez. 1995. Effect of sphingosine and stearylamine on the interaction of phosphatidylserine with calcium. A study using DSC, FT-IR and 45Ca2+-binding. Biochim. Biophys. Acta. 1236:279–288. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Garcia, F., J. Villalain, and J. C. Gomez-Fernandez. 1994. A phase behavior study of mixtures of sphingosine with zwitterionic phospholipids. Biochim. Biophys. Acta. 1194:281–288. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Garcia, F., V. Micol, J. Villalain, and J. C. Gomez-Fernandez. 1993. Interaction of sphingosine and stearylamine with phosphatidylserine as studied by DSC and NMR. Biochim. Biophys. Acta. 1153:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Koiv, A., P. Mustonen, and P. K. Kinnunen. 1993. Influence of sphingosine on the thermal phase behaviour of neutral and acidic phospholipid liposomes. Chem. Phys. Lipids. 66:123–134. [DOI] [PubMed] [Google Scholar]
- 12.Paukku, T., S. Lauraeus, I. Huhtaniemi, and P. K. Kinnunen. 1997. Novel cationic liposomes for DNA-transfection with high efficiency and low toxicity. Chem. Phys. Lipids. 30:23–29. [DOI] [PubMed] [Google Scholar]
- 13.Holopainen, J. M., M. Saily, J. Caldentey, and P. K. Kinnunen. 2000. The assembly factor P17 from bacteriophage PRD1 interacts with positively charged lipid membranes. Eur. J. Biochem. 267:6231–6238. [DOI] [PubMed] [Google Scholar]
- 14.Kinnunen, P. K., M. Rytomaa, A. Koiv, J. Lehtonen, P. Mustonen, and A. Aro. 1993. Sphingosine-mediated membrane association of DNA and its reversal by phosphatidic acid. Chem. Phys. Lipids. 66:75–85. [DOI] [PubMed] [Google Scholar]
- 15.Mustonen, P., J. Lehtonen, A. Koiv, and P. K. Kinnunen. 1993. Effects of sphingosine on peripheral membrane interactions: comparison of adriamycin, cytochrome c, and phospholipase A2. Biochemistry. 32:5373–5380. [DOI] [PubMed] [Google Scholar]
- 16.Siskind, L. J., S. Fluss, M. P. Bui, and M. Colombini. 2005. Mitochondrial sphingolipids and the induction of apoptosis. Biophys. J. 88:193A. (Abstr.) [Google Scholar]
- 17.Ruiz-Arguello, M. B., G. Basanez, F. M. Goni, and A. Alonso. 1996. Different effects of enzyme-generated ceramides and diacylglycerols in phospholipid membrane fusion and leakage. J. Biol. Chem. 271:26616–26621. [DOI] [PubMed] [Google Scholar]
- 18.Siskind, L. J., R. N. Kolesnick, and M. Colombini. 2002. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J. Biol. Chem. 277:26796–26803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siskind, L. J., and M. Colombini. 2000. The lipids C2- and C16-ceramide form large stable channels. Implications for apoptosis. J. Biol. Chem. 275:38640–38644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montes, L. R., M. B. Ruiz-Argüello, F. M. Goñi, and A. Alonso. 2002. Membrane restructuring via ceramide results in enhanced solute efflux. J. Biol. Chem. 277:11788–11794. [DOI] [PubMed] [Google Scholar]
- 21.Nieva, J. L., F. M. Goñi, and A. Alonso. 1989. Liposome fusion catalytically induced by phospholipase C. Biochemistry. 28:7364–7367. [DOI] [PubMed] [Google Scholar]
- 22.Steck, T. L., and J. A. Kant. 1974. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 31:172–180. [DOI] [PubMed] [Google Scholar]
- 23.Ellens, H., J. Bentz, and F. C. Szoka. 1985. H+ and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 24:3099–3106. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz, J., F. M. Goni, and A. Alonso. 1988. Surfactant-induced release of liposomal contents. A survey of methods and results. Biochim. Biophys. Acta. 937:127–134. [DOI] [PubMed] [Google Scholar]
- 25.Papahadjopoulos, D., K. Jacobson, S. Nir, and T. Isac. 1973. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 311:330–348. [DOI] [PubMed] [Google Scholar]
- 26.Helenius, A., and K. Simons. 1975. Solubilization of membranes by detergents. Biochim. Biophys. Acta. 415:29–79. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenberg, D., F. M. Goni, and H. Heerklotz. 2005. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30:430–436. [DOI] [PubMed] [Google Scholar]
- 28.Urbaneja, M. A., F. M. Goni, and A. Alonso. 1988. Structural changes induced by Triton X-100 on sonicated phosphatidylcholine liposomes. Eur. J. Biochem. 173:585–588. [DOI] [PubMed] [Google Scholar]
- 29.Slavik, J. 1982. Anilinonaphthalene sulphonate as a probe of membrane composition and function. Biochim. Biophys. Acta. 694:1–25. [DOI] [PubMed] [Google Scholar]
- 30.Goñi, F. M., A. Requero, and A. Alonso. 1996. Palmitoylcarnitine, a surface-active metabolite. FEBS Lett. 390:1–5. [DOI] [PubMed] [Google Scholar]
- 31.Sot, J., F. M. Goñi, and A. Alonso. 2005. Molecular associations and surface-active properties of short-and long-N-acyl chain ceramides. Biochim. Biophys. Acta. 1711:12–19. [DOI] [PubMed] [Google Scholar]
- 32.Veiga, M. P., J. L. Arrondo, F. M. Goni, and A. Alonso. 1999. Ceramides in phospholipid membranes: effects on bilayer stability and transition to nonlamellar phases. Biophys. J. 76:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contreras, F. X., G. Basanez, A. Alonso, A. Herrmann, and F. M. Goni. 2005. Asymmetric addition of ceramides but not dihydroceramides promotes transbilayer (flip-flop) lipid motion in membranes. Biophys. J. 88:348–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Contreras, F. X., A. V. Villar, A. Alonso, R. N. Kolesnick, and F. M. Goni. 2003. Sphingomyelinase activity causes transbilayer lipid translocation in model and cell membranes. J. Biol. Chem. 278:37169–37174. [DOI] [PubMed] [Google Scholar]
- 35.Goni, F. M., and A. Alonso. 2002. Sphingomyelinases: enzymology and membrane activity. FEBS Lett. 531:38–46. [DOI] [PubMed] [Google Scholar]
- 36.Cortajarena, A. L., F. M. Goñi, and H. Ostolaza. 2003. Asp-863 is a key residue for calcium-dependent activity of Escherichia coli RTX toxin alpha-haemolysin. FEBS Lett. 546:241–245. [DOI] [PubMed] [Google Scholar]
- 37.Krizo, J., E. Makrlik, and P. Vanura. 2006. NMR evidence of a valinomycin-proton complex. Biopolymers. 81:104–109. [DOI] [PubMed] [Google Scholar]
- 38.Sot, J., L. A. Bagatolli, F. M. Goñi, and A. Alonso. 2006. Detergent-resistant, ceramide-enriched domains in sphingomyelin/ceramide bilayers. Biophys. J. 90:903–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortiz, A., and J. C. Gomez-Fernandez. 1987. A differential scanning calorimetry study of the interaction of free fatty acids with phospholipid membranes. Chem. Phys. Lipids. 45:75–91. [DOI] [PubMed] [Google Scholar]
- 40.Micol, V., A. Ortiz, and J. C. Gomez-Fernandez. 1990. Factors contributing to the distribution of free fatty acids among phospholipid vesicles. Chem. Phys. Lipids. 55:245–251. [DOI] [PubMed] [Google Scholar]
- 41.Alanko, S. M., K. K. Halling, S. Maunula, J. P. Slotte, and B. Ramstedt. 2005. Displacement of sterols from sterol/sphingomyelin domains in fluid bilayer membranes by competing molecules. Biochim. Biophys. Acta. 1715:111–121. [DOI] [PubMed] [Google Scholar]
- 42.Goñi, F. M., M. A. Urbaneja, J. L. R. Arrondo, A. Alonso, A. A. Durrani, and D. Chapman. 1986. The interaction of phosphatidylcholine bilayers with Triton X-100. Eur. J. Biochem. 160:659–665. [DOI] [PubMed] [Google Scholar]
- 43.Heerklotz, H. 2002. Triton promotes domain formation in lipid raft mixtures. Biophys. J. 83:2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saily, V. M. J., J. M. Alakoskela, S. J. Ryhanen, M. Karttunen, and P. K. J. Kinnunen. 2003. Characterization of sphingosine-phosphatidylcholine monolayers: effects of DNA. Langmuir. 19:8956–8963. [Google Scholar]
- 45.Chen, Z., and R. P. Rand. 1997. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 73:267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Israelachvili, J. N., S. Marcelja, and R. G. Horn. 1980. Physical principles of membrane organization. Q. Rev. Biophys. 13:121–200. [DOI] [PubMed] [Google Scholar]
- 47.Birbes, H., S. E. Bawab, L. M. Obeid, and Y. A. Hannun. 2002. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv. Enzyme Regul. 42:113–129. [DOI] [PubMed] [Google Scholar]
- 48.Hannun, Y. A., and R. M. Bell. 1989. Regulation of protein kinase C by sphingosine and lysophoscholipids. Clin. Chim. Acta. 185:333–345. [DOI] [PubMed] [Google Scholar]
- 49.Sakane, F., K. Yamada, and H. Kanoh. 1989. Different effects of sphingosine R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS Lett. 255:409–413. [DOI] [PubMed] [Google Scholar]
- 50.Matecki, A., and T. Pawelczyk. 1997. Regulation of phospholipase C delta 1 by sphingosine. Biochim. Biophys. Acta. 1325:287–296. [DOI] [PubMed] [Google Scholar]
- 51.Nobe, K., H. Aizawa, H. Ohata, and K. Momose. 1995. Protein kinase C is involved in translocation of diacylglycerol kinase induced by carbachol in guinea pig taenia coli. Biochem. Pharmacol. 50:591–599. [DOI] [PubMed] [Google Scholar]
- 52.Harden, T. K. H., and J. Sondek. 2006. Regulation of phospholipase C isozymes by ras superfamily GTPases. Annu. Rev. Pharmacol. Toxicol. 46:355–379. [DOI] [PubMed] [Google Scholar]
- 53.Asaoka, Y., S. Nakamura, K. Yoshida, and Y. Nishizuka. 1992. Protein kinase C, calcium and phospholipid degradation. Trends Biochem. Sci. 17:414–417. [DOI] [PubMed] [Google Scholar]