Abstract
X-ray data are presented for the benchmark dipalmitoylphosphatidylcholine lipid bilayer in the most biologically relevant state in which the bilayers are fully hydrated and in the fluid (liquid-crystalline) phase. Form factors F(qz) are obtained from a combination of two sample preparations, oriented stacks of bilayers for qz extending to 0.85 Å−1 and unilamellar vesicles for smaller qz. Modeling obtains the electron density profile and values for the area per molecule, for the locations of the component groups, and for the different types of thicknesses of the bilayer, such as the hydrocarbon thickness and the steric thickness.
The most studied lipid bilayer is dipalmitoylphosphatidylcholine (DPPC), sometimes said to be the hydrogen atom of lipids. The spread in the literature results at 50°C emphasizes the difficulty of obtaining structural results for fully hydrated, fluid phase bilayers (1,2). This is especially serious for the area per molecule, A, which is a central quantity that plays a pivotal role in simulations (3,4).
The major difficulty in obtaining bilayer structure arises from the fluctuations that degrade the Bragg peaks that provide the basic data for the crystallographic approach to membrane structure. A decade ago, we used a liquid crystallographic method to recover the intensity lost from the peaks, and we reported a structure of DPPC (5). Since then, we have discovered a new method that focuses on the diffuse scattering from oriented stacks of bilayers (6,7). This method provides better primary data to obtain the bilayer form factor F(qz), mainly because the data extend to larger qz in reciprocal space, thereby providing better real space resolution to locate more features in the bilayer, but also because the data are continuous in qz instead of discrete as in liquid crystallography. Structures of several lipids have recently been reported using the new diffuse scattering method (8,9). We now use the new method, enhanced by data from unilamellar vesicles that are more robust for small qz, to report an improved structure of the benchmark lipid DPPC.
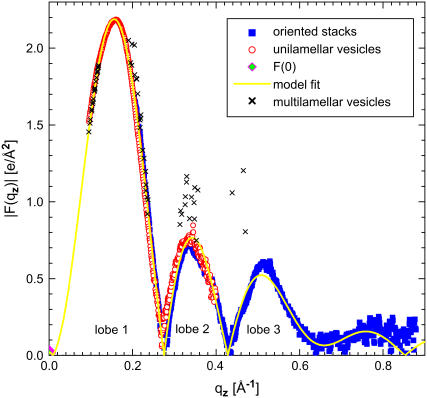
The basic F(qz) results are shown in Fig. 1. For unilamellar samples, background strongly exceeds signal as qz increases, so larger uncertainties are assigned and no data are used beyond the second lobe. Complementarily, the scattering from oriented stacks becomes problematic for F(qz) smaller than 0.2 Å−1 due to distortion from the very strong h = 2 order reflection (7), so these data are not used. Larger uncertainties are also applied near obvious distortions from the smooth behavior required by the sampling theorem. Comparison of the two types of data requires a scaling factor that is provided by modeling in Fig. 1, but the scaling factor depends mostly on the overlap of the data for 0.2 Å−1 < qz < 0.25 Å−1 in the first lobe. Although the two kinds of data do not overlap quite as well as for other lipids (8,9) (e.g., the minimum near 0.275 Å−1 occurs ∼0.005 Å−1 greater for the oriented samples than for the unilamellar vesicles), agreement is reasonable and consistent results, including these minor differences in the two types of data, are obtained from several data sets taken on two synchrotron runs.
FIGURE 1 .
Bilayer form factors F(qz) for DPPC at 50°C are from diffuse scattering from oriented stacks (blue squares) following Liu and Nagle (7) and Kučerka et al. (8,9) and from unilamellar vesicles of ∼60 nm diameter (red circles) following Kučerka et al. (8,9). F(0) is from volumetric measurements (1). Also shown are results from multilamellar vesicles (5). The yellow curve is the fit to the HB electron density model.
Fig. 1 also shows the F(qz) results obtained from multilamellar vesicles in our earlier liquid crystallography study (5) that used 21 different samples. Only three of those samples had strong enough fourth orders to provide F(qz) for qz > 0.4 Å−1, and those three samples had to be partially dehydrated with osmotic pressure. A major point was, due to the degradation of the fluctuations, the intensities had to be corrected by a factor that became increasingly >1 for greater lamellar repeat spacings D and as the x-ray order h increased. No correction led to the incorrect result that the structure of lipid bilayers changed precipitously as full hydration was approached. However, comparison with the results presented here indicates that the intensities were overcorrected, further motivating revisitation of the structure of DPPC in the fluid phase.
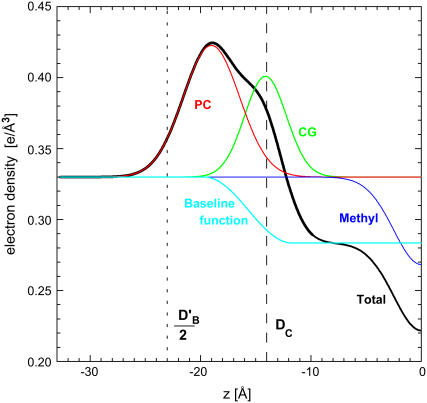
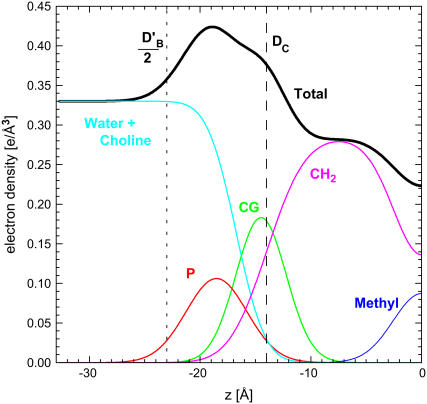
Structural results are obtained by modeling with functional forms for the electron density profiles. Fig. 2 shows the result of fitting our older hybrid baseline (HB) model and Fig. 3 shows the result using a recently developed H2 hybrid model (4). These forms have been shown to represent the electron density profiles of simulations very well (4). Both models employ the method of McIntosh and Simon (10) that uses well-established gel phase structure (11) as a reference. These figures show the distribution functions for the submolecular components and the Gibbs dividing surfaces for the various thicknesses of the bilayer.
FIGURE 2 .
Electron density profile for half the DPPC bilayer obtained by fitting the HB model to the F(qz) in Fig. 1. The phosphatidylcholine (PC), carbonyl-glycerol (CG), and the negative methyl trough Gaussians are added to the baseline function that represents the water and methylene plateaux to obtain the total electron density (black). The vertical dashed line (DC) shows half the hydrocarbon thickness (the Gibbs dividing surface for the hydrocarbon-headgroup interface) and the vertical dotted line (DB′/2) represents the steric thickness.
FIGURE 3 .
Electron density profile for half the DPPC bilayer obtained by fitting the H2 model to the F(qz) in Fig. 1. The phosphate (P), carbonyl-glycerol (CG), and methyl trough Gaussians are added to the methylene and water distributions based on error function to obtain the total electron density. The vertical dashed line (DC) shows half the hydrocarbon thickness (the Gibbs dividing surface for the hydrocarbon-headgroup interface) and the vertical dotted line (D′B/2) represents the steric thickness.
Numerical values are displayed in Table 1. The area A is not much changed from our previous value of 64 Å2 (1). This is perhaps surprising in view of the large differences in Fig. 1, but it was pointed out in Figs. 6 and 11 in Nagle et al. (5) that over or under correcting mostly affects the widths of the molecular distribution functions rather than their positions, so the gel phase reference method (10) appears to have obtained A reasonably well. However, the older analysis had to use a simpler, low spatial resolution model with only one Gaussian in the headgroup region because the data did not extend to high qz; more structural detail is now obtained.
TABLE 1 .
| Volume
|
Area
|
Hydrocarbon thickness
|
Steric thickness
|
Head-head thickness
|
Carbonyl-glycerol
|
|
|---|---|---|---|---|---|---|
| Model | VL | A | 2 DC | DB′ | DHH | 2 DCG |
| HB | 1228.5 | 64.2 | 27.9 | 45.9 | 37.8 | 28.2 |
| H2 | 1228.5 | 64.3 | 27.9 | 45.9 | 37.8 | 29.0 |
The agreement of the results in Table 1 for two rather different electron density models suggests that the exact choice of functional form is not crucial, provided that it includes the major features of the bilayer. The greatest difference is for the carbonyl-glycerol thickness. In the older HB model, about half the electrons in these groups are included in the baseline function. When the carbonyl-glycerol Gaussian is added to the sloping baseline function, the position of that group increases, consistent with the result for the H2 model, which, in this respect, is the better model.
Even though the older value of A (1,5) still appears satisfactory, it has been recently emphasized that the F(qz) are the most important x-ray structural results to compare to simulations (12,3,4), and this is a major reason to obtain better values of F(qz). Recent studies suggest that force fields carefully determined from extensive empirical data for small molecules may nevertheless result in poor agreement with experimental F(qz) when simulations are performed under the ideal condition of zero surface tension (3,4), because mismatches in competing interactions, such as the surface tension of the interfacial headgroup region versus the pressure produced by disordered hydrocarbon chains, produce incorrect area and thickness, which strongly affect F(qz). One alternative is to simulate at fixed area A determined by the above modeling. Another alternative is to run several simulations with different surface tensions or, equivalently, with different values of A. The simulation that best fits the F(qz) data then provides a model free method for determining A (4). Clearly, this would not have been feasible with the older F(qz) (5), but it has recently been shown that it is feasible when DMPC simulations are compared to data obtained by the new x-ray method (4). Better simulation force fields should ensue by comparing simulations to experimental F(qz) for a variety of bilayers, including the new DPPC results reported here.
Acknowledgments
This work was supported by National Institutes of Health grant GM 44976. Synchrotron x-ray beam time was provided by the Cornell High Energy Synchrotron Source funded by National Science Foundation grant DMR-0225180.
References
- 1.Nagle, J. F., and S. Tristram-Nagle. 2000. Structure of lipid bilayers. Biochim. Biophys. Acta. 1469:159–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagle, J. F., and S. Tristram-Nagle. 2000. Lipid bilayer structure. Curr. Opin. Struct. Biol. 10:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benz, R. W., F. Castro-Roman, D. J. Tobias, and S. H. White. 2005. Experimental validation of molecular dynamics simulations of lipid bilayers: a new approach. Biophys. J. 88:805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klauda, J. B., N. Kučerka, B. R. Brooks, R. W. Pastor, and J. F. Nagle. 2006. Simulation-based methods for interpreting x-ray data from lipid bilayers. Biophys. J. 90:2796–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagle, J. F., R. Zhang, S. Tristram-Nagle, W. Sun, H. I. Petrache, and R. M. Suter. 1996. X-ray structure determination of fully hydrated Lα phase dipalmitoylphosphatidylcholine bilayers. Biophys. J. 70:1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyatskaya, Y., Y. Liu, S. Tristram-Nagle, J. Katsaras, and J. F. Nagle. 2001. Method for obtaining structure and interactions from oriented lipid bilayers. Phys. Rev. E. 63:011907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu, Y., and J. F. Nagle. 2004. Diffuse scattering provides material parameters and electron density profiles of biomembranes. Phys. Rev. E. 69(4, Pt. 1):040901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kučerka, N., Y. Liu, N. Chu, H. I. Petrache, S. Tristram-Nagle, and J. F. Nagle. 2005. Structure of fully hydrated fluid phase DMPC and DLPC bilayers using x-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 88:2626–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kučerka, N., S. Tristram-Nagle, and J. F. Nagle. 2006. Structure of fully hydrated fluid phase lipid bilayers with monounsaturated chains. J. Membr. Biol. In press. [DOI] [PubMed]
- 10.McIntosh, T. J., and S. A. Simon. 1986. Area per molecule and distribution of water in fully hydrated dilauroylphosphatidylethanolamine bilayers. Biochemistry. 25:4948–4952. [DOI] [PubMed] [Google Scholar]
- 11.Tristram-Nagle, S., Y. Liu, J. Legleiter, and J. F. Nagle. 2002. Structure of gel phase DMPC determined by x-ray diffraction. Biophys. J. 83:3324–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs, J. N., H. I. Petrache, and T. B. Woolf. 2003. Interpretation of small angle X-ray measurements guided by molecular dynamics simulations of lipid bilayers. Chem. Phys. Lipids. 126:211–223. [DOI] [PubMed] [Google Scholar]