Abstract
Context
Breast cancer estrogen-receptor status is useful in predicting benefit from endocrine therapy. It may also help predict which patients benefit from advances in adjuvant chemotherapy.
Objective
Compare differences in benefits from adjuvant chemotherapy achieved by patients with ER-negative versus ER-positive tumors.
Design
Trial data from the Cancer and Leukemia Group B and U.S. Breast Cancer Intergroup analyzed; patient outcomes by ER status compared using hazards over time and multivariate models.
Setting
Randomized trials comparing (1): three regimens of cyclophosphamide, doxorubicin, and fluorouracil; (2) three doses of doxorubicin concurrent with cyclophosphamide, with/without subsequent paclitaxel; (3) sequential doxorubicin, paclitaxel, and cyclophosphamide with concurrent doxorubicin and cyclophosphamide followed by paclitaxel, and also three-week versus two-week cycles.
Patients
Total of 6644 node-positive breast cancer patients receiving adjuvant treatment.
Main Outcome Measures
Disease-free and overall survival.
Results
For ER-negative tumors, chemotherapy improvements reduced the relative risk of recurrence by 21, 25 and 23 percent in the three studies, respectively, and 55 percent comparing the lowest dose in the first study with biweekly cycles in the third study. Corresponding relative risk reductions for ER-positive tumors treated with tamoxifen were 9, 12, and 8 percent in the three studies, and 26 percent overall. The overall mortality rate reductions associated with chemotherapy improvements were 55 and 23 percent among ER-negative and ER-positive patients, respectively. All individual ER-negative comparisons and no ER-positive comparisons were statistically significant. Absolute benefits due to chemotherapy were greater for patients with ER-negative compared with ER-positive tumors: 22.8 percent more ER-negative patients survived to 5 years disease-free if receiving chemotherapy versus 7.0 percent for ER-positive patients; corresponding improvements for overall survival were 16.7 versus 4.0 percent.
Conclusions
Among patients with node-positive, ER-negative breast cancer, biweekly doxorubicin/cyclophosphamide plus paclitaxel lowers the rate of recurrence and death by more than 50 percent in comparison with low dose CAF as used in the first study.
Great strides have been made in the treatment of early-stage breast cancer. In patients with hormone-sensitive tumors, tamoxifen reduces the risk of recurrence and death by more than 30 percent.1 Moreover, treatment with aromatase inhibitors in place of or sequentially with tamoxifen further reduces the risk of recurrence in postmenopausal women with estrogen-receptor (ER)-positive tumors.2–4
In the absence of treatment, ER status is a weak prognostic factor, but it is a strong predictive factor in the sense that it identifies patients who benefit from endocrine therapy. With appropriate endocrine therapy, patients with ER-positive disease have substantially better prognoses as a group than do those with ER-negative disease.
Evidence is accumulating that improvements in chemotherapy disproportionately benefit patients with ER-negative tumors. The most recent meta-analysis of randomized trials showed that for patients aged 50 to 69 years with ER-negative tumors not receiving tamoxifen, the reduction in the risk of recurrence due to any kind of polychemotherapy was 33 percent; for mortality it was 26 percent. For patients with ER-positive tumors receiving tamoxifen the respective reductions were 15 and 11 percent.1 The corresponding reductions for patients younger than 50 years were between 32 and 39 percent.1 The apparent lack of an interaction between chemotherapy benefits and estrogen-receptor status in this younger age group may be due to the small sample size, but also could be related to the impact of chemotherapy on ovarian function in younger women.
The International Breast Cancer Study Group found that among patients with node-negative disease treated with tamoxifen, those with ER-negative tumors received substantial benefit from a three-cycle regimen of cyclophosphamide, methotrexate, and fluorouracil, with a 48 percent reduction in the risk of recurrence and a 49 percent reduction in the risk of death from any cause. This substantial benefit is in sharp contrast to those patients with ER-positive tumors for whom both risks were reduced by only 1 percent.5 The neoadjuvant setting provides further supportive evidence: tumors without hormone receptors are more sensitive to chemotherapy than are hormone-sensitive tumors, with markedly higher rates of pathological complete response.6–8
In a retrospective subset analysis, we addressed whether node-positive breast cancer patients with ER-negative disease benefit more from recent improvements in adjuvant chemotherapy than do those with ER-positive tumors treated with tamoxifen. We compared disease-free and overall survival according to ER status among patients enrolled in three consecutive randomized trials of chemotherapy conducted by the Cancer and Leukemia Group B (CALGB) and the U.S. Breast Cancer Intergroup (including the Eastern Cooperative Group, the Southwest Oncology Group, and the North Central Cancer Treatment Group).
METHODS
The three consecutive studies (8541, 9344, and 9741) were coordinated by CALGB. (Studies 9344 and 9741 were Intergroup trials 0148 and C9741.) The study protocols were approved by the institutional review boards of the participating centers, and all patients provided written informed consent. All three studies were randomized and addressed one or more question related to the optimal use of chemotherapy in women with node-positive breast cancer. Women on all arms of the three trials received doxorubicin-based chemotherapy. Tamoxifen was not randomized in any of the studies but instead was recommended for certain patient subgroups based on the prevailing standard of care. Each study showed a benefit for at least one of the factors under investigation. For the present analysis, the results of all three studies were updated as of August 16, 2005.
Clinical Trials
Figure 1 shows the study designs, and Table 1 the clinical and tumor characteristics for the three studies. The studies were designed sequentially based on the earlier studies’ results. In particular, the treatment group with the best results (or a slight variant thereof) in each of the first two studies served as the comparison group in the subsequent study.
Figure 1. Treatment Regimens in the Three Studies of Chemotherapy for Node-Positive Breast Cancer.
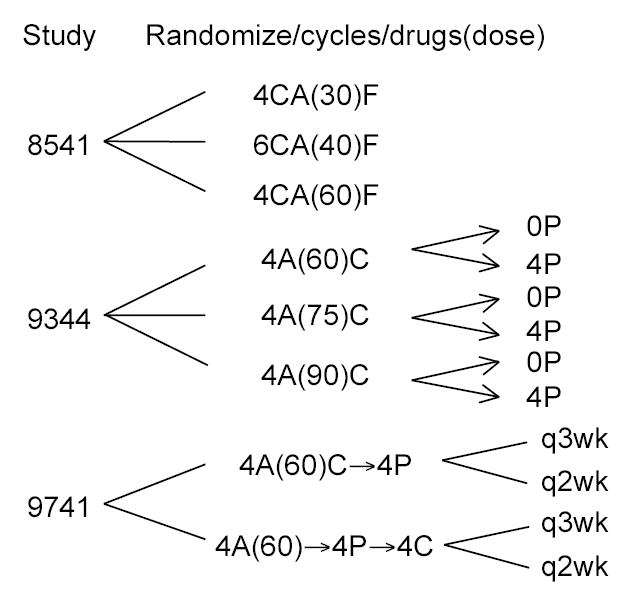
C denotes cyclophosphamide, A doxorubicin, F fluorouracil, and P paclitaxel. Study 8541 had three treatment arms, with doses of C, A and F in proportion to the dose of doxorubicin that is shown (in milligrams per square meter of body surface area). Study 9344 had a 3 by 2 factorial design, with randomization to one of 3 doses of A with a second randomization to 4 cycles of paclitaxel or not. Study 9741 had a 2 by 2 factorial design, with patients being randomized to biweekly vs triweekly schedules and separately to concurrent AC followed by P vs sequential A followed by P then C.
Table 1.
Clinical and Tumor Characteristics in the Three Studies for Each Regimen and ER status. Denominators are numbers of patients within ER-status, study/regimen group. Numerators are the numbers of patients in that group having the characteristic.
| Characteristic | |||||||
|---|---|---|---|---|---|---|---|
| Postmenopausal
|
Tumor size, ≥ 2 cm
|
Positive nodes, ≥ 4
|
|||||
| Study | Regimen | ER- negative | ER- positive | ER- negative | ER- positive | ER- negative | ER- positive |
| 8541 | Low dose | 102/189 (54) | 128/154 (83) | 123/187 (66) | 94/153 (61) | 76/189 (40) | 60/154 (39) |
| Moderate dose | 90/178 (51) | 133/155 (86) | 119/177 (67) | 91/154 (59) | 75/178 (42) | 64/155 (41) | |
| High dose | 90/159 (57) | 131/162 (81) | 115/159 (72) | 100/161 (62) | 68/159 (43) | 69/162 (43) | |
| 9344 | No paclitaxel | 241/640 (38) | 331/871 (38) | 436/639 (68) | 544/866 (63) | 325/639 (51) | 484/871 (56) |
| Paclitaxel | 235/623 (38) | 356/901 (40) | 424/621 (68) | 555/893 (62) | 317/623 (51) | 502/900 (56) | |
| 9741 | Every 3 wk | 154/318 (48) | 291/569 (51) | 213/321 (66) | 334/565 (59) | 133/327 (41) | 225/575 (39) |
| Every 2 wk | 154/325 (47) | 305/571 (53) | 218/333 (65) | 314/579 (54) | 128/336 (38) | 235/585 (40) | |
Study 85419 involved the treatment of 1,550 patients between January 1985 and April 1991. Patients were randomly assigned to one of three schedules of cyclophosphamide, doxorubicin, and fluorouracil (CAF): high-dose (600, 60, and 600 mg per square meter, respectively, given in four cycles), moderate-dose (400, 40, and 400 mg per square meter in six cycles), or low-dose (300, 30, and 300 mg per square meter in four cycles). The high- and moderate-dose groups received the same total dose, and the low-dose group received half of that dose. Forty-five percent of patients with ER-positive tumors received tamoxifen (not randomized) after chemotherapy, and 68 percent of these patients were postmenopausal. Disease-free survival and overall survival were significantly increased in the moderate- and high-dose groups. The median follow-up at the time of our analysis was 17 years.
Study 934410 enrolled a total of 3,121 patients who were treated between May 1994 and April 1997. In this 3x2 factorial study, patients were randomly assigned to doxorubicin at a dose of 60, 75, or 90 mg per square meter, given concurrently with cyclophosphamide at a fixed dose of 600 mg per square meter, with or without subsequent treatment with paclitaxel (175 mg per square meter). Chemotherapy was given every three weeks for a total of four cycles. Ninety-four percent of the patients with ER-positive tumors received tamoxifen at the completion of chemotherapy, as recommended by the protocol. Disease-free and overall survival were significantly increased in the group that received paclitaxel, but there was no evidence of a relationship between dose of doxorubicin and survival. At the time of our analysis, the median follow-up was nine years.
Study 974111 involved the treatment of 1,973 patients between September 1997 and March 1999. All patients received doxorubicin, cyclophosphamide, and paclitaxel at respective doses of 60, 600, and 175 mg per square meter. The study had a 2x2 factorial design to assess two levels of dose density (two-week vs. three-week cycles) and two treatment sequences (concurrent administration of doxorubicin and cyclophosphamide followed by paclitaxel vs. sequential administration of doxorubicin, paclitaxel, and cyclophosphamide). Ninety-one percent of patients with ER-positive tumors received tamoxifen after chemotherapy. There was a significant improvement in disease-free and overall survival for the dose-dense group (two-week cycles) but no difference depending on treatment sequence. The median follow-up at the time of our analysis was six years.
Statistical Analysis
Our primary objective was to assess the cumulative survival benefit of chemotherapy improvements over time according to ER status. We compared low-dose with high-dose CAF in study 8541, no paclitaxel with paclitaxel in study 9344, and three-week with two-week cycles in study 9741. We considered patients with ER-positive tumors only if they received tamoxifen.
We used Kaplan-Meier methods to determine survival according to ER status in each of the three studies. Events included in the analysis of disease-free survival were local, regional, and distant recurrences of breast cancer and deaths from any cause. Confidence intervals for hazard ratios were based on Cox multivariate regression models comparing treatment groups, adjusted for menopausal status, number of positive axillary lymph nodes, and tumor size (with the square-root transformation used for the latter two variables, which is CALGB’s standard practice).
In considering the cumulative effects of chemotherapy over the three studies, not all pairs of therapies have within-study comparisons. The process of comparing therapies across studies adds an extra level of variability.12–14 We addressed the comparability of similar treatments across consecutive studies using Cox models within ER categories: the high-dose CAF group in study 8541 with the no-paclitaxel group in study 9344, and the paclitaxel group in study 9344 with the group treated every three weeks in study 9741. The cumulative hazard ratio of improvements in chemotherapy (from low-dose CAF in study 8541 to the every-two-week regimens in study 9741) was calculated as the product of the hazard ratios from the individual studies.
To address statistical significance of the differences in chemotherapy effects for the ER-positive and ER-negative subsets we incorporated a chemotherapy/ER interaction factor in Cox proportional hazards modeling; for the totality of the three studies we also included study as a factor. We estimated the risks of recurrence over time according to ER status and compared these risks to determine which patients benefited from chemotherapy and when these benefits occurred.
RESULTS
Disease-free and overall survival did not differ significantly between the group that received high-dose CAF in study 8541 and the group that received doxorubicin and cyclophosphamide without paclitaxel in study 9344, after adjustment for covariates, including ER status. Similarly, there were no significant differences in survival between the group that received doxorubicin and cyclophosphamide followed by paclitaxel in study 9344 and the groups given chemotherapy every three weeks in study 9741.
Figure 2 shows the results of multivariate Cox models for disease-free survival according to ER status. Overall, about 60 percent of the patients had ER-positive tumors. The ability to detect treatment differences was slightly greater in the ER-positive group because that group experienced more events, 1472, as opposed to 1152 in the ER-negative group. In each of the three studies, the differences in outcome by treatment were statistically significant among ER-negative patients but not among ER-positive patients who received tamoxifen (see Table 2)—however, the ER-positive group still had numerically lower risks of recurrence and death with the more intensive treatment.
Figure 2. Kaplan-Meier Curves for Disease-free Survival According to ER Status in the Three Studies.
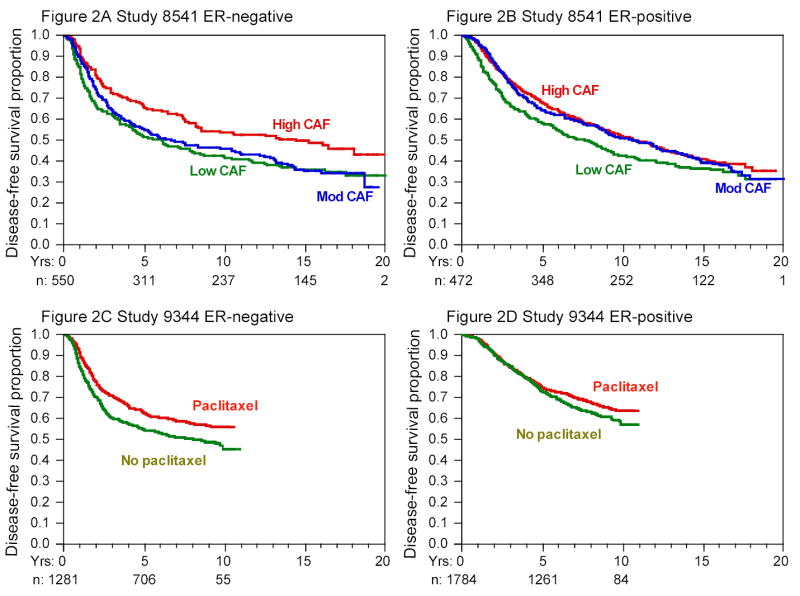
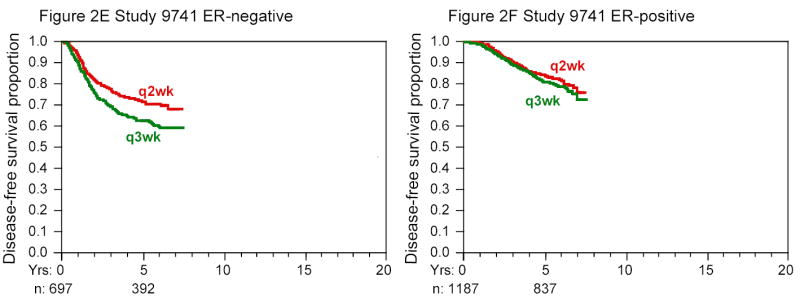
Cumulative at-risk sample sizes over time are shown in each panel. The initial sample sizes were approximately equally divided among the groups in question. The dose effect of doxorubicin in study 9344 and the comparison of sequential or concurrent therapy in study 9741 are not shown; neither factor was associated with disease-free survival in unadjusted or adjusted analyses, nor were there significant differences within ER subgroups.
Table 2.
Reductions in Relative Risks of Recurrence and Death and Absolute Differences in Five-Year Disease-free and Overall Survival, According to ER Status. Relative risks were assessed from multivariate proportional hazards models (adjusted for menopausal status, number of positive axillary lymph nodes, and tumor size). Absolute differences for the individual studies were estimated from Kaplan-Meier survival curves. Absolute differences overall were calculated from the relative risk reduction comparing patients in low-dose CAF of Study 8541 vs those patients modeled as though they receiving biweekly AC followed by P as in Study 9741—see Figure 5 as regards DFS.
| Reduction in Risk | Absolute Difference in 5-Yr Survival | |||
|---|---|---|---|---|
| Recurrence | Death | Disease-free | Overall | |
| Study and ER Status | % (95% Confidence Interval) | % | ||
| 8541 (high dose vs. low dose) | ||||
| ER-negative | 21 (9 to 31) | 17 (4 to 29) | 13.9 | 6.6 |
| ER-positive | 9 (−6 to 22) | 6 (−11 to 20) | 6.6 | 4.0 |
| 9344 (paclitaxel vs. no paclitaxel) | ||||
| ER-negative | 25 (12 to 36) | 24 (10 to 37) | 8.2 | 7.4 |
| ER-positive | 12 (−3 to 25) | 11 (−8 to 26) | 2.1 | 0.0 |
| 9741 (every 2 wk vs. every 3 wk) | ||||
| ER-negative | 24 (1 to 42) | 28 (1 to 47) | 9.1 | 7.4 |
| ER-positive | 8 (−20 to 29) | 8 (−28 to 35) | 2.8 | −0.2 |
| Overall (every 2 wk in 9741 vs low dose in 8541) | ||||
| ER-negative* | 55 (37 to 68) | 55 (38 to 69) | 22.8 | 16.7 |
| ER-positive* | 26 (−4 to 48) | 23 (−17 to 49) | 7.0 | 4.0 |
The interaction between higher chemotherapy and ER status was assessed using proportional hazards model, with added terms for study and main effects for higher chemotherapy and ER status. The interaction was statistically significant (p = 0.02 for recurrence and p = 0.05 for survival) for the overall comparison but not when comparing the studies separately.
The every-two-week regimens of study 9741, as compared with low-dose CAF in study 8541, resulted in a 55 percent reduction in the risk of a recurrence among ER-negative patients (95 percent confidence interval, 37 to 68); the risk reduction was 26 percent (95 percent confidence interval, −4 to 48) for ER-positive patients. The respective overall reductions in the risk of death were 55 percent (95 percent confidence interval, 38 to 69) and 23 percent (95 percent confidence interval, −17 to 49).
The pattern of risks over time for ER-negative patients was similar across the three studies, with a high risk of an event in the first two to three years after treatment (Figure 3). The risks for ER-positive patients were also similar across the studies, but in contrast to the pattern for ER-negative patients, the risk of an event in the first few years was very low. A comparison of risks among ER-positive patients in study 8541 who received tamoxifen and those who did not (Figure 4) suggests that this low early risk was due to tamoxifen and not to the patients’ ER status. The risks for ER-positive patients who did not receive tamoxifen were similar to those for ER-negative patients, with the former group experiencing a reduction of 67 percent in year 1 for high-dose CAF as compared with low-dose CAF.
Figure 3. Empirical Risk of Recurrence or Death over Time According to ER Status.


Risk was calculated as the number of events during the year divided by the number of patients at risk for experiencing the event at the beginning of the year. Cumulative at-risk sample sizes over time are shown in the respective panels of Figure 2. In the rightmost parts of the curves the samples were relatively small, and the standard errors (not shown) were correspondingly large. Not all patients have reached the later follow-up times. In addition, patients with earlier recurrences have been eliminated from the at-risk group.
Figure 4. Empirical Risk of Recurrence or Death over Time for ER-Positive Patients in Study 8541.
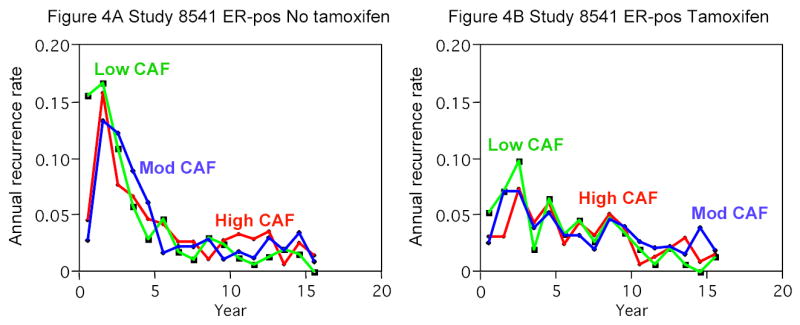
Panel A shows the risks for patients not receiving tamoxifen (initial n=552), and Panel B shows the risks for patients receiving tamoxifen (same as Panel B of Figure 3) (initial n=472).
From the standpoint of the patient, absolute benefit associated with a treatment program is far more important than the relative risk reduction. The ER-negative group experienced substantially greater absolute improvements in disease-free and overall survival rates. Figure 5 compares DFS (panels A and B) and OS (panels C and D) for patients in the low-dose regimen of CAF in Study 8541 with the same patients modeled as receiving biweekly AC followed by paclitaxel as in Study 9741. For ER-negative disease, the improvement is 22.8 percent at 5 years (see Table 2) and 25.8 percent at 10 years. The corresponding improvements for ER-positive disease treated with tamoxifen are 7.0 percent at 5 years and 10.0 percent at 10 years.
Figure 5. Disease-free and Overall Survival for Patients in Study 8541: Actual Patients in Lowdose CAF vs Modeled as though Receiving Biweekly Doxorubicin, Cyclophosphamide and Paclitaxel as in Study 9741.
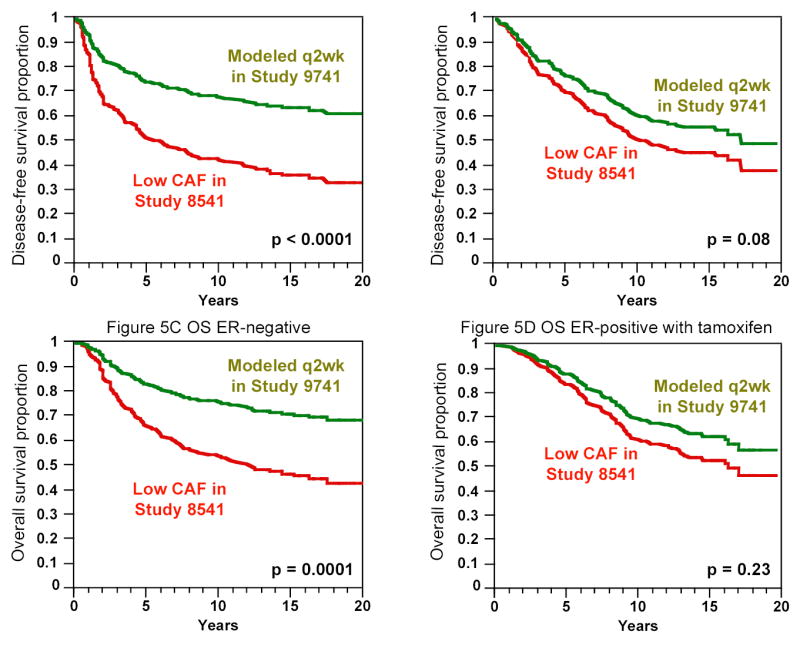
Patients have mean tumor size, number of positive lymph nodes and menopausal status as in the low-dose arm of Study 8541. The “Modeled q2wk in Study 9741” curves do not apply to the patients in Study 9741 but instead for patients having the characteristics of the low-dose arm of Study 8541. Both panels A (DFS) and C (OS) show an average reduction in hazard of 55 percent for ER-negative disease while panels B (DFS) and D (OS) show average reductions of 26 and 23 percent for ER-positive disease receiving tamoxifen (see Table 2). The proportional hazards model assumption is that average reduction in hazard applies over the entire 20-year period. The p-values shown are from multivariate models for given ER status. The ER status by chemotherapy interaction p-value for DFS is 0.02 and for OS is 0.05.
DISCUSSION
Advances in the treatment of primary breast cancer include the use of chemotherapy, tamoxifen and, more recently, aromatase inhibitors for patients with ER-positive tumors, as well as trastuzumab for those with HER2-positive disease.15–18 Breast cancer is heterogeneous, with differences among subtypes in treatment responses and outcomes. Patients with ER-positive tumors who receive adjuvant hormonal therapy have better disease-free and overall survival than do those with ER-negative tumors, particularly during the first five years after diagnosis. Our results indicate that advances in chemotherapy have lessened the survival differences between ER-positive patients who receive hormonal therapy and ER-negative patients, with the latter group deriving much greater benefit from modern improvements in chemotherapy regimens.
Since early and overall event rates are higher for patients with ER-negative tumor status than for those with ER-positive status, relative reductions in these rates due to improvements in chemotherapy translate into even greater absolute benefits for patients with ER-negative tumors. The absolute improvement in five-year disease-free survival was 22.8 percent for ER-negative patients, as compared with 7.0 percent for ER-positive patients, and the difference for overall survival was also pronounced: an improvement of 16.7 percent for ER-negative patients versus 4.0 percent for ER-positive patients (Table 2 and Figure 5).
The risks of recurrence and death vary over time, as shown in Figure 3, and so do the reductions in risk that are attributable to improved chemotherapy. The high risk of an event in the first two to three years after treatment for ER-negative patients coincides with the period of time when the benefit of the more effective chemotherapy regimens is manifest. For example, the reduction in risk with high-dose CAF as compared with low-dose CAF was 55 percent in the first year and 30 percent in the second year. There was little or no advantage of high-dose CAF for ER-negative patients after three years. However, the benefit was durable in that there was no sign of a rebound in risk in the high-dose group in later years, a finding reflected as well by the persistent separation in the corresponding survival curves in Figure 2.
The benefit of chemotherapy in women with ER-positive tumors is far less dramatic, possibly because the benefits of improved adjuvant chemotherapy are due mostly, if not entirely, to a reduction in the risk of recurrence in the first few years after treatment. In this early period, the risk of recurrence is so small in ER-positive patients who are treated with tamoxifen, and presumably other hormonal therapies, that any additional risk reduction due to chemotherapy would be very difficult to detect statistically. Moreover, based on data from the preoperative setting, there is reason to believe that many ER-positive tumors are intrinsically less responsive to short courses of chemotherapy than ER-negative tumors. Despite the lack of a statistically significant benefit from improved chemotherapy regimens in ER-positive patients, some of these patients almost certainly derive an added benefit from such regimens. Identifying such patients is complex. In study 8541, HER2/neu overexpression or amplification identified patients who derived a benefit from high-dose CAF (600, 60, and 600 mg per square meter, respectively, per cycle), which is now the standard CAF regimen.19,20 This benefit was independent of ER status (results not shown). Subgroup analyses according to HER2 status from studies 9344 and 9741 are pending. Although the findings will be of interest, they will have to be interpreted in light of recent data demonstrating a benefit of adjuvant and neoadjuvant trastuzumab therapy in patients with HER2-positive tumors.15–18,21–24 Of interest, recent studies have documented the benefits of chemotherapy in subsets of tamoxifen-treated patients who have hormone-responsive tumors,25,26 but they have also suggested that patients with ER-positive tumors who are not in these subsets derive little or no benefit from adjuvant chemotherapy.
No method of assessing ER status is perfect, and some tumors labeled ER-positive may actually be ER-negative.27 In the same vein, an obvious question is whether the benefits of chemotherapy depend on the degree of ER positivity. Unfortunately, quantitative ER measurements were not routinely collected in our studies.
As Figure 3 illustrates, the risk of recurrence in both ER groups was between 2 percent and 4 percent per year after approximately three to five years and did not appear to be influenced by treatment. This level of risk is similar to that for women with node-negative disease. Moreover, the risk of recurrence decreased dramatically after about year 3, irrespective of the number of positive nodes and tumor size (data not shown). Tumors that have not recurred are those that are less aggressive or are sensitive to therapy: in effect, nonrecurrence trumps baseline clinical characteristics.
The long-term hazards were slightly higher in the ER-positive group than in those with ER-negative disease (Figure 3). Possible explanations for this finding are the development of resistance to initial hormonal therapy, differences in the underlying biology of the disease, or both.28
The Oxford Overview1 suggested a possible role of age in any interaction between ER status and benefits of early polychemotherapy regimens, although the subset sample sizes were small. Our study also suffers from a sample size limitation. However, we addressed this interaction and found little evidence that age plays a role. If anything, in our studies the interaction between ER status and chemotherapy benefits is stronger in younger women. In particular, for women younger than 50 who had ER-positive tumors treated with tamoxifen there was no suggestion of a benefit for the accumulation of more intensive and extensive chemotherapies over the three studies.
Our analysis has several limitations. One is that patients admitted to our clinical trials may not be typical of patients generally. (Although there is evidence from models of breast cancer that adjuvant therapy benefits observed in clinical trials applies as well to the general U.S. population.29) We combined the results of three trials with a total of 13 treatment regimens. The trials were randomized separately, and each patient was eligible to receive only a subgroup of the 13 regimens. Each trial was confounded with any covariates that were temporally related, limiting the ability to draw definitive conclusions based on cross-study analyses. For example, the use of screening mammography increased over the course of these studies. There is a well-understood stage shift for cancers detected on screening, but such cancers are also associated with an improved prognosis, even after accounting for known prognostic factors.30,31 We accounted for known patient and tumor characteristics using multivariate analyses, but we did not have information about how the patients’ cancers were detected.
Another limitation is that tamoxifen therapy was not randomly assigned in the studies we considered. In studies 9344 and 9741, almost all ER-positive patients received tamoxifen, and we compared ER-positive patients who received tamoxifen with ER-negative patients. In a separate analysis of study 8541, the benefit of more intensive chemotherapy was similar in ER-positive patients not treated with tamoxifen and in ER-negative patients. There may have been a bias in the assignment of tamoxifen in study 8541; however, with the exception of menopausal status, the most important determinant of tamoxifen use was time, with much greater use after the announcement of the Oxford Overview results in 1988.32
Multiple comparisons are a concern in any subgroup analysis: examining enough subgroups will identify spurious treatment correlations, with the effects tending to be stronger with greater numbers of subgroups examined.33,34 Subgroup analyses based on hypotheses established from previous trials are less problematic. In our study, the three trials independently show the same effect. Viewing one study as hypothesis-generating leaves the other two as confirmatory. The fact that the specific chemotherapy question differed across the three trials is an important consideration but may not be relevant. Our analysis suggests that the biologic subtype of breast cancer is the most important predictor of the benefits of an improved chemotherapy regimen, regardless of the specific regimen.
Our study has two substantive clinical implications. First, although patients with ER-positive breast tumors may reasonably opt for chemotherapy, they should recognize that the benefits are not great as compared with those for patients with ER-negative disease. The benefits of intensive and extensive chemotherapy for unselected patients who have ER-positive disease treated with tamoxifen are modest at best. Whether such patients should opt for chemotherapy will depend on their attitudes toward the associated negative sequelae. In the years ahead, it is likely that we will have better predictors that will allow clinicians to determine which patients with ER-positive disease truly benefit from the addition of chemotherapy.25,26 Second, with advances in chemotherapy, patients with ER-negative tumors have had sequentially improved outcomes, and their prognoses now approach those of optimally treated patients with ER-positive disease. For patients with ER-negative disease, the overall disease-free survival and overall survival benefits of modern intensive and extensive chemotherapy considered in our study are substantial.
Footnotes
Supported in part by a grant (CA31946) from the National Cancer Institute to the CALGB.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359(9324):2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 3.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 4.Coombes RC, Hall E, Gibson LJ, et al. for the Intergroup Exemestane Study. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 5.International Breast Cancer Study Group (IBCSG) Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2002;94(14):1054–1065. doi: 10.1093/jnci/94.14.1054. [DOI] [PubMed] [Google Scholar]
- 6.Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res. 2004;10(19):6622–6628. doi: 10.1158/1078-0432.CCR-04-0380. [DOI] [PubMed] [Google Scholar]
- 7.Gianni L, Zambetti M, Clark K, et al. Gene expression profiles of paraffin-embedded core biopsy tissue predict response to chemotherapy in patients with locally advanced breast cancer [abstract]. J Clin Oncol 2004;22(suppl 14). Abstract 501. [DOI] [PubMed]
- 8.Buzdar AU, Valero V, Theriault RL, et al. Pathological complete response to chemotherapy is related to hormone receptor status [abstract on the Internet]. 26th Annual San Antonio Breast Cancer Symposium, Thursday Session Index; Treatment and Response Predictions; 2003. Abstract 302. Available at: http://www.sabcs.org/SymposiumOnline/index.asp#abstracts Accessed October 10, 2005.
- 9.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998;90(16):1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 10.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21(6):976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 11.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 12.Ballesteros J. Orphan comparisons and indirect meta-analysis. J Clin Psychopharmacol. 2005;25(2):127–131. doi: 10.1097/01.jcp.0000155826.05327.c1. [DOI] [PubMed] [Google Scholar]
- 13.Dominici F, Parmigiani G. Combining studies with continuous and dichotomous responses: a latent-variables approach. In: Stangl DK, Berry DA, eds. Meta-analysis in Medicine and Health Policy New York: Marcel-Dekker, Inc.; 2000:112–120.
- 14.Berry DA, Berry SM, McKellar J, Pearson TA. Comparison of the dose-response relationships of two lipid-lowering agents: a Bayesian meta-analysis. Am Heart J. 2003;145(6):1036–1045. doi: 10.1016/S0002-8703(03)00106-6. [DOI] [PubMed] [Google Scholar]
- 15.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353(16):1659–1672. [DOI] [PubMed]
- 17.Perez EA, Suman VJ, Davidson NE, et al. Interim cardiac safety analysis of NCCTG N9831 Intergroup Adjuvant Trastuzumab Trial. J Clin Oncol. 2005;23(16S):556. [abstract] [Google Scholar]
- 18.Robinson A, Ellard S, Speers C, et al. Clinical and molecular predictors of sustained response to trastuzumab in metastatic breast cancer. J Clin Oncol. 2005;23(16S):500. [abstract] [Google Scholar]
- 19.Muss HB, Thor A, Berry DA, et al. c-erbB-2 expression predicts response to adjuvant therapy in women with node positive early breast cancer. N Engl J Med. 1994;330(18):1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- 20.Thor A, Berry DA, Budman D, et al. erbB-2, p53 and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst. 1998;90(18):1346–1360. doi: 10.1093/jnci/90.18.1346. [DOI] [PubMed] [Google Scholar]
- 21.Rueckert S, Ruehl I, Kahlert S, Konecny G, Untch M. A monoclonal antibody as an effective therapeutic agent in breast cancer: trastuzumab. Expert Opin Biol Ther. 2005;5(6):853–866. doi: 10.1517/14712598.5.6.853. [DOI] [PubMed] [Google Scholar]
- 22.Jones RL, Smith IE. Efficacy and safety of trastuzumab. Expert Opin Drug Saf. 2004;3(4):317–327. doi: 10.1517/14740338.3.4.317. [DOI] [PubMed] [Google Scholar]
- 23.Emens LA, Davidson NE. Trastuzumab in breast cancer. Oncology. 2004;18(9):1117–1128. [PubMed] [Google Scholar]
- 24.Tan AR, Swaim SM. Ongoing adjuvant trials with trastuzumab in breast cancer. Semin Oncol. 2003;30(5 Suppl 16):54–64. doi: 10.1053/j.seminoncol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Paik S, Shak S, Tang G, et al. Expression of the 21 genes in the Recurrence Score assay and prediction of the clinical benefit from tamoxifen in NSABP study B-14 and chemotherapy in NSABP study B-20 [abstract]. Breast Cancer Res Treat. 2004;88(Suppl 1)S15. Abstract 24.
- 26.Albain K, Barlow W, O'Malley F, et al. Concurrent (CAFT) versus sequential (CAF-T) chemohormonal therapy (cyclophosphamide, doxorubicin, 5-fluorouracil, tamoxifen) versus T alone for postmenopausal, node-positive estrogen (ER) and/or progesterone (PgR) receptor-positive breast cancer: mature outcomes and new biologic correlates on phase III intergroup trial 0100 (SWOG-8814) [abstract]. Breast Cancer Res Treat 2004;88(Suppl 1). Abstract 37.
- 27.Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005;23(22):5148–5154. doi: 10.1200/JCO.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 28.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14(10):2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 29.Berry DA, Cronin KA, Plevritis SK, et al. (2005). Effect of screening and adjuvant therapy on breast cancer mortality. N Eng J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 30.Joensuu H, Lehtimaki T, Holli K, et al. Risk for distant recurrence of breast cancer detected by mammography screening or other methods. JAMA. 2004;292(9):1064–1073. doi: 10.1001/jama.292.9.1064. [DOI] [PubMed] [Google Scholar]
- 31.Shen Y, Yang Y, Inoue LYT, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97(16):1195–1203. doi: 10.1093/jnci/dji239. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: An overview of 61 randomized trials among 28,896 women. N Engl J Med. 1988;319(26):1681–1692. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 33.Berry DA. Subgroup analyses. Biometrics. 1990;46(4):1227–1230. [PubMed] [Google Scholar]
- 34.Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]


