Abstract
Changes in the immunoglobulin G1 (IgG1)/IgG2 ratio following vaccination can indicate the activation of cellular control mechanisms typical of a T-cell-dependent response. We examined IgG subclass ratios in 17 healthy adults (26 to 55 years of age) before and 4 to 6 weeks following immunization with a quadrivalent meningococcal-polysaccharide diphtheria toxoid conjugate vaccine against serogroups A, C, Y, and W135. Serologic responses were determined by serum bactericidal antibody assay and serogroup-specific IgG, IgG1, and IgG2 enzyme-linked immunosorbent assay. Prevaccination serogroup A-specific IgG1/IgG2 ratios were <1 for all subjects and differed by subject for C, Y, and W-135. Postvaccination, significant increases in IgG, IgG1, and IgG2, were observed for all serogroups. Serogroup-specific IgG1/IgG2 ratios increased for group A (14/17 subjects, 88%), decreased in more than half of subjects for groups C (9/17, 53%) and W135 (12/17, 71%) and decreased for serogroup Y (16/17, 94%). IgG1/IgG2 ratios differed between individual vaccinees and were similar to the responses of adults who received pneumococcal conjugate vaccines or a monovalent C conjugate vaccine. Further studies on IgG subclasses following meningococcal polysaccharide and conjugate vaccination are needed.
A quadrivalent serogroup A, C, W135, and Y polysaccharide conjugate vaccine (Menactra) has recently been licensed in those of ages 11 to 55 years in the United States on the basis of safety and immunogenicity data collected from American children and adults (2). Conjugation of four meningococcal polysaccharides to a protein carrier produces T-cell-dependent responses, unlike the T-cell-independent responses induced by plain polysaccharide vaccines. Avidity indices have been used to measure immune responses to vaccines in infants and young children to demonstrate antibody maturation (3); however, such indices are not as useful in adults, most of whom have had previous exposure to pathogens, with the result that vaccination provides a booster, rather than a primary, immune response in this age group (6). It has been suggested that a vaccine's ability to increase the immunoglobulin G1 (IgG1)/IgG2 ratio may indicate the activation of cellular control mechanisms typical for T-cell-dependent responses, as has been observed for pneumococcal conjugate vaccines in children (12, 19, 20). However, available data for meningococcal vaccines are scant; a single study of IgG subclasses after monovalent meningococcal group C conjugate vaccine has been published (8). In general, IgG subclass data for adults have been equivocal (8, 12, 19, 20), making the acquisition of further data of interest. We report the IgG1 and IgG2 subclass response to Menactra vaccine in 17 healthy adults evaluated in the United Kingdom.
MATERIALS AND METHODS
Ethical approval for the study was obtained from the Central and South Bristol research ethics committee (E5554). Seventeen healthy adults were recruited from the Bristol HPA laboratory, University of Bristol, and United Bristol Healthcare NHS Trust.
One dose of vaccine, Menactra (Sanofi Pasteur), was administered as a standard 0.5-ml dose (containing 4 μg each of serogroup A, C, W135, and Y polysaccharides and 48 μg of diphtheria toxoid formulated into 10 mM sodium phosphate-buffered physiological saline) intramuscularly in the left deltoid. Blood samples were obtained by venipuncture before and 4 to 6 weeks after vaccination. Separated sera were stored below −70°C for subsequent analysis. Any participant with a serogroup C serum bactericidal antibody (SBA) reciprocal titer of <8, a level associated with a lack of protection (1), based on postvaccination sera, was offered a dose of MCC vaccine and a subsequent (4 to 6 weeks later) reassay of antibody levels.
Subjects completed a health diary to record oral temperature and any local or systemic reactions daily for the week following vaccination. Serious adverse events were monitored using standard adverse event questionnaires completed by study personnel at each postvaccination visit.
Serogroup A-, C-, W135-, and Y-specific IgG antibody levels.
Sera were tested for serogroup-specific IgG antibodies using a standardized enzyme-linked immunosorbent assay described by Carlone et al. (5) for serogroup A, except reference serum CDC 1992 and monoclonal-PAN anti-human Fcγ peroxidase (Stratech Scientific) antibody were used. For the reference serum, we used previously assigned serogroup-specific IgG concentrations (7, 10). The polysaccharide and methylated human serum albumin concentrations used for microtiter plate coating were 5 μg/ml for serogroups A and C and 2 μg/ml and 1 μg/ml, respectively, for serogroups W135 and Y.
Serogroup A-, C-, W135-, and Y-specific IgG1 and IgG2 antibody levels.
Sera were tested for serogroup-specific IgG1 and IgG2 antibodies by enzyme-linked immunosorbent assay as described by Joseph et al. (10). Following nonspecific protein binding blocking, reference serum (CDC1992), an in-house quality control serum and unknown samples were added in duplicate to a Costar EIA/RIA (Corning Life Sciences, Schiphol, The Netherlands) medium binding plate coated with the required polysaccharide and eight twofold dilutions made directly in the plate, leaving two wells at the base of the quality control as buffer blanks. Following overnight serum incubation, plates were incubated sequentially with mouse monoclonal antibodies (MAbs) to human IgG subclasses for 3 h at room temperature, with alkaline phosphatase-conjugated rabbit anti-mouse antibody for 2.5 h at room temperature and with p-nitrophenyl phosphate (1 mg/ml) in 1 M diethanolamine buffer containing 0.5 mM MgCl2 (pH 9.8) for 2 h at room temperature. The antibodies used were anti-human MAbs anti-IgG1 (HP6069), anti-IgG2 (HP6200), and rabbit anti-mouse IgG1-alkaline phosphatase (Zymed Laboratories, CA). Both the MAbs and conjugated anti-mouse antibody were diluted in serum conjugate buffer at predetermined dilutions. The reaction was stopped by the addition of 50 μl 3 M sodium hydroxide and absorbances read at 405 nm with a reference filter at 690 nm.
Serum bactericidal antibody.
Sera were tested for SBA in a standardized protocol as previously described for serogroups A and C (14). The target strains were serogroup A (CDC no. F8238, phenotype A:4,21:P1.20,9), serogroup C strain C11 (C:16:P1.7-1,1), serogroup W135 (laboratory number M01 240070) (W:NT:P1.18-1,3) and serogroup Y (laboratory number M01 242975) (Y:2a:P1.5,2). The complement source was serum from 3- to 4-week-old rabbits (Pel-Freez Biologicals, WI). SBA titers were expressed as the reciprocal serum dilutions yielding ≥50% killing after 90 min for serogroup A and 60 min for serogroups C, W135, and Y.
Statistics.
Serogroup-specific IgG, IgG1, and IgG2 concentrations were calculated by interpolation from a reference serum curve plotted using a four-parameter, logistic curve model in the SOFTmax PRO data analysis software program (Molecular Devices, Wokingham, United Kingdom). Samples were assigned the lower limit of detection if the optical density of the first dilution was lower than the mean of the optical density of the last dilution of antibody in the reference serum. The lower limit of detection was set by the assay itself, based upon the lowest concentration of the reference standard.
Serogroup-specific IgG, IgG1, and IgG2 concentrations were log transformed and geometric mean concentrations (GMCs) calculated with 95% confidence intervals (CI).
SBA titers below 4, the lower limit of detection, were assigned a value of 2 for computational purposes. SBA titers were log transformed and geometric mean titers (GMTs) with 95% CI calculated. Rises (n-fold) in titer response from the baseline were calculated for each participant.
Paired t tests were used to test for differences between time points.
RESULTS
Participants.
The age range was 26 to 55 years; the median age was 31.5 years. Three participants had received a meningococcal plain polysaccharide (serogroups A and C or A, C, W135, and Y) vaccine at least one year prior to enrollment.
Safety monitoring.
Six subjects reported adverse events following vaccination; three reported injection site reactions, which were considered definitely related to vaccination. Two subjects had adverse events that were not considered related to vaccination (paronychia and athlete's foot), and two asthmatic subjects reported chest tightness that was considered possibly related to vaccination.
Serogroup-specific IgG responses.
Serogroup-specific IgG GMCs significantly increased postvaccination for all four serogroups (P < 0.01). See Table 1.
TABLE 1.
Serologic responses following vaccination with quadrivalent meningococcal conjugate vaccine by serogroup and IgG subclass
| Serogroup (n = 17) | SBA GMT (95% CI)
|
GMC (95% CI) (μg/ml)
|
Postvaccination n-fold rises in GMC
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IgG
|
IgG1
|
IgG2
|
||||||||
| Prevaccination | Postvaccination | Prevaccination | Postvaccination | Prevaccination | Postvaccination | Prevaccination | Postvaccination | IgG1 | IgG2 | |
| A | 37.7 (8.5-166.0) | 2,048 (832.8-5,036.4) | 1.1 (0.5-2.4) | 9 (4.1-19.5) | 0.1 (0.04-0.2) | 2.9 (1.08-7.8) | 1.5 (0.7-3.5) | 14.7 (4.8-44.7) | 29.0 | 9.8 |
| C | 22.2 (4.9-99.6) | 801.8 (267.4-2,403.9) | 1.6 (0.5-4.7) | 10.1 (4.5-22.6) | 0.4 (0.1-1.1) | 1.9 (0.8-4.6) | 0.9 (0.3-2.6) | 4.4 (1.5-12.8) | 4.8 | 4.9 |
| W135 | 7.1 (2.3-22.0) | 1,111.0 (355.3-3,474.4) | 0.6 (0.3-1.2) | 7.3 (2.3-22.6) | 0.6 (0.3-1.2) | 2.3 (1.1-4.9) | 0.3 (0.1-0.7) | 1.9 (0.5-6.7) | 3.8 | 6.3 |
| Y | 16.7 (3.5-78.9) | 1,670.3 (754.6-3,697.1) | 0.9 (0.5-1.9) | 17.7 (7.9-39.9) | 1.1 (0.6-1.8) | 6.7 (4.6-10.7) | 0.5 (0.2-1.5) | 17.7 (7.4-42.1) | 6.1 | 35.4 |
Serogroup-specific IgG subclass responses.
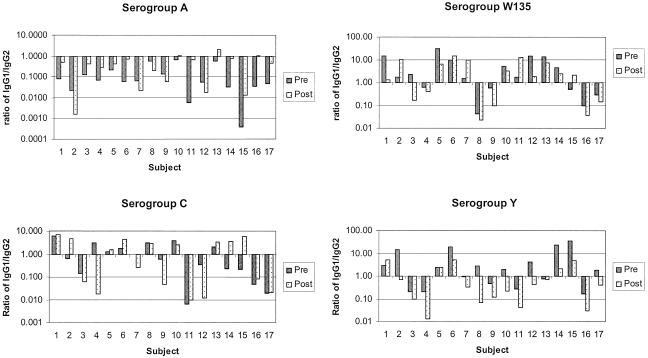
Before vaccination, IgG1/IgG2 ratios varied in individual subjects for all four serogroups (Fig. 1). No significant difference between IgG1 and IgG2 GMCs was noted for serogroups C, W135, and Y; however, the serogroup A-specific IgG2 GMC was significantly higher than that of IgG1 (P < 0.001).
FIG. 1.
Individual serogroup A, C, W135, and Y IgG1/IgG2 ratios pre- and postvaccination with meningococcal serogroup A, C, W135, and Y conjugate vaccine. An IgG1/IgG2 ratio of >1 indicates a higher proportion of IgG1; an IgG1/IgG2 ratio of <1 indicates a higher proportion of IgG2.
One month following vaccination, serogroup-specific IgG1 and IgG2 responses were induced to all four serogroups with significant increases in both IgG subclass GMCs (P < 0.05, for IgG1 and IgG2 for all four serogroups) (Table 1). In most individuals, the IgG antibody response was heterogeneous, consisting of both IgG1 and IgG2, and the ratios of IgG1/IgG2 differed by individual and serogroup (Fig. 1).
The serogroup A-specific IgG2 GMC was significantly higher than that of IgG1 (P = 0.004) postvaccination (Table 1); 14/17 (82%) subjects had a higher proportion of serogroup A-specific IgG2, i.e., an IgG1/IgG2 ratio of <1 (Fig. 1). Twelve (71%) individuals had an increase in IgG1/IgG2 ratio; of these, three had IgG1/IgG2 ratios of >1 postvaccination.
The serogroup C-specific response following vaccination varied by individual (Fig. 1), with IgG1/IgG2 ratios increasing in 8 (47%) subjects but decreasing in 9 (53%). In three subjects, the IgG1/IgG2 ratio increased from <1 prevaccination to >1 postvaccination.
GMCs for serogroup W135-specific IgG1 and IgG2 were not significantly different from each other postvaccination (P = 0.7). Serogroup W135-specific responses varied by subject. The IgG1/IgG2 ratio increased in 5 (29%) subjects and decreased in 12 (71%).
Following vaccination, serogroup Y-specific IgG2 GMC was higher, but not significantly so, than that for IgG1 (P = 0.17). Sixteen subjects (94%) had a decrease in IgG1/IgG2 ratio, with ratios declining to less than 1 in 12 (70%) subjects and decreasing from >1 prevaccination to <1 postvaccination in 5 subjects.
Serum bactericidal antibody.
Compared with baseline values, SBA GMTs significantly increased postvaccination for all serogroups (P < 0.01). Postvaccination SBA titers of ≥8 for serogroups C and W135 were observed in 16/17 (94%) subjects; all 17 (100%) subjects had SBA titers of ≥8 for serogroups A and Y. Fourfold rises in SBA following vaccination were seen in 15 (88%) subjects for serogroups A and C and in 16 (94%) for serogroups W135 and Y (Table 1). The one subject who did not achieve an SBA titer of 8 to the serogroup C portion of the vaccine was offered a dose of monovalent meningococcal C conjugate vaccine.
DISCUSSION
Different IgG subclasses have distinct effector functions, and in the context of vaccination, some antigenic stimuli induce mostly IgG1 antibodies, while others stimulate IgG2 antibodies preferentially (17). Serogroup-specific IgG2 represented a higher proportion of IgG in some participants in two previous studies of plain polysaccharide meningococcal vaccines (6, 17). In published literature, the relative proportions of IgG1 and IgG2 induced by vaccines have been shown to differ based on recipient age, vaccine type (plain polysaccharide versus protein conjugate), the specific bacterial polysaccharide type or subtype administered, and the protein used for conjugation (8, 12, 13, 15, 17, 19, 20). In some cases, responses to different components of the same vaccine varied with regard to subgroup or polysaccharide-specific response within an individual age group (8, 12, 19, 20). Although these differences in IgG subclass responses are incompletely understood, determination of the IgG subclass response to vaccination may eventually provide an insight into the type of immune response induced.
We observed significant increases in both IgG1 and IgG2 subclasses in 17 healthy adults following vaccination with a quadrivalent meningococcal conjugate vaccine against serogroups A, C, Y, and W135. Serogroup-specific IgG responses included both IgG1 and IgG2 in all cases, and the concentration of one subclass was higher in the majority of subjects. We found that IgG1/IgG2 ratios varied between individual vaccinees. In five subjects, postvaccination IgG concentrations had a higher proportion of IgG2 to all four serogroups. In the 12 remaining subjects, the predominant IgG subclass varied by serogroup. These findings are not surprising, given previous observations with the monovalent C conjugate vaccine (8).
All 17 subjects had SBA titers of ≥8, a level associated with protection against serogroup C disease, to serogroups A and Y postvaccination, with 16 of 17 (94%) having similarly high levels for serogroups C and W135. Our SBA results were comparable to those in previous studies of the quadrivalent meningococcal conjugate vaccine used (4, 11, 16); nevertheless, the relationship between functional antibody activity and IgG subclass is unknown.
The IgG subclass ratio findings in our study are difficult to interpret, particularly given the small sample size and the preliminary nature of the study. While the serogroup A-specific n-fold rise in IgG1 was greater than that for IgG2, which could be explained by a T-cell-dependent response, the rise in IgG2, which predominated over IgG1 in most sera, may indicate a T-cell-independent response. We do not yet have a satisfactory explanation for this apparent contradiction. Preexisting immunity through cross-reactive determinants carried on other bacteria (18) in combination with an IgG1 response that reflects a systemic immune response independent from the IgG2 recall could be a factor. Insel and Anderson (9) demonstrated that most of the antibodies produced following Haemophilus influenzae type b conjugate vaccination were produced by a few clones committed to a specific IgG subclass, which were present prior to vaccination. Preexisting antibody due to prior vaccination or natural immunity could also affect findings. This may also be an age-related phenomenon, consistent both with reported results within this age group immunized with pneumococcal conjugate vaccines and with results following monovalent C conjugate vaccine (12, 13). While it has been suggested that IgG2 production following meningococcal conjugate vaccine could be due to free oligosaccharide, there are no available data on the current vaccine to support this hypothesis (13). Studies of the quadrivalent conjugate vaccine may provide further information.
Our results imply that in some adults, cellular control mechanisms thought to be typical of a T-cell-dependent response have been activated. Further work to elucidate the IgG subclass responses of meningococcal polysaccharide or conjugate vaccination and natural priming are warranted.
Acknowledgments
We thank Nick Andrews (Statistics Unit, Communicable Disease Surveillance Centre, HPA Centre for Infections, London) for critical reading of the manuscript and statistical analysis. Thomas Papa and Lisa DeTora of Sanofi Pasteur also provided scientific and editorial guidance in the construction of the manuscript.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilukha, O. O., and N. E. Rosenstein. 2005. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. 54:1-21. [PubMed] [Google Scholar]
- 3.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for the use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of the correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, J. D., R. Edelman, J. C. King, Jr., T. Papa, R. Ryall, and M. B. Rennels. 2002. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J. Infect. Dis. 186:1848-1851. [DOI] [PubMed] [Google Scholar]
- 5.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S.H.Turner, B. D. Pilkaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, B. Swaminthan, G. Arakere, C. Thompson, D. Phipps, D. Madore, and C. V. Broome. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldblatt, D., R. Borrow, and E. Miller. 2002. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J. Infect. Dis. 185:397-400. [DOI] [PubMed] [Google Scholar]
- 7.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huo, Z., R. Sinha, E. A. McNeela, R. Borrow, R. Giemza, C. Cosgrove, P. T. Heath, K. H. Mills, R. Rappuoli, G. E. Griffin, and D. J. Lewis. 2005. Induction of protective serum meningococcal bactericidal and diphtheria-neutralizing antibodies and mucosal immunoglobulin A in volunteers by nasal insufflations of the Neisseria meningitidis serogroup C polysaccharide-CRM197 conjugate vaccine mixed with chitosan. Infect. Immun. 73:8256-8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insel, R. A., and P. W. Anderson. 1986. Response to oligosaccharide-protein conjugate vaccine against Haemophilus influenzae b in two patients with IgG2 deficiency unresponsive to capsular polysaccharide vaccine. N. Engl. J. Med. 315:499-503. [DOI] [PubMed] [Google Scholar]
- 10.Joseph, H., P. Balmer, M. Bybel, T. Papa, R. Ryall, and R. Borrow. 2004. Assignment of Neisseria meningitidis serogroups A, C, W135, and Y anticapsular total immunoglobulin G (IgG), IgG1, and IgG2 concentrations to reference sera. Clin. Diagn. Lab. Immunol. 11:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keyserling, H., T. Papa, K. Koranyi, R. Ryall, E. Bassily, M. J. Bybel, K. Sullivan, G. Gilmet, and A. Reinhardt. 2005. Safety, immunogenicity, and immune memory of a novel meningococcal (groups A, C, Y, and W-135) polysaccharide diphtheria toxoid conjugate vaccine (MCV-4) in healthy adolescents. Arch. Pediatr. Adolesc. Med. 159:907-913. [DOI] [PubMed] [Google Scholar]
- 12.Lottenbach, K. R., C. M. Mink, S. J. Barenkamp, E. L. Anderson, S. M. Homan, and D. C. Powers. 1999. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect. Immun. 67:4935-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maclennan, J., S. Obaro, J. Deeks, D. Lake, C. Elie, G. Carlone, E. R. Moxon, and B. Greenwood. 2001. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J. Infect. Dis. 183:97-104. [DOI] [PubMed] [Google Scholar]
- 14.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, et al. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxelius, V. A. 1979. IgG subclass levels in infancy and childhood. Acta Paediatr. Scand. 68:23-27. [DOI] [PubMed] [Google Scholar]
- 16.Pichichero, M., J. Casey, M. Blatter, E. Rothstein, R. Ryall, M. Bybel, G. Gilmet, and T. Papa. 2005. A comparative trial of the safety and immunogenicity of quadrivalent (A, C, Y, W-135) meningococcal polysaccharide-diphtheria conjugate vaccine versus quadrivalent polysaccharide vaccine in two ten-year-old children. Pediatr. Infect. Dis. J. 24:57-62. [DOI] [PubMed] [Google Scholar]
- 17.Rautonen, N., J. Pelkonen, S. Sipinen, H. Kayhty, and O. Makela. 1986. Isotype concentrations of human antibodies to group A meningococcal polysaccharide. J. Immunol. 137:2670-2675. [PubMed] [Google Scholar]
- 18.Robbins, J., L. Myerowitz, J. K. Whisnant, M. Argaman, R. Schneerson, Z. T. Handzel, and E. C. Gotschlich. 1972. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect. Immun. 6:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikkema, D. J., N. A. Ziembiec, T. R. Jones, S. W. Hildreth, D. V. Madore, and S. A. Quataert. 2005. Assignment of weight-based immunoglobulin G1 (IgG1) and IgG2 units in antipneumococcal reference serum lot 89-S(F) for pneumococcal polysaccharide serotypes 1, 4, 5, 7F, 9V, and 18C. Clin. Diagn. Lab. Immunol. 12:218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soininen, A., I. Seppala, T. Nieminen, J. Eskola, and H. Kayhty. 1999. IgG subclass distribution of antibodies after vaccination of adults with pneumococcal conjugate vaccines. Vaccine 17:1889-1897. [DOI] [PubMed] [Google Scholar]