Abstract
Serial measurement of antibodies has not been used to provide evidence of active viral replication of human papillomavirus (HPV). Serum specimens from sequential study visits contributed by 642 human immunodeficiency virus (HIV)-positive and 116 HIV-negative participants enrolled in the Women's Interagency HIV Study were used to detect significant rises in HPV type 16 (HPV-16) antibody levels. Factors associated with a significant rise were identified using multivariable logistic regression models with generalized estimating equations. Among HIV-positive women, 8.3% of 1,997 pairs showed antibody rises, compared to 6.1% of 361 pairs among HIV-negative women (P = 0.191). For HIV-positive women, rises were associated with current (odds ratio [OR], 23.4; P < 0.001) or past (OR, 8.9; P < 0.001) HPV-16 infection relative to never being HPV-16 infected and with CD4+ cell counts (OR per 100-cell increase, 0.8; P < 0.001) but not with sexual behavior. For HIV-negative women, rises were associated with past (OR, 10.9; P = 0.033) HPV-16 infection relative to no HPV-16, current cigarette smoking (OR, 5.0; P = 0.029) relative to no smoking history, and having 6 to 10 lifetime sexual partners compared to 0 to 5 partners (OR, 9.9; P = 0.036). Serial measurement of HPV-16 serum antibodies is a useful tool for identifying active HPV-16 viral replication. Rises among HIV-positive women may more often result from reactivation of a latent HPV infection in the context of HIV-induced immunosuppression, while rises among HIV-negative women may more often result from reinfection with HPV.
Human papillomavirus (HPV) is the most common sexually transmitted viral infection in humans and the etiological agent of cervical and other anogenital cancers (for a review, see reference 5). Diagnosis of HPV infection has relied primarily on detection of the viral genome by PCR or hybrid capture, a non-amplification-based nucleic acid detection method. In longitudinal studies with infrequent sampling, HPV infection may be underestimated by DNA-based methods alone because infections are usually transient (13, 19, 21).
Detection of virus-specific serum antibodies is a well-established biomarker of viral infection. Over the past decade, serological assays for HPV based on virus-like particles (VLP) have been validated by numerous studies (for a review, see reference 11). HPV type 16 (HPV-16) VLP-based enzyme-linked immunosorbent assays (ELISA) in general, including our own (31, 32, 36), have a sensitivity of 50% or greater for current HPV-16 infections detected by PCR. The absence of detectable serum antibodies in all individuals with an infection documented by PCR is probably multifactorial, including misclassification of infection by PCR, delayed seroconversion, viral-mediated immune evasion, and antibody responses below the level of detection of available assays. The type specificity of VLP-based ELISA is strongly supported by experimental studies with sera of known specificity and human studies demonstrating stronger associations of seroreactivity with detection of homologous DNA than with DNA of other types. In human studies, the smaller but significant associations with some other HPV types may be explained by the fact that different genital HPV types are transmitted similarly and the fact that multiple infections are very common. The most consistent finding from epidemiological studies of the determinants of VLP seroreactivity is the strong correlation with lifetime number of sexual partners, thus documenting the validity of HPV antibody measurement as a marker of past HPV exposure.
Although serial measurements of HPV antibodies have been utilized to document the kinetics of the serum antibody response to infection (8), paired samples have not been used to identify active viral replication. We used serial measurements of antibodies to HPV-16 to identify significant rises in antibody levels between two study visits for participants enrolled in a large prospective cohort study of human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. We then investigated factors associated with significant rises to assess the value of this marker as a measure of active viral replication.
MATERIALS AND METHODS
Study population.
The Women's Interagency HIV Study (WIHS) is a multicenter prospective cohort study consisting of 2,059 HIV-positive and 569 HIV-negative women enrolled from 1994 to 1995. At baseline and at each 6-month follow-up visit, participants completed interviewer-administered questionnaires to assess sociodemographic characteristics, medical/health history, obstetric and gynecologic history and contraceptive use, tobacco, alcohol, and drug use, and sexual behaviors. In addition, women had a physical and gynecologic examination, which included a Papanicolaou smear and the collection of blood, urine, and cervicovaginal lavage samples. A more detailed description of the WIHS cohort characteristics, recruitment methods, and protocols has been published previously (4, 18). Our analyses included participants who contributed one or more pairs of consecutive study visits with HPV-16 antibody results available. The unit of observation for analyses was a pair of consecutive study visits. Participants could contribute up to five pairs corresponding to consecutively attended visits at yearly intervals between WIHS visits 3 and 13.
Laboratory methods.
All women at study baseline with HPV-16, -18, -31, -6, or -11 detected by HPV DNA and a random sample of women without prevalent DNA of the above types were tested for HPV-16 antibodies by HPV-16 VLP-based ELISA as previously described (35). To reduce interassay variation, all samples from a woman were tested on the same microtiter plate. The technician performing the test was blinded to the identity of samples from the same woman. At yearly intervals beginning with the third study visit through WIHS visit 13, a total of 3,246 serum samples from these women were tested in duplicate (coefficient of variation of 11%). Analyses used optical density (OD) units to quantify antibody levels and used a previously determined cutoff of 0.05 OD units to define HPV-16 seropositivity (34). For descriptive purposes, OD units were transformed to immunoglobulin G (IgG) levels by use of the following formula developed using standard serial twofold dilutions of IgG: y = 1.1x + 2.6 (where y equals the log10 IgG protein concentration and x equals the log10 mean OD). The relationship between OD value and IgG concentration was linear from 2 to 1,000 μg/ml IgG protein.
Cervicovaginal cells were tested for the presence of HPV DNA by PCR with MY09/MY11 L1 consensus primers as described previously (25). Plasma HIV RNA was measured using the isothermal nucleic acid sequence-based amplification (Nuclisens) method (bioMérieux, Boxtel, The Netherlands). CD4+ T-lymphocyte counts (cells/μl) were determined using standardized flow cytometry (7). Pap smears were read centrally by two cytotechnologists who used the 1991 Bethesda system for cytologic diagnosis, and any abnormal specimens were diagnosed by a cytopathologist (23).
Primary outcome.
The outcome of interest was a significant rise in OD units within a pair of study visits, typically 1 year apart. We determined HPV-16 antibody levels, measured in OD units, at both the index visit (t0) and the follow-up visit (t1) for all pairs. We standardized the change in OD units by dividing the difference in OD values between t0 and t1 by the amount of time between t0 and t1.
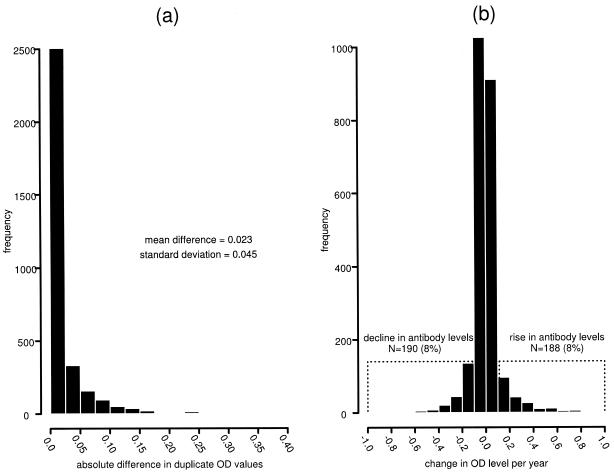
To distinguish true rises in antibody levels from random variation, we examined differences in duplicate samples tested at a given visit, which can be due only to random variation in the assay. The average absolute difference in OD units between duplicate samples was 0.023, with a standard deviation of 0.045. A significant rise in antibody levels for a pair of visits was defined as a change greater than 0.113 OD units (mean + 2 standard deviations). Significant declines were determined similarly, as a decrease of 0.113 OD units or more. Changes occurring between the threshold of a rise and a decline were considered nonsignificant fluctuations in antibody levels. Figure 1a displays a histogram of the absolute differences found in duplicate samples, and Fig. 1b displays the histogram of changes in OD units for all pairs. The magnitudes of the changes in antibody levels were similar across the operational range of the assay at t0 (data not shown). In addition, we were interested in longitudinal changes in antibody in the year following the identification of a rise, among women contributing a second pair after a rise. Rises or nonsignificant fluctuations in antibody levels in the second pair were considered sustained rises. Declines in antibody levels in the second pair were considered transient rises.
FIG. 1.
Distribution of the absolute differences in OD units for HPV-16 IgG levels for 3,246 samples tested in duplicate samples (a) and distribution of changes in OD units for 2,358 pairs of visits (1,997 from HIV-positive participants and 361 from HIV-negative participants) (b).
Changes in HPV-16 antibody levels over time could occur because of nonspecific changes in serum immunoglobulins due to HIV disease or other causes. Therefore, we measured BK virus (BKV) antibody levels by a VLP-based ELISA, as previously described (12, 33), among pairs with a rise in HPV-16 antibody and a random sample of an equal number of pairs without a rise. Exposure to BKV occurs in childhood, and seroprevalence reaches nearly 100% by early adulthood; antibody responses to BKV are robust and persist for life (20). Significant rises in BKV antibody levels (cutoff of 0.202 OD units) were determined using the same method as that described for HPV-16 antibody levels. As a measure of change in serum immunoglobulins, an ELISA for a viral antibody should provide greater precision than standard nephelometric methods for measuring total immunoglobulins. In addition, BKV antibodies were measured by a method technically identical to that used to detect HPV-16 antibodies.
Exposures.
The primary exposure of interest was HPV-16 infection categorized as current, past, or never. Current infection was defined as HPV-16 DNA at t0. Past infection was defined in one of three ways: (i) HPV-16 DNA prior to t0 (accounted for 5% of past HPV-16 infection), (ii) HPV-16 seropositivity at or prior to t0 (accounted for 80% of past HPV-16 infection), and (iii) HPV-16 DNA prior to t0 and HPV-16 seropositivity at or prior to t0 (accounted for 15% of past HPV-16 infection). Never HPV infected was defined as the absence of HPV-16 DNA and HPV-16 seropositivity at t0 and prior to t0.
Another exposure of interest was sexual behavior, with the following categorizations: recent male partners in the prior 6 months (>1, 1, or 0 partners), any male sex partners in the previous year, lifetime number of male sex partners (>10, 6 to 10, or 0 to 5 partners), and a composite of lifetime and recent sexual behavior. HAART use in the WIHS was defined according to DHHS guidelines (30) and has been described in prior publications (22, 26). Other factors considered in analyses and measured at t0 were abnormal versus normal Pap smear results, CD4+ cells/mm3 (per 100 cells), HIV RNA copies/ml (per log10), prior clinical AIDS diagnosis, age (per 10 years), race/ethnicity, injection drug use history, cigarette smoking history, and oral contraceptive use.
Statistical analyses.
Using logistic regression, we first computed univariable odds ratios (OR) for the outcome of a rise in HPV-16 antibody levels stratified by HIV serostatus. Multivariable models were constructed separately for HIV-positive and HIV-negative women by including all covariates with a P of <0.10 in HIV-stratified univariable analyses. Next, covariates with a P of ≥0.10 were added to the multivariable model one at a time and included if they were significant at the 0.10 level. The final multivariable model was obtained by removing covariates from the last iteration one at a time, starting with the highest P value, until all covariates had a P of <0.10. For all logistic regression analyses, generalized estimating equations (10) were used to account for the correlation between pairs of study visits contributed by the same individual. Kaplan-Meier plots and Cox proportional-hazards models (9) were used to illustrate differences by HIV serostatus in the cumulative incidence of HPV-16 infection defined both serologically (defined as the first pair to exhibit a rise in antibody levels after one or more pairs without a rise) and by PCR (defined as the first visit to be HPV-16 DNA positive after one or more visits without HPV-16 DNA).
RESULTS
The study population consisted of 642 HIV-positive and 116 HIV-negative women contributing a median of three pairs of visits (range, one to five pairs), with HIV-positive and HIV-negative women contributing 1,997 and 361 pairs of visits, respectively. The median time between index and follow-up visits among pairs was 1.0 years (interquartile range, 0.9 to 1.1) for both HIV-positive and HIV-negative women. Compared to WIHS participants not included in analyses, study participants had similar races and ages but were more likely to be HIV positive and positive for any HPV DNA (including HPV-16 DNA) at baseline.
Among all pairs contributed by HIV-positive women, 166 (8.3%) showed antibody rises, 169 (8.5%) had declines, and 1,662 (83.2%) had no significant change (Table 1). Among all pairs of visits contributed by HIV-negative women, 22 (6.1%) were rises, 21 (5.8%) were declines, and 318 (88.1%) exhibited no significant change. No differences in the proportion of pairs with a rise (P = 0.191) or decline (P = 0.120) by HIV serostatus were found. Among HIV-positive and HIV-negative women, 14 (8%) and 1 (5%) of all antibody rises, respectively, were considered seroconversions, that is, the rise in antibody level crossed the previously defined cutoff point for seropositivity (0.05 OD units) and occurred in a participant who had not been seropositive at any prior visit. Table 2 presents changes in antibody results in the year following a rise (i.e., typically over a total of three study visits or 2 years). Of 188 total rises, 128 (115 HIV positive and 13 HIV negative) had a subsequent pair of visits available for analysis. Declines following a rise were observed to occur in 47.0% and 61.4% (P = 0.308) of pairs for HIV-positive and HIV-negative women, respectively, suggesting that many observed rises were transient and not sustained. We did not evaluate longitudinal changes in HPV-16 serology beyond 2 years because of the small number of women contributing data. Furthermore, sampling of antibody levels annually precluded a more precise determination of the duration of the response.
TABLE 1.
Proportion of visit pairs with a rise, decline, or no change in HPV-16 IgG levels by HIV serostatus
| Change in IgG levelsa for a pair of visits | Results for HIV-positive women (n = 642)
|
Results for HIV-negative women (n = 116)
|
||
|---|---|---|---|---|
| No. (%) of pairsc | Median (IQRe) change in IgG levels | No. (%) of pairsc | Median (IQRe) change in IgG levels | |
| Riseb | 166d (8.3) | 88.3 (61.4, 147.7) | 22d (6.1) | 72.2 (62.1, 91.6) |
| Declineb | 169 (8.5) | −71.8 (−111.6, −54.2) | 21 (5.8) | −90.6 (−117.7, −49.9) |
| No change | 1,662 (83.2) | −0.2 (−4.4, 3.1) | 318 (88.1) | 0.1 (−2.5, 3.2) |
| Total | 1,997 | 361 | ||
For descriptive purposes, OD units were transformed to IgG levels by use of the following formula developed using standard serial twofold dilutions of IgG: y = 1.1x + 2.6 (where y equals the log10 IgG protein concentration and x equals the log10 mean OD). The relationship between OD value and IgG concentration was linear from 2 to 1,000 μg/ml IgG protein.
A significant rise or decline corresponds to an increase or decrease, respectively, of >0.113 in OD level.
No difference was found by HIV serostatus for percentages with a rise (P = 0.191) or decline (P = 0.120), based on generalized estimating equations.
Fourteen rises (8%) and one rise (5%) among HIV-positive and HIV-negative women, respectively, were seroconversions.
IQR, interquartile range.
TABLE 2.
Changes in HPV-16 IgG levels by HIV serostatus after a rise
| Parameter | No. (%) of instances
|
|
|---|---|---|
| HIV positive | HIV negative | |
| No. of pairs of visits with a rise in IgG levels | 166 | 22 |
| No. with second pair following initial rise | 115 | 13 |
| Change in IgG levels for second pair | ||
| Rise | 12 (10.4) | 0 (0.0) |
| Decline | 54 (47.0) | 8 (61.4) |
| No change | 49 (42.6) | 5 (38.5) |
| Sustained risea | 61 (53.0) | 5 (38.5) |
Defined as a rise or no change in second pair. P = 0.308 by HIV serostatus, based on generalized estimating equations.
The univariable results for HIV-positive women shown in Table 3 demonstrate that current and past HPV-16 infections were associated with OR of a rise in HPV-16 IgG levels of 27.0 (95% confidence interval [CI], 9.9, 73.5) and 9.2 (95% CI, 3.7, 22.7), respectively, relative to having no evidence of infection. Supporting the specificity of a rise in antibodies as a measure of HPV-16 infection, we observed a greater likelihood of an antibody rise for subjects having HPV-16 DNA (OR, 5.3; 95% CI, 2.9, 9.6) than for subjects being HPV DNA negative at the index visit, but we found no statistical significance for other HPV types. We also found a greater likelihood of a rise in antibodies following an abnormal Pap smear at the index visit (OR, 1.8; 95% CI, 1.3, 2.6) and with higher HIV RNA levels (OR per log10 level, 1.6; 95% CI, 1.3, 2.0), and we discovered a lower likelihood of an antibody rise with higher CD4+ cell counts (OR per 100 cells, 0.8; 95% CI, 0.8, 0.9). There was no association between a rise in HPV-16 antibody levels and recent or past sexual history. In the multivariable models for HIV-positive women, also presented in Table 3, there was a strong association between rises in antibody levels for current (OR, 23.4; 95% CI, 8.7, 62.8) and past (OR, 8.9; 95% CI, 3.6, 22.2) HPV-16 infection relative to never being HPV-16 infected. Higher CD4+ cell count levels were associated with a lower likelihood of a rise (OR per 100 cells, 0.8; 95% CI, 0.7, 0.9). Finally, sexual behavior was not associated with HPV-16 seroconversion, although with 14 total seroconversions among HIV-positive women (data not shown), we had limited statistical power to examine this question.
TABLE 3.
Factors associated with a rise in HPV-16 IgG levels for HIV-positive women
| Variable | na | % Rise | Univariable results
|
Multivariable resultsb
|
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| HPV-16 infectionc | ||||||
| Current | 108 | 24.1 | 27.0 (9.9, 73.5) | <0.001 | 23.4 (8.7, 62.8) | <0.001 |
| Past | 1,131 | 9.7 | 9.2 (3.7, 22.7) | <0.001 | 8.9 (3.6, 22.2) | <0.001 |
| Never | 430 | 1.2 | 1 | 1 | ||
| HPV DNA | ||||||
| HPV-16 | 108 | 24.1 | 5.3 (2.9, 9.6) | <0.001 | ||
| Other HPV | 1,064 | 8.2 | 1.5 (0.9, 2.4) | 0.092 | ||
| No HPV | 497 | 5.6 | 1 | |||
| Pap smear | ||||||
| Abnormal | 918 | 10.7 | 1.8 (1.3, 2.6) | 0.001 | ||
| Normal | 906 | 6.2 | 1 | |||
| HAART use | ||||||
| Yes | 736 | 7.3 | 0.8 (0.6, 1.1) | 0.234 | 0.7 (0.5, 1.0) | 0.063 |
| No | 1,260 | 8.9 | 1 | 1 | ||
| CD4+ cell count, cells/mm3 (per 100 cells) | 0.8 (0.8, 0.9) | <0.001 | 0.8 (0.7, 0.9) | <0.001 | ||
| HIV RNA, copies/ml (per log10) | 1.6 (1.3, 2.0) | <0.001 | ||||
| Prior AIDS diagnosis | ||||||
| Yes | 895 | 8.4 | 1.0 (0.7, 1.4) | 0.924 | ||
| No | 1,102 | 8.3 | 1 | |||
| Age (per 10 yr) | 0.8 (0.7, 1.0) | 0.061 | 0.8 (0.6, 1.0) | 0.098 | ||
| Race/ethnicity | ||||||
| White | 350 | 9.1 | 1.1 (0.7, 1.7) | 0.594 | ||
| Hispanic | 438 | 8.2 | 1.0 (0.7, 1.5) | 0.990 | ||
| Other | 38 | 5.3 | 0.6 (0.2, 2.3) | 0.470 | ||
| African-American | 1,171 | 8.2 | 1 | |||
| Injection drug use | ||||||
| Yes | 97 | 8.3 | 1.0 (0.5, 2.2) | 0.983 | ||
| No | 1,889 | 8.3 | 1 | |||
| Cigarette smoking | ||||||
| Current | 1,115 | 8.8 | 1.2 (0.8, 1.7) | 0.434 | ||
| Former | 192 | 7.8 | 1.0 (0.6, 1.8) | 0.943 | ||
| Never | 679 | 7.7 | 1 | |||
| Oral contraceptive use | ||||||
| Yes | 64 | 12.5 | 1.6 (0.7, 3.5) | 0.250 | ||
| No | 1,847 | 8.2 | 1 | |||
| No. of male sex partners in last 6 mo | ||||||
| >1 | 220 | 6.8 | 0.8 (0.4, 1.4) | 0.382 | ||
| 1 | 1,113 | 8.5 | 1.0 (0.7, 1.4) | 0.910 | ||
| 0 | 651 | 8.6 | 1 | |||
| Any male sex partners in last yr | ||||||
| Yes | 1,464 | 8.1 | 1.0 (0.6, 1.4) | 0.847 | ||
| No | 439 | 8.4 | 1 | |||
| No. of lifetime male sex partners | ||||||
| >10 | 1,009 | 8.4 | 1.0 (0.7, 1.5) | 0.989 | ||
| 6-10 | 349 | 8.3 | 1.0 (0.6, 1.6) | 0.945 | ||
| 0-5 | 592 | 8.5 | 1 | |||
| No. of lifetime male sex partners/no. of male sex partners in last 6 mo | ||||||
| >10/>0 | 731 | 7.9 | 1.0 (0.6, 1.6) | 0.985 | ||
| >10/0 | 273 | 9.9 | 1.3 (0.7, 2.3) | 0.426 | ||
| ≤10/>0 | 569 | 8.6 | 1.1 (0.7, 1.7) | 0.721 | ||
| ≤10/0 | 364 | 8.0 | 1 | |||
Missing data resulted in different total numbers for each variable.
Final multivariable model adjusted for other factors with results in column. See the text for procedure to determine final model.
Current infection was defined as HPV-16 DNA at t0. Past infection was defined in one of three ways: (i) HPV-16 DNA prior to t0, (ii) HPV-16 seropositivity at or prior to t0, or (iii) HPV-16 DNA prior to t0 and HPV-16 seropositivity at or prior to t0. Never HPV infected was defined as the absence of HPV-16 DNA and HPV-16 seropositivity at t0 and prior to t0.
The univariable and multivariable results for HIV-negative women are presented in Table 4. We demonstrated that current and past HPV-16 infections were associated with an OR of a rise in HPV-16 IgG levels of 11.6 (95% CI, 0.7, 186.5) or 9.7 (95% CI, 1.2, 78.3), respectively, relative to having no evidence of infection. The nonsignificant finding for current HPV-16 infection was likely a result of small sample size in that group (n = 10). Current smoking was associated with increased odds of a rise relative to lifetime nonsmoking (OR, 5.4; 95% CI, 1.3, 23.2). ORs for all levels of sexual activity were greater than 2.0 relative to the reference group. However, because of the relatively small number of visit pairs (n = 22) with a significant antibody rise contributed by HIV-negative women, the only statistically significant (P < 0.05) result was a greater likelihood of a rise in antibody levels for a lifetime number of male sex partners of >10 than for a number of partners from 0 to 5. In multivariable models, there was a statistically significant association between past (OR, 10.9; 95% CI, 1.2, 98.3) but not current HPV-16 infection relative to never being HPV-16 infected. Current smoking relative to lifetime nonsmoking (OR, 5.0; 95% CI, 1.2, 20.8) and 6 to 10 lifetime male sex partners relative to 0 to 5 partners (OR, 9.9; 95% CI, 1.2, 85.4) were other factors significantly related to a rise in HPV-16 antibody levels. We did not examine associations with HPV-16 seroconversions since there was only one event in this group.
TABLE 4.
Factors associated with a rise in HPV-16 IgG levels for HIV-negative women
| Variable | na | % Rise | Univariable results
|
Multivariable resultsb
|
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| HPV-16 infectionc | ||||||
| Current | 10 | 10.0 | 11.6 (0.7, 186.5) | 0.085 | 9.6 (0.4, 214.7) | 0.152 |
| Past | 199 | 8.5 | 9.7 (1.2, 78.3) | 0.033 | 10.9 (1.2, 98.3) | 0.033 |
| Never | 105 | 1.0 | 1 | 1 | ||
| HPV DNA | ||||||
| HPV-16 | 10 | 10.0 | 2.0 (0.3, 15.3) | 0.505 | ||
| Other HPV | 76 | 7.9 | 1.5 (0.6, 3.8) | 0.340 | ||
| No HPV228 | 228 | 5.3 | 1 | |||
| Pap smear | ||||||
| Abnormal | 44 | 9.1 | 1.7 (0.5, 5.4) | 0.389 | ||
| Normal | 284 | 5.6 | 1 | |||
| Age (per 10 yr) | 1.0 (0.7, 1.4) | 0.962 | ||||
| Race/ethnicity | ||||||
| White | 41 | 7.3 | 1.1 (0.3, 3.6) | 0.849 | ||
| Hispanic | 76 | 4.0 | 0.6 (0.1, 2.7) | 0.490 | ||
| Other | 16 | 6.3 | 0.9 (0.1, 7.7) | 0.959 | ||
| African-American | 228 | 6.6 | 1 | |||
| Injection drug use | ||||||
| Yes | 19 | 5.3 | 0.8 (0.1, 7.2) | 0.877 | ||
| No | 340 | 6.2 | 1 | |||
| Cigarette smoking | ||||||
| Current | 206 | 8.7 | 5.4 (1.3, 23.2) | 0.023 | 5.0 (1.2, 20.8) | 0.029 |
| Former | 38 | 5.3 | 3.1 (0.5, 21.5) | 0.244 | 1.4 (0.2, 12.6) | 0.775 |
| Never | 115 | 1.7 | 1 | 1 | ||
| Oral contraceptive use | ||||||
| Yes | 26 | 7.7 | 1.4 (0.4, 5.6) | 0.621 | ||
| No | 324 | 5.6 | 1 | |||
| No. of male sex partners in last 6 mo | ||||||
| >1 | 80 | 8.8 | 2.9 (0.8, 11.1) | 0.117 | ||
| 1 | 185 | 6.5 | 2.1 (0.6, 7.5) | 0.252 | ||
| 0 | 94 | 3.2 | 1 | |||
| Any male sex partners in last yr | ||||||
| Yes | 289 | 6.9 | 4.2 (0.6, 29.4) | 0.152 | ||
| No | 57 | 1.8 | 1 | |||
| No. of lifetime male sex partners | ||||||
| >10 | 164 | 7.9 | 8.2 (1.1, 62.8) | 0.043 | 5.4 (0.7, 41.6) | 0.107 |
| 6-10 | 96 | 8.3 | 8.6 (1.0, 77.0) | 0.053 | 9.9 (1.2, 85.4) | 0.036 |
| 0-5 | 96 | 1.0 | 1 | 1 | ||
| No. of lifetime male sex partners/no. of male sex partners in last 6 mo | ||||||
| >10/>0 | 130 | 8.5 | 5.4 (0.7, 39.2) | 0.098 | ||
| >10/0 | 32 | 6.3 | 3.9 (0.4, 40.2) | 0.257 | ||
| ≤10/>0 | 133 | 6.0 | 3.7 (0.4, 30.9) | 0.225 | ||
| ≤10/0 | 59 | 1.7 | 1 | |||
Missing data resulted in different total numbers for each variable.
Final multivariable model adjusted for other factors with results in column. See the text for procedure to determine final model.
Current infection was defined as HPV-16 DNA at t0. Past infection was defined in one of three ways: (i) HPV-16 DNA prior to t0, (ii) HPV-16 seropositivity at or prior to t0, or (iii) HPV-16 DNA prior to t0 and HPV-16 seropositivity at or prior to t0. Never HPV infected was defined as the absence of HPV-16 DNA and HPV-16 seropositivity at t0 and prior to t0.
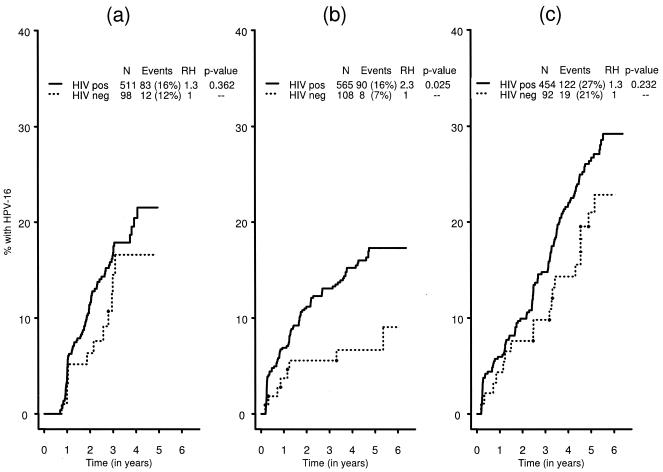
We further explored differences in risk of infection between HIV-positive and HIV-negative women by identifying newly detected rises in HPV-16 antibodies or newly detected HPV-16 DNA. The cumulative incidence of newly detected rises in HPV-16 antibodies was not significantly higher in HIV-positive women (22%) than in HIV-negative women (17%) (relative hazard [RH], 1.3; P = 0.362) (Fig. 2a). In contrast, the cumulative incidence of HPV-16 infection as detected by PCR was significantly higher for HIV-positive (17%) than for HIV-negative (9%) women (RH, 2.3; P = 0.025) (Fig. 2b). A higher incidence of HPV-16 infection was found when using a definition that included either a rise in HPV-16 antibodies or detection of HPV-16 DNA in cervicovaginal cells, but there was no difference by HIV serostatus (RH, 1.3; P = 0.232) (Fig. 2c).
FIG. 2.
Cumulative incidence of HPV-16 infection, defined by an incident rise in HPV-16 antibody levels (a), incident HPV-16 DNA detected by PCR (b), or by either method (c). pos, positive; neg, negative.
We also performed a secondary analysis measuring changes in antibody level to BKV to confirm the specificity of rises in HPV-16 antibody levels. In univariable models (data not shown), HIV infection, low CD4+ cell count levels, higher HIV RNA levels, and 0 to 5 lifetime sexual partners (but not >5 partners) were associated with a rise in BKV antibody levels. Importantly, there was a null association between a rise in BKV antibody levels and a rise in HPV-16 antibody levels, current or past HPV-16 infection, and detection of HPV-16 DNA. In multivariable models, only immunosuppression remained a predictor of BKV antibody rises (data not shown). Finally, we further examined significant declines in HPV-16 IgG levels. As described above and in Table 2, declines were commonly observed in the year following a rise. Multivariable analysis confirmed that only a prior rise in HPV-16 IgG levels was associated with a subsequent decline in antibody levels (data not shown). These data suggest a decline is likely due to the waning of the antibody response to HPV over time.
DISCUSSION
We propose that a rise in HPV-16 antibodies is a marker of active viral replication during the sampling interval. Support for the validity of the marker rests on the well-established performance characteristics of HPV VLP ELISA (11), general immunological principles which hold that a rise in antibody level above baseline is indicative of viral infection, and the associations demonstrated herein. Serum immunoglobulin levels are tightly regulated and generally remain stable for prolonged periods of time (3, 16). In the absence of immune system dysfunction, rises in antiviral antibodies reflect antigenic stimulation and thus comparison of the levels of virus-specific antibodies in acute- and convalescent-phase serum samples is a time-honored method of viral diagnosis (37). In the context of prior infection, typically, repeat exposures to viral antigen will result in an anamnestic immune response reflected as a rise in antibody level, such as is observed upon vaccine boost. Following natural infection, a serologic profile of a rising antibody level has been used to diagnosis reactivation of Epstein-Barr virus (24), varicella-zoster virus (14, 15), and human herpesvirus 6 (27). Here, we applied this approach to HPV-16 infections and identified increases in virus-specific antibody levels above an a priori threshold meant to distinguish between real changes and random variations in paired serum samples.
As expected for a type-specific HPV biomarker, there was a strong association of a rise in serum HPV-16 VLP antibody level with detection of HPV-16 DNA, a weak association with detection of other HPV types, and an association with Pap abnormalities at the index visit. Rises in HPV-16 IgG levels were typically not sustained, as declines in IgG levels were commonly observed to occur following a rise. The specificity of rises in HPV-16 antibody level is further supported by the null association with rises in antibody to BKV, indicating that rises in HPV antibody level were not due to nonspecific changes in immunoglobulins. The association of a rise in BKV antibody levels with a decline in CD4+ cell count is consistent with the known reactivation of latent polyomaviruses in immunocompromised HIV-infected individuals. As expected, among HIV-positive women, our serological marker of HPV infection, similarly to that reported for HPV DNA, was associated with low CD4+ cell count and high HIV RNA (2, 25, 28, 29). The majority of paired samples (∼85%) did not have a significant rise or decline in antibody levels, indicating that in general antibody levels in both HIV-positive and HIV-negative women are stable over time, confirming a prior report (1).
An unexpected finding in our study was that HPV-16 infections defined by a rise in antibodies are as common among HIV-negative women as among HIV-positive women. The cumulative incidence of newly detected rises was not significantly higher in HIV-positive women than in HIV-negative women. In contrast, the cumulative incidence of HPV-16 infection as detected by PCR was significantly higher in HIV-positive women than in HIV-negative women, similar to prior studies (17, 25, 29). A possible reason for the difference in incidence of HPV-16 infections detected by serology and PCR is that infections in HIV-negative women may go undetected by PCR due to infrequent sampling, a shorter duration of viral shedding, or lower levels of viral replication. In fact, a recent study that included weekly self-collected genital tract samples found that many HPV infections were detectable only for a few weeks (6). In contrast, increases in antibody levels capture infections occurring at any time between the index and follow-up visits.
Our data also suggest that the mechanisms for rises in HPV-16 antibody levels are different for HIV-positive and HIV-negative women, which may further explain the differences between serologic and PCR results described above. For all women, the majority of rises occurred in those who were already HPV-16 seropositive and thus were likely to have been previously infected with HPV-16. In fact, a past HPV-16 infection was one of the strongest correlates with a rise in HPV-16 antibody levels. These rises may be due to reinfection or reactivation. For HIV-positive women, sexual behavior was not associated with a rise in HPV-16 antibody levels. However, lower CD4+ cell counts were associated with a rise, suggesting that infections in HIV-positive women may be more commonly due to reactivation of a latent infection in the context of HIV-induced immunosuppression. In contrast, HIV-negative women who currently are or in the past were more sexually active were more likely to have a rise in HPV-16 antibody levels than those who reported less sexual activity, although the difference between sexually active and less active women reached statistical significance in multivariable models only for those who reported 6 to 10 lifetime partners. Thus, HPV-16 infections in HIV-negative women may result more often from reinfection with sexually transmitted HPV.
There were limitations to our study. First, we had a relatively small sample of HIV-negative women (n = 116). Despite the reduced statistical power, we detected important differences by HIV serostatus which warrant confirmation in future studies. Second, for a serological diagnosis of infection, the site of infection is unknown. Therefore, we cannot exclude the possibility that some serologically defined infections were the result of HPV-16 infections in the anal canal, oral cavity, or other anogenital or aerodigestive tissues. Also, our estimate of the cumulative incidence of HPV-16 infection may be somewhat inflated because women in our study population were more likely to have HPV-16 DNA at baseline than WIHS participants not included. Conversely, because not all infections induce a detectable antibody response, we may have underestimated the cumulative incidence of HPV-16 infection. Relative estimates of effect, however, should be unbiased. A further limitation is the possibility of misclassification of our outcome of a rise in antibody levels. We repeated our analysis using a more stringent cutoff, defined as the mean plus 3 standard deviations (cutoff of 0.158 OD units), and the inferences did not change despite a modest decrease in statistical significance (data not shown). Finally, distinguishing between reinfection and reactivation of infection is difficult since documenting sexual exposure to HPV from an infected male partner is problematic. Our conclusion that some infections among HIV-positive women are a result of reactivation of HPV is supported by a recent study of the natural history of HPV indicating that a substantial fraction of incident HPV DNA detection in immunocompromised HIV-positive women was not related to recent sexual activity (28).
In summary, we describe a serological marker that likely detects HPV-16 viral replication occurring between annual measurements. Confirmation of infection, however, would require frequent HPV DNA measurements during the interval between serological assays and at multiple anatomic sites. Using a rise in antibodies as a marker of infection, our results suggest that HPV-16 infections may be more common than previously estimated by DNA detection methods, particularly in HIV-negative women. Another important observation from our study was that most HPV-16 infections occurred in women who had serological or virological evidence of prior exposure to HPV-16 and thus are due to either reinfection or reactivation of HPV-16. Since rises were not associated with sexual behavior among HIV-positive women, infections may reflect reactivation of latent infection in these women, although more carefully designed studies are needed to distinguish reinfection from reactivation.
Acknowledgments
Data in the manuscript were collected by the WIHS Collaborative Study Group at the following centers (principal investigators): New York City/Bronx Consortium (Kathryn Anastos), Brooklyn, NY (Howard Minkoff), Washington, DC Metropolitan Consortium (Mary Young), The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt), Los Angeles County/Southern California Consortium (Alexandra Levine), Chicago Consortium (Mardge Cohen), and Data Coordinating Center (Stephen Gange).
The WIHS is funded by the National Institute of Allergy and Infectious Diseases, with supplemental funding from the National Cancer Institute and the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-CH-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, and MO1-RR-00083). HPV DNA test data and partial funding for repeat HPV-16 VLP testing were provided by an NCI grant (2R01 CA85178-05). This work was also supported by a grant from the National Institute of Allergy and Infectious Diseases (RO1-AI-42058).
We would also like to thank Joachim Dillner and Patti Gravitt for helpful discussions during the analysis and manuscript preparation.
REFERENCES
- 1.af Geijersstam, V., M. Kibur, Z. Wang, P. Koskela, E. Pukkala, J. Schiller, M. Lehtinen, and J. Dillner. 1998. Stability over time of serum antibody levels to human papillomavirus type 16. J. Infect. Dis. 177:1710-1714. [DOI] [PubMed] [Google Scholar]
- 2.Ahdieh, L., R. S. Klein, R. Burk, S. Cu-Uvin, P. Schuman, A. Duerr, M. Safaeian, J. Astemborski, R. Daniel, and K. Shah. 2001. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J. Infect. Dis. 184:682-690. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, R., and C. A. Biron. 1999. Immunity to viruses, p. 1295-1334. In W. E. Paul (ed.), Fundamental immunology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 4.Barkan, S. E., S. L. Melnick, S. Preston-Martin, K. Weber, L. A. Kalish, P. Miotti, M. Young, R. Greenblatt, H. Sacks, J. Feldman, et al. 1998. The Women's Interagency HIV Study. Epidemiology 9:117-125. [PubMed] [Google Scholar]
- 5.Bosch, F. X., A. Lorincz, N. Munoz, C. J. Meijer, and K. V. Shah. 2002. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 55:244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, D. R., M. L. Shew, B. Qadadri, N. Neptune, M. Vargas, W. Tu, B. E. Juliar, T. E. Breen, and J. D. Fortenberry. 2005. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J. Infect. Dis. 191:182-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvelli, T., T. N. Denny, H. Paxton, R. Gelman, and J. Kagan. 1993. Guideline for flow cytometric immunophenotyping: a report from the National Institute of Allergy and Infectious Diseases, Division of AIDS. Cytometry 14:702-715. [DOI] [PubMed] [Google Scholar]
- 8.Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. [DOI] [PubMed] [Google Scholar]
- 9.Cox, D. R., and D. Oates. 1984. Analysis of survival data. Chapman and Hall, London, United Kingdom.
- 10.Diggle, P. J., K. Y. Liang, and S. L. Zeger. 1995. Analysis of longitudinal data. Oxford University Press, New York, N.Y.
- 11.Dillner, J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9:423-430. [DOI] [PubMed] [Google Scholar]
- 12.Engels, E. A., D. E. Rollison, P. Hartge, D. Baris, J. R. Cerhan, R. K. Severson, W. Cozen, S. Davis, R. J. Biggar, J. J. Goedert, and R. P. Viscidi. 2005. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int. J. Cancer 117:1013-1019. [DOI] [PubMed] [Google Scholar]
- 13.Evander, M., K. Edlund, A. Gustafsson, M. Jonsson, R. Karlsson, E. Rylander, and G. Wadell. 1995. Human papillomavirus infection is transient in young women: a population-based cohort study. J. Infect. Dis. 171:1026-1030. [DOI] [PubMed] [Google Scholar]
- 14.Furuta, Y., F. Ohtani, H. Aizawa, S. Fukuda, H. Kawabata, and T. Bergstrom. 2005. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr. Infect. Dis. J. 24:97-101. [DOI] [PubMed] [Google Scholar]
- 15.Furuta, Y., F. Ohtani, H. Kawabata, S. Fukuda, and T. Bergstrom. 2000. High prevalence of varicella-zoster virus reactivation in herpes simplex virus-seronegative patients with acute peripheral facial palsy. Clin. Infect. Dis. 30:529-533. [DOI] [PubMed] [Google Scholar]
- 16.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9:1131-1137. [DOI] [PubMed] [Google Scholar]
- 17.Hankins, C., F. Coutlee, N. Lapointe, P. Simard, T. Tran, J. Samson, L. Hum, et al. 1999. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Can. Med. Assoc. J. 160:185-191. [PMC free article] [PubMed] [Google Scholar]
- 18.Hessol, N. A., M. Schneider, R. M. Greenblatt, M. Bacon, Y. Barranday, S. Holman, E. Robison, C. Williams, M. Cohen, and K. Weber. 2001. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am. J. Epidemiol. 154:563-573. [DOI] [PubMed] [Google Scholar]
- 19.Hildesheim, A., M. H. Schiffman, P. E. Gravitt, A. G. Glass, C. E. Greer, T. Zhang, D. R. Scott, B. B. Rush, P. Lawler, M. E. Sherman, et al. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. J. Infect. Dis. 169:235-240. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, H. H., and J. Steiger. 2003. Polyomavirus BK. Lancet Infect. Dis. 3:611-623. [DOI] [PubMed] [Google Scholar]
- 21.Ho, G. Y., R. Bierman, L. Beardsley, C. J. Chang, and R. D. Burk. 1998. Natural history of cervicovaginal papillomavirus infection in young women. N. Engl. J. Med. 338:423-428. [DOI] [PubMed] [Google Scholar]
- 22.Kirstein, L. M., R. M. Greenblatt, K. Anastos, A. Levine, A. L. French, H. Minkoff, S. Silver, and S. J. Gange. 2002. Prevalence and correlates of highly active antiretroviral therapy switching in the Women's Interagency HIV Study. J. Acquir. Immune Defic. Syndr. 29:495-503. [DOI] [PubMed] [Google Scholar]
- 23.Kurman, R. J., D. E. Henson, A. L. Herbst, K. L. Noller, M. H. Schiffman, et al. 1994. Interim guidelines for management of abnormal cervical cytology. JAMA 271:1866-1869. [PubMed] [Google Scholar]
- 24.Maurmann, S., L. Fricke, H. J. Wagner, P. Schlenke, H. Hennig, J. Steinhoff, and W. J. Jabs. 2003. Molecular parameters for precise diagnosis of asymptomatic Epstein-Barr virus reactivation in healthy carriers. J. Clin. Microbiol. 41:5419-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palefsky, J. M., H. Minkoff, L. A. Kalish, A. Levine, H. S. Sacks, P. Garcia, M. Young, S. Melnick, P. Miotti, and R. Burk. 1999. Cervicovaginal human papillomavirus infection in human immunodeficiency virus-1 (HIV)-positive and high-risk HIV-negative women. J. Natl. Cancer Inst. 91:226-236. [DOI] [PubMed] [Google Scholar]
- 26.Silverberg, M. J., M. E. Gore, A. L. French, M. Gandhi, M. J. Glesby, A. Kovacs, T. E. Wilson, M. A. Young, and S. J. Gange. 2004. Prevalence of clinical symptoms associated with highly active antiretroviral therapy in the Women's Interagency HIV Study. Clin. Infect. Dis. 39:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloots, T. P., J. P. Kapeleris, I. M. Mackay, M. Batham, and P. L. Devine. 1996. Evaluation of a commercial enzyme-linked immunosorbent assay for detection of serum immunoglobulin G response to human herpesvirus 6. J. Clin. Microbiol. 34:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strickler, H. D., R. D. Burk, M. Fazzari, K. Anastos, H. Minkoff, L. S. Massad, C. Hall, M. Bacon, A. M. Levine, D. H. Watts, M. J. Silverberg, X. Xue, N. F. Schlecht, S. Melnick, and J. M. Palefsky. 2005. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J. Natl. Cancer Inst. 97:577-586. [DOI] [PubMed] [Google Scholar]
- 29.Sun, X. W., L. Kuhn, T. V. Ellerbrock, M. A. Chiasson, T. J. Bush, and T. C. Wright, Jr. 1997. Human papillomavirus infection in women infected with the human immunodeficiency virus. N. Engl. J. Med. 337:1343-1349. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services. 29. October 2004. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Online.] http://aidsinfo.nih.gov/guidelines.
- 31.Viscidi, R. P., L. Ahdieh-Grant, B. Clayman, K. Fox, L. S. Massad, S. Cu-Uvin, K. V. Shah, K. M. Anastos, K. E. Squires, A. Duerr, D. J. Jamieson, R. D. Burk, R. S. Klein, H. Minkoff, J. Palefsky, H. Strickler, P. Schuman, E. Piessens, and P. Miotti. 2003. Serum immunoglobulin G response to human papillomavirus type 16 virus-like particles in human immunodeficiency virus (HIV)-positive and risk-matched HIV-negative women. J. Infect. Dis. 187:194-205. [DOI] [PubMed] [Google Scholar]
- 32.Viscidi, R. P., K. L. Kotloff, B. Clayman, K. Russ, S. Shapiro, and K. V. Shah. 1997. Prevalence of antibodies to human papillomavirus (HPV) type 16 virus-like particles in relation to cervical HPV infection among college women. Clin. Diagn. Lab. Immunol. 4:122-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viscidi, R. P., D. E. Rollison, E. Viscidi, B. Clayman, E. Rubalcaba, R. Daniel, E. O. Major, and K. V. Shah. 2003. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin. Diagn. Lab. Immunol. 10:278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Viscidi, R. P., M. Schiffman, A. Hildesheim, R. Herrero, P. E. Castle, M. C. Bratti, A. C. Rodriguez, M. E. Sherman, S. Wang, B. Clayman, and R. D. Burk. 2004. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol. Biomark. Prev. 13:324-327. [DOI] [PubMed] [Google Scholar]
- 35.Viscidi, R. P., B. Snyder, S. Cu-Uvin, J. W. Hogan, B. Clayman, R. S. Klein, J. Sobel, and K. V. Shah. 2005. Human papillomavirus capsid antibody response to natural infection and risk of subsequent HPV infection in HIV-positive and HIV-negative women. Cancer Epidemiol. Biomark. Prev. 14:283-288. [PubMed] [Google Scholar]
- 36.Wang, S. S., M. Schiffman, T. S. Shields, R. Herrero, A. Hildesheim, M. C. Bratti, M. E. Sherman, A. C. Rodriguez, P. E. Castle, J. Morales, M. Alfaro, T. Wright, S. Chen, B. Clayman, R. D. Burk, and R. P. Viscidi. 2003. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br. J. Cancer 89:1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods, G. L., and J. A. Washington. 1995. The clinician and the microbiology laboratory, p. 169-199. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 4th ed. Churchill Livingstone, Philadelphia, Pa.