Abstract
Resistance to glucocorticoid (GC) treatment in some patients with idiopathic nephrotic syndrome (INS) is a significant clinical problem. Heat shock protein 90 (HSP90) is the chaperon protein of the GC receptor, which is supposed to be the key factor of GC response. Therefore, we conducted this study to define the mechanisms of GC resistance related to HSP90. INS patients and cell lines with differing GC responses were included in the present study. We found that the level of HSP90 mRNA expression in INS patients was significantly higher than that in healthy controls and that HSP90 expression in GC-resistant INS patients was higher than that in GC-sensitive INS patients. A confocal immunofluorescence test was performed to investigate the subcellular localization of HSP90, and we found that the distribution of HSP90 in the GC-resistant INS group was greater in the nuclei than that of the GC-sensitive INS group. When the function of HSP90 was blocked by the HSP90-specific inhibitor, the GC sensitivity of GC-sensitive cells decreased remarkably. These results indicate that HSP90 plays a vital role in GC response. In addition, the abnormality in the mRNA level and subcellular distribution of HSP90 in GC-resistant INS patients may be etiologically significant in terms of endogenous/synthetic GC resistance. On one hand, it may disturb immunoendocrine regulation via endogenous GC and immune homeostasis and thus be involved in the occurrence of the immune-mediated disease; on the other hand, it may influence the patient's response to synthetic GC treatment and result in treatment failure.
Idiopathic nephrotic syndrome (INS) is a very common immune-mediated disease. Glucocorticoid (GC) has powerful anti-inflammatory and immunomodulatory effects and has been used as a first-line treatment in immune-mediated/inflammatory diseases. However, the therapeutic effect of GC depends on the GC response of the patients, and considerable interindividual variation in GC sensitivity exists in such patients. In fact, GC, as a key mediator of the hypothalamo-pituitary-adrenal (HPA) axis, is also important in the maintenance of immune homeostasis of the body. Our previous study (12) found that intrinsic resistance to endocrine GC may be involved in the disturbance of the regulation of neuroendocrine immune networks and the occurrence of lupus nephritis. GC sensitivity is also an important prognostic factor in INS, and GC insensitivity/GC resistance poses a dilemma, particularly since few alternative therapies are available.
GC exerts its physiologic effects via the glucocorticoid receptor (GR). Heat shock protein 90 (HSP90) is the chaperon protein of GR (18). HSP90 binds to the GR, keeping it in a partially unfolded state, which is conceived as a “pocket” for the GC binding with high affinity. HSP90 can help activated GR's nuclear translocation (4); after dissociation from HSP90, GR acquires DNA-binding activity. Because HSP90 is necessary for proper GC action in vivo, we presume that HSP90 plays a key role in GC resistance. Exact knowledge of the various molecular aspects of GC resistance that disturb immunoendocrine communication will be of etiologic significance and may help in the development of novel strategies for the treatment of immune-mediated/inflammatory diseases in humans.
MATERIALS AND METHODS
Patients and controls.
The diagnoses of 51 INS patients (18 females and 33 males; mean age, 32 ± 11.9 years) were all confirmed by laboratory and renal biopsy examinations. A standard prednisone regimen for nephritic syndrome begins with prednisone at 1 mg kg of body weight−1 day−1 for 8 weeks, and then the dose is gradually tapered off. INS patients took prednisone in the morning, once a day. According to the clinical response to treatment, 25 patients who had complete remission of proteinuria and reduced edema with GC therapy were classified as GC sensitive. Twenty-six patients who failed to achieve a remission on completion of this regimen were considered as GC resistant (5). None of these subjects had features of primary cortisol resistance (hypercortisolism without Cushing's syndrome), which has been reported to be related to lymphocyte resistance to GC due to GR abnormalities (19). All patients had normal renal function, and none had hepatic disease or uncontrolled hypertension.
In order to exclude the possibility that the study could be confounded by the medication, we collected the blood samples from the patients between 6 and 7 a.m., before the patients took prednisone and other medicine. Blood samples were also obtained from 23 healthy volunteers (9 females and 14 males; mean age, 31 ± 10.6 years). These volunteers had no history of taking GCs or any other immunosuppressive agents. All subjects gave their written informed consent, and the Ethics Committee of Sun Yat-sen University approved the study. Peripheral blood mononuclear cells (PBMCs) were subsequently isolated by Ficoll-Hypaque density gradient centrifugation. A portion of the PBMCs was added to 1 ml TRIzol (Invitrogen Life Technologies) and stored at −70°C until analysis, and another portion of the PBMCs was spun down onto a glass slide to detect the expression and subcellular distribution of HSP90.
Reverse transcription-PCR analysis.
Total RNA was extracted and cDNA synthesis was performed following the manufacturer's protocol (Invitrogen Life Technologies). The integrity of the RNA was determined by visualization of the 28S and 18S rRNA samples through a 1% agarose formaldehyde gel. The purity and quantity of RNA were assessed with a biophotometer (Eppendorf Company, Germany).
For selection of the primers of GR, we referred to the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/). The primers were as follows: GR sense, 5′-TTA CCA CAA CTC ACC CCT ACC-3′; GR antisense, 5′-GGC TCT TCA GAC CGT CCT TA-3′. We selected the primers of HSP90 and performed PCR as previously described (22). DNA contamination was controlled for by using primers for HSP90, GR, and β-actin, which span introns giving a detectable product only with cDNA.
Confocal immunofluorescence analysis.
PBMCs were fixed and permeabilized in acetone at 4°C for 10 min. HSP90 was detected by incubation with primary mouse anti-human HSP90 monoclonal antibody for 2 h at 37°C, washed twice with phosphate-buffered saline, and further incubated with a fluorescein isothiocyanate-conjugated sheep anti-mouse antibody. Subcellular localization of HSP90 was investigated with confocal immunofluorescence microscopy (LSM510 META; Carl Zeiss, Germany).
The above-listed antibodies were purchased from Stressgen Biotechnologies Inc., Canada. Images were collected, and for the evaluation of HSP90 location, the relative nuclear and cytoplasmic fluorescence levels in about 80 cells per condition per subject were determined and scored according to the following five-grade nuclear location score: exclusive nuclear fluorescence, +2; nuclear fluorescence exceeding cytoplasmic fluorescence, +1; equivalence in the levels of nuclear and cytoplasmic fluorescence, 0; cytoplasmic fluorescence exceeding nuclear fluorescence, −1; and exclusive cytoplasmic fluorescence, −2. Since HSP90 is present mainly in the cytoplasm under physiological conditions, the cells were divided into two groups according their scores, i.e., the cytoplasm>nucleus group, with scores of ≤−1, and the nucleus>cytoplasm group, with scores of >−1.
Cells.
GC-sensitive CEM-C7H2 cells and GC-resistant CEM-C1 cells were a kind gift from Reinhard Kofler (Tyrolean Research Institute, Innsbruck, Austria). The phenotypic characterizations of CEM-C7H2 and CEM-C1 cells are that these leukemia cells are sensitive or insensitive/resistant to GC-induced apoptosis (7). The cells in logarithmic growth phase were cultured in phenol red-free RPMI 1640 medium (HyClone Labs). Cell viability, assessed by a trypan blue exclusion test, was always >99%. To study the role of HSP90 in different GC responses, cells at a concentration of 1 × 106/ml were cultured in medium alone, in dexamethasone (DEX) (1 μM; Sigma), in the HSP90 inhibitor geldanamycin (GA) (1 μM; Alexis Biochemicals), or in DEX and GA. Because GA was dissolved in dimethyl sulfoxide (0.5%), the same concentration of dimethyl sulfoxide was added into a medium-only well and a DEX-treated well as a parallel control. Studies were performed in triplicate and repeated at least three times. After the cells were incubated for 48 h, the apoptosis status of the cells was qualitatively or quantitatively analyzed as described below.
Apoptosis analysis.
The procedure of flow cytometric analysis of DNA content was as described in the work of McCloskey et al (16). Propidium iodide (PI) fluorescence of each cell was assessed by analysis on an ELITE flow cytometer (Beckman-Coulter ELITE) for the presence of a sub-G1 peak. Morphological changes in apoptosis were assessed by acridine orange (AO)/ethidium bromide (EtBr) staining. The cells were stained as previously described (3). The cells were observed with a fluorescence microscope. The peak excitation wavelength was 480 nm.
Statistical analysis.
Measurement data were finally presented as means ± standard deviations, and the differences between groups were compared by analysis of variation. Chi-square tests were used for analyzing categorical variables. All statistical tests were performed with SPSS 10.0 for Windows software (SPSS, Chicago, Ill.). A P value of <0.05 indicated a statistically significant difference, and all reported P values are two sided.
RESULTS
HSP90 and GR mRNA expression in different GC response subjects.
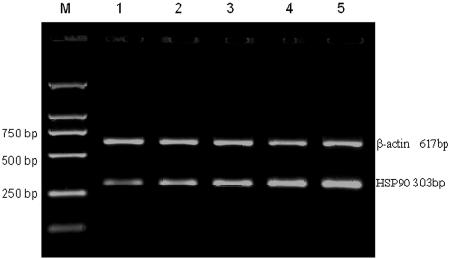
RNA electrophoresis and the A260-to-A280 ratio indicated that the integrity and purity of isolated RNA were fine. Reverse transcription-PCR analysis indicated the presence of specific bands of the correct sizes (303 bp for HSP90, 475 bp for GR, and 617 bp for β-actin).
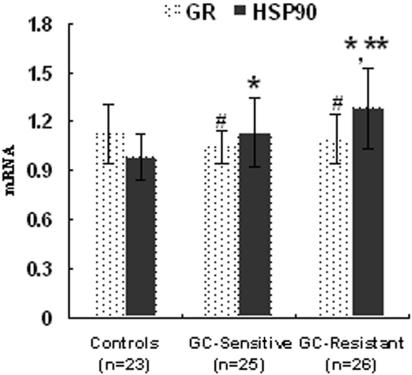
HSP90 is a very abundant cytosolic protein that is expressed under both normal and stressful conditions. The results indicated that the expression of HSP90 mRNA of healthy subjects was the lowest (0.98 ± 0.14), and the expression of HSP90 mRNA of GC-resistant INS patients was the highest (Fig. 1), even higher than that of GC-sensitive INS patients (1.28 ± 0.25 versus 1.13 ± 0.21; P < 0.05). There was no significant difference between the levels of GR mRNA for patients and those for healthy subjects (P > 0.05). The data are shown in Fig. 2.
FIG. 1.
The relative level of HSP90 mRNA of GC-resistant INS patients was the highest, higher than that of GC-sensitive INS patients and that of healthy controls. Lanes: M, DNA marker; 1, healthy controls; 2 and 3, GC-sensitive INS patients; 4 and 5, GC-resistant INS patients.
FIG. 2.
Levels of GR and HSP90 mRNA in INS patients and healthy controls. The bars indicate means ± standard deviations. Symbols: #, compared to the GR mRNA level for healthy controls (P > 0.05); *, compared to the HSP90 mRNA level for healthy controls (P < 0.05); **, compared to the HSP90 mRNA level for the GC-sensitive INS group (P < 0.05).
For INS patients, the ratio of HSP90 mRNA to GR mRNA is 1.09 ± 0.17 for the GC-sensitive group and 1.18 ± 0.23 for the GC-resistant group, whereas in healthy controls, the ratio of HSP90 mRNA to GR mRNA is 0.89 ± 0.16. The mRNA expression ratio of HSP90 to GR of INS patients was higher than that of healthy controls too (P < 0.05).
Subcellular distribution of HSP90 in PBMC from healthy subjects and GC-sensitive and GC-resistant INS patients.
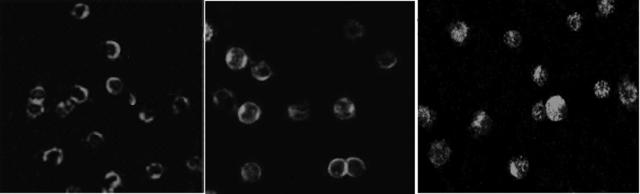
As far as the subcellular distribution of HSP90 was concerned, the results of confocal microscopic immunofluorescence assay indicated that, in PBMC of healthy volunteers and GC-sensitive INS patients, HSP90 was located mainly in the cytoplasm (Fig. 3, left and middle panels), whereas for GC-resistant INS groups, the subcellular distribution of HSP90 was greater in the nuclei (Fig. 3, right panel) than that for the GC-sensitive INS group (P < 0.001). The results of the nuclear location scoring are summarized in Table 1.
FIG. 3.
The subcellular distribution of HSP90 in the GC-resistant INS group (right panel) was greater in the nuclei than that in healthy controls (left panel) and that in the GC-sensitive INS group (middle panel). Magnification, ×400.
TABLE 1.
Subcellular distribution of HSP90 in PBMC of INS patient groups with differing GC responses
| Patient groupa | HSP90 distribution (no. of cells) in group with score ofb:
|
|
|---|---|---|
| ≤−1 | >−1 | |
| GC-S | 970 | 455 |
| GC-R | 900 | 748 |
| χ2 value | 25.973 | |
| P value | <0.001 | |
GC-S, GC-sensitive INS patients; GC-R, GC-resistant INS patients.
See text for scoring system.
The role of HSP90 in GC response.
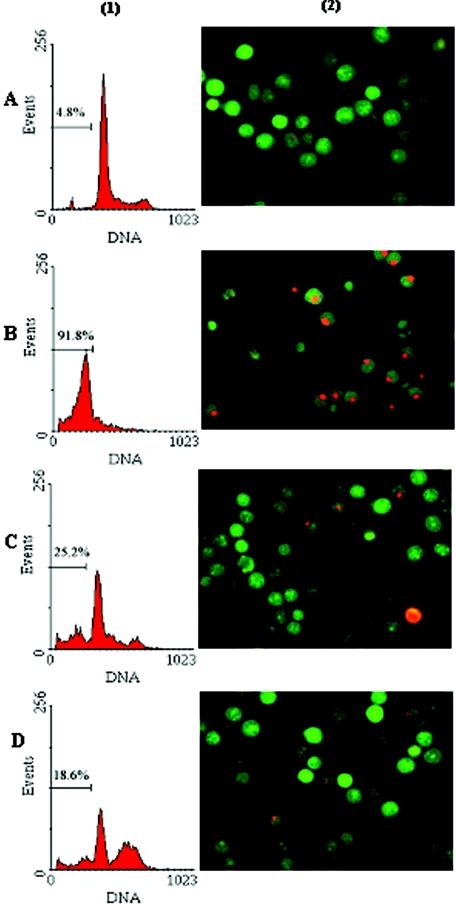
One of the apparent phenomena of GC response in vitro is the apoptosis of cells treated with GC. Analysis of DNA content, which can be precisely assessed by flow cytometry after cell PI staining, presents one of the criteria used to quantify apoptosis. We found that DEX induced apoptosis in most GC-sensitive C7H2 cells (91.8%) after 48 h of incubation at 37°C, whereas DEX induced apoptosis in only 15.2% of GC-resistant C1 cells. Along with GC, we added HSP90 inhibitor GA and found that GA blocked apoptosis of C7H2 cells induced by DEX significantly (91.8% versus 18.6%; P < 0.01). The data shown are representative of three independent experiments. Furthermore, morphological and nuclear changes occurring during apoptosis were assessed following AO and EtBr dual staining. This staining allows apoptotic but still-living cells to be distinguished from dead cells (3). The live cells have normal green nuclei, early apoptotic cells have bright green nuclei with condensed or fragmented chromatin, late apoptotic cells display condensed and fragmented orange chromatin, and cells that have died from direct necrosis have structurally normal orange/red nuclei. A good correlation was found when the assessment of apoptotic cells by flow cytometry analysis was compared to the results of AO/EtBr staining (Fig. 4).
FIG. 4.
HSP90 inhibitor GA blocked apoptosis of C7H2 cells induced by DEX. C7H2 cells were incubated for 48 h at 37°C either in culture medium alone (A), with 1 μM DEX (B), with 1 μM HSP90 inhibitor, GA (C), or with 1 μM DEX and 1 μM GA (D). (1) Quantitative flow cytometric analysis of DNA content in C7H2 cells by PI staining; (2) morphological change of apoptosis in C7H2 cells showed by dual staining with AO/EtBr (400×).
DISCUSSION
The occurrence of many immune-mediated diseases is due at least in part to a disturbance in immune homeostasis (20). According to the theory of neuroendocrine immunology, immune response is regulated by the neuroendocrine system. In the neuroendocrine immune networks, HPA axis regulation plays a critical role in maintaining immune homeostasis (11). Endogenous GC, acting as the key molecule of the HPA axis, takes part in coordinating immune response, and its immunosuppressive and anti-inflammatory actions in particular explain the widespread use of synthetic GC in the treatment of a variety of immune-mediated/inflammatory diseases, such as INS and asthma.
Two distinct clinical features of GC sensitivity and GC resistance have been noted. Our previous study (12) suggested that susceptibility to lupus nephritis is related to immune regulation disorder caused by resistance to endogenous GC. In clinical practice, it has been found that resistance to synthetic GC intervention will result in GC treatment failure. GC resistance is thus a phenomenon of major significance in a number of clinical situations, and exact knowledge of GC resistance will be of etiologic significance and allow for the establishment of a more-specific scheme for the treatment of immune-mediated diseases.
It has been demonstrated that there is no difference in the levels of adrenocorticotropin, GC, and affinity of GR to GC between GC-resistant patients and controls (9, 12). As the chaperon protein of GR, HSP90 is significant in GC action (18). We conducted this study to determine if HSP90 plays an important role in GC resistance.
PBMCs, which are mainly lymphocytes and monocytes, are not only the target cells of immune modulation but also the executive of immune response. At a cellular level, there is evidence for a defective response of peripheral blood cells to GC, which correlates with their clinical resistance to GC treatment (1). Our study found that the level of HSP90 mRNA expression in PBMC from INS patients was significantly higher than that in healthy controls, a difference which might be due to pathophysiologic (notably inflammatory) processes (15). We further found that HSP90 mRNA expression in GC-resistant INS patients was higher than that in GC-sensitive INS patients (P < 0.05). We inferred that the GC-resistant INS patients might have endogenous GC resistance as well, which brought about worse pathological processes and more-elevated HSP90 levels accordingly. On the other hand, the elevated HSP90 level may interfere with GR function directly or indirectly and contribute to such pathological processes by not allowing GC to exert its physiologic and treatment effects (14).
We further analyzed the ratio of HSP90 to GR expression in order to understand the relationship between HSP90 and GR. We found INS patients have a higher ratio of HSP90 to GR than that in controls. However, there was no significant difference in HSP90-to-GR ratios between GC-sensitive and GC-resistant INS patients (1.18 ± 0.23 versus 1.09 ± 0.17; P > 0.05). In addition, there was no significant difference in GR mRNA levels between patients and healthy subjects. The data suggested that the elevated HSP90 level affected GR function and GC response not exactly via changing the GR expression or the expression ratio of HSP90 to GR.
On this account, we further observed the distribution of HSP90 using confocal microscopy. We found that in PBMC from GC-resistant INS patients, the subcellular distribution of HSP90 was greater in the nuclei than that of the GC-sensitive INS group. As we know, it is traditionally considered the case that upon GC binding, the GR undergoes an allosteric change which results in dissociation from the GR-HSP90 complex and ligand-bound GR translocates into the nucleus, while HSP90 still stays in the cytoplasm. However there is increasing indirect (10, 13) and direct (2, 6) evidence showing that after GC binding, the association of GR with HSP90 is dynamic in the delivery of GR to its nuclear site of action. Several possibilities to explain the higher level of nuclear HSP90 in GC-resistant INS patients may be raised. In cytoplasm, there may exist a decreased binding of HSP90 to GR, resulting in more “free” HSP90 moving into nuclei; or upon ligand binding, HSP90 may not dissociate from cytosolic GR-HSP90 heterocomplexes at all, so that GR-HSP90 heterocomplexes translocate into nuclei as a whole; or there may exist an abnormality in HSP90 trafficking, such that HSP90 accumulates in the nuclei and cannot go back to cytoplasm. Consequently, it may interfere with GR function and GC action through the following mechanisms. (i) Decreased binding of HSP90 to GR will decrease or prevent binding of GR to GC (17). (ii) Translocation of GR-HSP90 into nuclei as a whole will mask the DNA-binding domain of GR and prevent the binding of GR to the DNA response element. (iii) Elevated nuclear HSP90 will rebind to GR and interfere with GR cytoplasmic-nuclear shuttling or even GR recycling (21). Kang et al. (14) have also demonstrated that an abnormally high HSP90-to-GR ratio in nuclei can negatively interfere with the GC-dependent response. Tago et al. (21) also found that overmuch nuclear HSP90 can retain GR in the nucleus. Thus, the Hsp90-GR interaction, although dynamically shifted toward dissociation by the specific steroid, may take place in the nuclear compartment not only before but also after receptor activation to a DNA-binding form, thus modulating the responsiveness to hormone.
In order to verify what we had found in the INS study, GC-sensitive and -resistant cell lines and an HSP90-specific inhibitor, GA, were included in our vitro experiment. GA, a benzoquinone ansamycin, binds to the amino-terminal domain of HSP90 and regulates HSP90 conformation, thus inhibiting its chaperon function (8). The sensitivity of GC-sensitive C7H2 cells decreased significantly when they were treated with GA (GC-induced apoptosis in C7H2 cells decreased from 91.8% to 18.6%), indicating that inhibition of HSP90 in vitro will change cellular GC responsiveness. Our results confirmed that HSP90 did play a prominent role in GC response. To further investigate this issue, it is necessary to establish a cell model expressing an elevated level of nuclear HSP90 and then study the exact interaction of nuclear HSP90 and GR and the downstream effects.
In summary, there exists abnormality in the mRNA level and subcellular location of HSP90 in GC-resistant INS patients; this abnormality may be responsible for endogenous/synthetic GC resistance. The occurrence of immune-mediated diseases and GC resistance is a very complicated pathophysiologic process. Although large numbers of studies targeting GC resistance focus mainly on GR defects, the current study illustrates a new insight into the explanation of different GC responses. That will be of etiologic significance and will be useful in carrying out GC response-based individualized GC therapy.
Acknowledgments
We gratefully acknowledge Reinhard Kofler for kindly providing CEM-C7H2 and CEM-C1 cell lines. We thank Qing-yu Kong for insightful discussions and valuable technical advice.
REFERENCES
- 1.Adcock, I. M., S. J. Lane, C. R. Brown, M. J. Peters, T. H. Lee, and P. J. Barnes. 1995. Differences in binding of glucocorticoid receptor to DNA in steroid-resistant asthma. J. Immunol. 154:3500-3505. [PubMed] [Google Scholar]
- 2.Caamano, C. A., M. I. Morano, F. C. Dalman, W. B. Pratt, and H. Akil. 1998. A conserved proline in the hsp90 binding region of the glucocorticoid receptor is required for hsp90 heterocomplex stabilization and receptor signaling. J. Biol. Chem. 273:20473-20480. [DOI] [PubMed] [Google Scholar]
- 3.Coligan, J. E., A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.). 1995. Current protocols in immunology. John Wiley & Sons, Inc., New York, N.Y.
- 4.Elbi, C., D. A. Walker, G. Romero, W. P. Sullivan, D. O. Toft, G. L. Hager, and D. B. DeFranco. 2004. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc. Natl. Acad. Sci. USA 101:2876-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk, R. J., J. C. Jennette, and P. H. Nachman. 1999. Primary glomerular disease, p. 1266-1297. In B. M. Brenner (ed.), The kidney, 6th ed. W. B. Saunders, Philadelphia, Pa.
- 6.Galigniana, M. D., P. R. Housley, D. B DeFranco, and W. B. Pratt. 1999. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J. Biol. Chem. 274:16222-16227. [DOI] [PubMed] [Google Scholar]
- 7.Geley, S., B. L. Hartmann, M. Hala, E. M. Strasser-Wozak, K. Kapelari, and R. Kofler. 1996. Resistance to glucocorticoid-induced apoptosis in human T-cell acute lymphoblastic leukemia CEM-C1 cells is due to insufficient glucocorticoid receptor expression. Cancer Res. 56:5033-5038. [PubMed] [Google Scholar]
- 8.Grenert, J. P., W. P. Sullivan, P. Fadden, T. A. Haystead, J. Clark, E. Mimnaugh, H. Krutzsch, H. J. Ochel, T. W. Schulte, E. Sausville, L. M. Neckers, and D. O. Toft. 1997. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 272:23843-23850. [DOI] [PubMed] [Google Scholar]
- 9.Haack, D., K. Schärer, A. Asam-Tauscher, and P. Vecsei. 1999. Glucocorticoid receptors in idiopathic nephrotic syndrome. Pediatr. Nephrol. 13:653-656. [DOI] [PubMed] [Google Scholar]
- 10.Hache, R. J. G., R. Tse, T. Reich, J. G. A. Savory, and Y. A. Lefebvre. 1999. Nucleocytoplasmic trafficking of steroid-free glucocorticoid receptor. J. Biol. Chem. 274:1432-1439. [DOI] [PubMed] [Google Scholar]
- 11.Harbuz, M. S., A. J. Chover-Gonzalez, and D. S. Jessop. 2003. Hypothalamo-pituitary-adrenal axis and chronic immune activation. Ann. N. Y. Acad. Sci. 992:99-106. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, T., S. Liu, M. Tan, F. Huang, Y. Sun, X. Dong, W. Guan, L. Huang, and F. Zhou. 2001. The phase-shift mutation in the glucocorticoid receptor gene: potential etiologic significance of neuroendocrine mechanisms in lupus nephritis. Clin. Chim. Acta 313:113-117. [DOI] [PubMed] [Google Scholar]
- 13.Kang, K. I., J. Devin, F. Cadepond, N. Jibard, A. Guichon-Mantel, E. E. Baulieu, and M. G. Catelli. 1994. In vivo functional protein-protein interaction: nuclear targeted hsp90 shifts cytoplasmic steroid receptor mutants into the nucleus. Proc. Natl. Acad. Sci. USA 91:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang, K. I., X. Meng, J. Devin-Leclerc, I. Bouhouche, A. Chadli, F. Cadepond, E. E. Baulieu, and M. G. Catelli. 1999. The molecular chaperone Hsp90 can negatively regulate the activity of a glucocorticosteroid-dependent promoter. Proc. Natl. Acad. Sci. USA 96:1439-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann, S. H. 1990. Heat shock proteins and immune response. Immunol. Today 11:129-136. [DOI] [PubMed] [Google Scholar]
- 16.McCloskey, T. W., N. Oyaizu, M. Coronesi, and S. Pahwa. 1994. Use of a flow cytometric assay to quantitate apoptosis in human lymphocytes. Clin. Immunol. Immunopathol. 71:14-18. [DOI] [PubMed] [Google Scholar]
- 17.Picard, D., B. Khursheed, M. J. Garabedian, M. G. Fortin, S. Lindquist, and K. R. Yamamoto. 1990. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 348:166-168. [DOI] [PubMed] [Google Scholar]
- 18.Pratt, W. B., and D. O. Toft. 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrinol. Rev. 18:306-360. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz, M., U. Lind, M. Gafvels, G. Eggertsen, J. Carlstedt-Duke, L. Nilsson, M. Holtmann, P. Stierna, A. C. Wikstrom, and S. Werner. 2001. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin. Endocrinol. 55:363-371. [DOI] [PubMed] [Google Scholar]
- 20.Sternberg, E. M. 2001. Neuroendocrine regulation of autoimmune/inflammatory disease. J. Endocrinol. 169:429-435. [DOI] [PubMed] [Google Scholar]
- 21.Tago, K., F. Tsukahara, M. Naruse, T. Yoshioka, and K. Takano. 2004. Regulation of nuclear retention of glucocorticoid receptor by nuclear Hsp90. Mol. Cell. Endocrinol. 213:131-138. [DOI] [PubMed] [Google Scholar]
- 22.Vachier, I., G. Chiappara, A. M. Vignola, R. Gagliardo, E. Altieri, B. Terouanne, P. Vic, J. Bousquet, P. Godard, and P. Chanez. 1998. Glucocorticoid receptors in bronchial epithelial cells in asthma. Am. J. Respir. Crit. Care Med. 158:963-970. [DOI] [PubMed] [Google Scholar]