Abstract
This study was performed to determine the feasibility of using whole serum to detect antibodies to canine parvovirus (CPV) under nonlaboratory conditions and to evaluate the performance characteristics of an immunochromatography assay kit. Precise detection of levels of antibody against CPV in puppies can be used to determine a vaccination schedule, because maternal antibodies frequently result in the failure of protective vaccination, and can also be used to determine the antibody levels of infected puppies. Several methods for the titration of CPV antibodies have been reported, including the hemagglutination inhibition (HI) assay, which is considered the “gold standard.” These methods, however, require intricate and time-consuming procedures. In this study, a total of 386 serum specimens were tested. Compared to the HI assay, the rapid assay had a 97.1% sensitivity and a 76.6% specificity (with a cutoff HI titer of 1:80). This single-step assay could be performed rapidly and easily without special equipment. The kit provides a reliable method for detection of anti-CPV antibody where laboratory support and personnel are limited.
Canine parvovirus (CPV) is a member of the feline parvovirus subgroup and is classified as an autonomous parvovirus of the family Parvoviridae (14). After being detected in dogs in 1978 (1, 2, 7), CPV was found to be globally distributed and is now endemic in populations of domestic and wild canids (9, 13). Young puppies are very susceptible to infection by CPV, particularly because the natural immunity provided by maternal antibodies in the colostrum may wear off before the puppies' own immune systems become mature enough to fight off infection. If a puppy is exposed to CPV during this gap in protection, it may be infected by CPV and become ill. Maternal antibodies provided by colostrum can interfere with an effective immune response to vaccination and may even cause vaccinated puppies to succumb to parvovirus infection. To narrow gaps in protection and provide optimal protective immunity against parvovirus during the first few months of life, a series of puppy vaccinations could be scheduled. However, interference caused by maternal antibodies is considered a major cause of CPV vaccination failure (5, 6, 8, 12, 17), and it is therefore very important to know the antibody level before vaccination. Antibody can be titrated by a serum neutralization test (11), a hemagglutination inhibition (HI) test (4), or an enzyme-linked immunosorbent assay which is available commercially (16, 17). Serum neutralization and HI tests, however, require laboratory facilities to perform and a long period of time to obtain results. Immunocomb testing based on an enzyme-linked immunosorbent assay (16) provides rapid results within 30 min but requires substantial handling.
In the present study, a one-step rapid test kit using purified CPV antigen, a monoclonal anti-CPV antibody detector, and an anti-canine antibody capture was developed and compared with the HI assay, often regarded as the gold standard of tests used to quantify antibody titers. Changes in serum antibody level during recovery from CPV infection in dogs were also measured with the one-step rapid test kit.
MATERIALS AND METHODS
Cells and viruses.
The CRFK cell line (CCL-94; ATCC) was used to propagate CPV. CRFK cells were grown as monolayer culture in Dulbecco modified Eagle medium (catalog no. 12100-046; Gibco) supplemented with 10% fetal calf serum and antibiotics. The C-780916 strain of CPV (VR-953; ATCC) was propagated using Dulbecco modified Eagle medium containing 2% fetal calf serum. The cell culture supernatant was harvested 3 to 4 days after infection and inactivated with a solution of 0.2% formaldehyde. The inactivated CPV was treated with polyethylene glycol 6000 (catalog no. 96245-1201; Junsei, Japan), followed by ultracentrifugation on a discontinuous sucrose density gradient as previously described (3).
Monoclonal antibody production.
Hybridomas producing mouse monoclonal antibodies to CPV were produced as follows. Spleen cells from BALB/c mice (female, 6 to 8 weeks old) immunized with purified CPV were fused to Sp 2/0 myeloma cells. Briefly, cell culture-grown CPV was highly purified and concentrated by affinity chromatography up to 215 hemagglutinating units (HAU). This CPV was mixed with complete Freund's adjuvant for the first immunization and mixed with incomplete Freund's adjuvant for the second and third immunizations. The fourth immunization was carried out with a 0.1-ml injection of intact CPV into the spleen directly. All immunizations were performed at seven intervals. Serum was taken from the tail of a mouse and screened for the presence of an HI titer. When the serum had an HI titer above 1:640, fusion with Sp 2/0 myeloma cells was performed. Hybridomas producing positive monoclonal antibodies in the screening test were selected and subcloned three times from a single cell by limiting dilution. Mouse ascites fluid was produced in BALB/c mice, and immunoglobulin G (IgG) was prepared by affinity chromatography using protein A-Sepharose (catalog no. 20365ZZ; Pierce). Western blotting was carried out as previously described (15) to confirm the specificities of the monoclonal antibodies (MAbs). Subtyping of cloned MAbs was carried out using goat anti-mouse IgGs (catalog nos. M5532, M5657, M5782, M5907, M6157, and M6032; Sigma). Among the antibodies produced by the cloned hybridomas, one MAb IgG1 subtype, designated CPV MAb 4c3, was selected and used as a CPV antigen detector after being conjugated to colloidal gold.
Polyclonal antibody production.
Goat anti-canine IgGs and goat anti-porcine IgGs were prepared after immunization of goats with canine IgG and porcine IgG, respectively. Canine and porcine IgGs were isolated from sera of adult animals and purified by affinity chromatography using protein A-Sepharose (catalog no. 20365ZZ; Pierce). The test and control lines were coated with the anti-canine IgG and anti-porcine IgG, respectively, of the assay kit.
Conjugation with colloidal gold.
CPV MAb 4c3 was conjugated to 30-nm colloidal gold beads as described previously (15). Briefly, the CPV MAb 4c3 was dialyzed against 2 mM borate buffer (pH 9.0) for 1 h at 4°C and then centrifuged. The colloidal gold was adjusted to pH 8.9 with 0.2 M H2CO3 and mixed with CPV MAb 4c3 for 2 min and then incubated for 8 min at room temperature. The gold conjugate (which forms the test line [T line] detector) was stabilized with 1% bovine serum albumin and 0.05% 20 M Carbowax. Before use, the detector was washed three times with phosphate-buffered saline containing 1% bovine serum albumin. Porcine IgGs purified by affinity chromatography were conjugated to colloidal gold by the same methods.
One-step rapid assay. (i) Test principle.
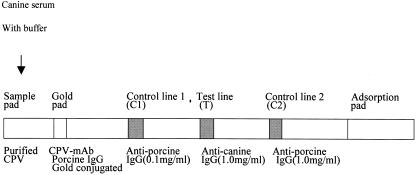
This test is based on immunochromatography using two antibodies. The test kit includes a test strip in a plastic cassette (Fig. 1). The capture antibodies were bound to three different lines on a nitrocellulose membrane: control line 1 (C1 line; 0.1 mg/ml goat anti-porcine IgG), the test line (1.0 mg/ml goat anti-canine IgG), and control line 2 (C2 line; 1.0 mg/ml goat anti-porcine IgG). Gold-conjugated CPV MAb 4c3 and gold-conjugated porcine IgG were dried on a glass fiber (Fig. 1, gold pad). Purified CPV (final titer, 210 HAU) was dried on cellulose paper (Fig. 1, sample pad). The test strip was assembled in the order shown in Fig. 1: sample pad, gold pad, nitrocellulose paper, and adsorption pad (cellulose paper). All pads overlapped to enable migration of the CPV-antibody complexes along the test strip.
FIG. 1.
Diagram of the test strip for the detection of anti-canine parvovirus antibody. Serum is added to the sample pad where serum antibodies can interact with CPV. Addition of buffer enables the complex to migrate along the test strip where gold-conjugated antibodies are captured by the immobilized anti-porcine or anti-canine IgG.
(ii) Test procedure.
Serum was diluted 1:25 with test buffer (0.4 M Tris, 1% Tween 20 [pH 7.5]), and 150 μl of the diluted serum was added to the sample hole of the kit. CPV antibodies in the canine serum were bound by purified CPV and then complexed with the colloidal gold-conjugated anti-CPV MAb 4c3. When these complexes migrated to the immobilized antibody (goat anti-canine IgG) in the T line, they were bound by the anti-canine IgG and a purple band formed (goat anti-canine IgG-canine serum antibody-purified CPV-CPV MAb-colloidal gold). The color density is proportional to the antibody titer. The immobilized anti-porcine IgG in the C1 and C2 lines binds the gold-conjugated porcine IgG independently of the CPV antibody. The bands on the control lines confirm that the test was performed correctly and additionally are equivalent to high and low titers of antibody, as measured by the HI assay, so that the band density of the T line can be compared to known titers. The reagents were adjusted such that the band density of the C1 line was equivalent to a 1:80 HI titer and that of the C2 line to a 1:640 HI titer.
(iii) Results.
The band density of the T line is compared to those of the C1 and C2 lines 20 min after sample application. If the band strength of the T line is higher than that of the C2 line, a high (above 1:640) antibody titer to CPV is present in the serum. This is indicative of a good immune status. However, if the band strength of the T line is between those of the C1 and C2 lines, the antibody titer to CPV is moderate (between 1:80 and 1:320), as is the immune protection status. If the band strength of the T line is lower than that of the C1 line, the antibody titer to CPV is low (below 1:80), and a weakly protected immune status is indicated. All experiments were performed with the samples coded and blinded.
(iv) Serum samples.
The reference sera were obtained by courtesy of Y.-K. Lim of Cheju University. The HI titers of the reference canine sera were 1:10, 1:40, 1:160, and 1:2,560. Serum samples from a total of 386 dogs were tested for CPV titer with the rapid assay. All sera were provided by animal hospitals in Korea. Serum samples were taken from dogs between 4 weeks to 5 years of age, regardless of vaccination history.
(v) Hemagglutination inhibition test.
CPV antibody titer was also determined by use of the HI assay, as described by Carmichael et al. (4). Briefly, all tests were carried out at 4°C using 1% pig erythrocytes and 8 HAU of CPV (C-780916). Sera were pretreated with kaolin and pig erythrocytes to remove nonspecific background. Sera were serially diluted in phosphate-buffered saline (pH 7.2), starting with a 1:10 dilution. Titers were expressed as the reciprocal of the highest serum dilution that completely inhibited the hemagglutination. Sera with HI titers of 1:80 or above were considered anti-CPV positive, and those with HI titers below 1:40 were considered anti-CPV negative.
(vi) Sera of dogs recovering from CPV infection.
To identify the patterns of antibody titers through CPV infection and return the dogs to healthy status, paired serum samples from two dogs were evaluated with both the HI assay and the new rapid assay kit. Two hospitalized dogs which had been infected by CPV were intensively medicated with fluid therapy, antibiotics, and a nausea control agent. Serum samples were collected daily until the dogs recovered. All sera were stored at −20°C before testing.
RESULTS
Monoclonal antibody production.
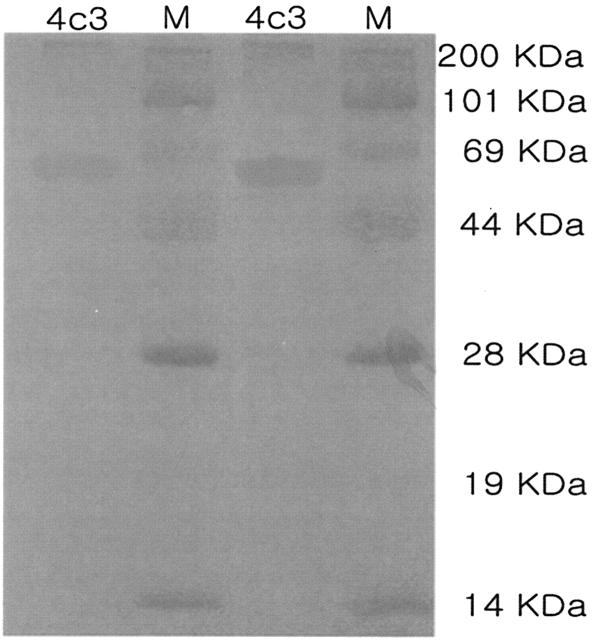
After screening hybridoma cells secreting anti-CPV antibodies into the culture supernatant, we cloned several hybridoma cell lines by limiting dilution and selected a suitable clone, which we designated CPV MAb 4c3. The prepared monoclonal antibodies bound a 64-kDa CPV protein by Western blotting (Fig. 2) and were identified as being of an IgG2a isotype.
FIG. 2.
Western blotting with CPV MAb 4c3. CPV was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with CPV MAb 4c3. The CPV MAb 4c3 bound to the 64-kDa protein of CPV. M, molecular markers.
Rapid assay kit application.
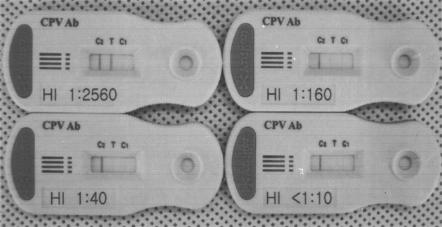
The results of our newly developed assay were compared to those of the HI assay to measure antibody titers. The intensity of the colored band at the T line was proportional to the HI titer of the reference serum sample (Fig. 3). Serum which had an HI titer below 1:10 did not produce a colored band at the T line. Serum which had a 1:40 HI titer showed a weaker band at the T line than the C1 line, and serum which had an intermediate antibody titer (1:160) produced a band whose density was intermediate between that of the C1 and C2 lines. Serum with a 1:2,560 HI titer developed a higher-density band strength at the T line than at the C2 line.
FIG. 3.
The intensity of the color developed at the T line (T) correlated with the HI assay-determined titer of the reference serum sample shown on each test strip.
Correlation with HI titer.
Sera from a total of 386 dogs were tested for CPV antibody using the rapid assay kit, and the results were compared to those obtained by an HI assay. The band at the T line became denser as the HI titer of the serum sample became higher (Table 1). Table 2 shows the sensitivity and specificity of the rapid assay, determined using positive and negative serum samples. The sensitivity and specificity of the rapid assay were 97.1% and 76.6%, respectively, compared to the HI assay.
TABLE 1.
Correlation of band density and HI titer with the 386 serum samples
| HI titer | No. of serum samples | No. of serum samples with indicated band strengtha
|
||
|---|---|---|---|---|
| T < C1 | C1 < T ≤ C2 | T > C2 | ||
| <1:10 | 5 | 5 | 0 | 0 |
| 1:10 | 9 | 9 | 0 | 0 |
| 1:20 | 9 | 9 | 0 | 0 |
| 1:40 | 14 | 13 | 1 | 0 |
| 1:80 | 14 | 8 | 6 | 0 |
| 1:160 | 85 | 3 | 75 | 7 |
| 1:320 | 57 | 0 | 52 | 5 |
| 1:640 | 70 | 0 | 48 | 22 |
| 1:1,280 | 59 | 0 | 13 | 46 |
| >1:2,560 | 64 | 0 | 0 | 64 |
| Total | 386 | 47 | 195 | 144 |
T, band strength of T line; C1, band strength of C1 line; C2, band strength of C2 line.
TABLE 2.
Sensitivity and specificity of the rapid assay in detecting anti-CPV antibodies in serum samples
| Result by new rapid assaya | No. of samples with indicated result by HI assay
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive (T ≥ C1) | 338 | 1 | 339 |
| Negative (T < C1) | 11 | 36 | 47 |
| Total | 349 | 37 | 386 |
The sensitivity and specificity of the rapid assay were 97.1% (338/349 samples) and 76.6% (36/47 samples), respectively. The band strength of the C1 line is equivalent to a 1:80 HI assay titer. T, band strength of T line; C1, band strength of C1 line.
Anti-CPV antibodies in sera from recovering dogs.
Two dogs which had recovered from CPV infection showed increased antibody titers. The HI titers of the two dogs were between 1:10 and 1:20 when they were hospitalized. However, from 3 to 4 days after hospitalization, the antibody titers had increased and finally reached between 1:160 and 1:320 when the puppies were fully recovered. The newly developed kit also showed that the band density of the T line increased with increasing health (Table 3). On day 0, the band density was faint. On day 1, however, the band density of the T line became clear but appeared less dense than that of the C1 line. By day 2, the band density was the same as that of the C1 line. By day 7, the T line was denser than the C2 line.
TABLE 3.
Pattern of increasing CPV antibody levels in dogs during recovery from CPV infection
| Dog | No. of days after hospitalization | HI titer | Band strengtha | Status of dog |
|---|---|---|---|---|
| 1 | 0 | 1:20 | T < C1 | Ill |
| 1 | 1:40 | T < C1 | Ill | |
| 2 | 1:80 | T = C1 | Ill | |
| 3 | 1:80 | T = C1 | Ill | |
| 4 | 1:320 | C1 < T < C2 | Healthy | |
| 5 | 1:640 | T = C2 | Healthy | |
| 2 | 0 | 1:10 | T < C1 | Ill |
| 1 | 1:40 | T < C1 | Ill | |
| 2 | 1:80 | T = C1 | Ill | |
| 3 | 1:160 | C1 < T < C2 | Healthy | |
| 4 | 1:640 | T = C2 | Healthy | |
| 5 | 1:1,280 | T > C2 | Healthy |
T, band strength of T line; C1, band strength of C1 line; C2, band strength of C2 line.
DISCUSSION
The failure of anti-CPV vaccination in puppies is due primarily to maternal antibodies provided in the colostrum. Carmichael et al. (5) demonstrated that more than 95% of the dogs with HI titers less than 1:10 responded to the vaccine, but only 50% responded when the HI titers were approximately 1:20. No animal with a titer greater than 1:80 at the time of vaccination became actively immunized. In a previous study, obvious clinical signs of CPV infection were observed only in dogs with maternal antibody titers of <80; conversely, dogs with an antibody titer of 1:160 were not clinically affected, despite the apparent infection (10). Additionally, Pratelli et al. (11, 12) showed that the CPV 2b vaccine was able to elicit protective immunity in 100% of the pups whose HI titers were 1:10 to 1:40, 83% of the pups with a titer of 1:80, 57% of the pups with a titer of 1:160, and 60% of the pups with a titer of 1:320. Therefore, it is very important to know the antibody level to eliminate vaccination failure caused by high levels of maternal antibodies in puppies.
In order to provide a simple, easy-to-read, rapid test with excellent sensitivity and specificity for the detection of anti-CPV antibodies, we have developed a new kit using an immunochromatography membrane strip. The principle of the one-step rapid assay described in this paper is as follows. Any CPV-specific antibodies in the canine sera react with CPV directly in the sample pad. The CPV bound to canine serum antibodies binds the gold-conjugated anti-CPV MAb. The anti-canine sera- CPV-anti-CPV MAb migrates on the nitrocellulose membrane and binds the goat anti-canine IgG in the test line, resulting in a purple band whose intensity is proportional to the specific CPV antibody level. To interpret the results, the observer compares the color intensities of the test line and the control lines.
In this study, a variety of hybridoma cells which produced CPV-specific monoclonal antibodies were screened. We selected one hybridoma producing a secreted MAb that showed excellent reactivity in our test system. The selected MAb reacted with a 63-kDa protein of CPV which we assumed was VP2. VP2 is the major immunogen of CPV and is able to elicit the production of neutralizing antibodies. In the rapid assay, we used purified CPV for MAb binding to increase the sensitivity and specificity, because a partially purified CPV preparation increased the nonspecific background in the assay (data not shown). The partially purified CPV interfered with the reactions. For example, components from fetal bovine serum give false reactions.
We were particularly interested in determining whether the rapid assay kit could be used to quantify CPV antibodies. The assay yielded rapid results, was easy to perform without any specialized equipment, and showed a 97.1% sensitivity and 76.6% specificity, compared to HI assay results. The interpretation of test results, however, was equivocal in a few cases. Therefore, a further study for increasing the specificity is needed. Particularly, samples with 1:80 and 1:160 HI titers should be tested on a large scale. According to the criteria established in this paper, technicians who were conducting this assay experienced some difficulties in discriminating the band densities, particularly when the band density of the T line was similar to that of C1 line. None of the 386 serum samples analyzed in this study presented a “not valid” result when the newly developed rapid assay kit was used. Overall, this kit is very useful for determining maternal antibody levels and determining a vaccine program for puppies as well as evaluating the need to revaccinate adult dogs. Also, this kit could be used in the prognosis of CPV infection and the determination of appropriate medications at animal hospitals.
In conclusion, we have developed a new assay kit based on an immunochromatography method and have found that the kit could determine CPV antibody level and was easy to use.
Acknowledgments
We are very grateful for the highly skilled and enthusiastic support of J. E. Yoo and K. S. Na. Their input and dedication helped to make this work possible.
REFERENCES
- 1.Appel, M. J. G., W. F. Scott, and L. E. Carmichael. 1979. Isolation and immunization studies of a canine parvo-like virus from dogs with haemorrhagic enteritis. Vet. Rec. 105:156-159. [DOI] [PubMed] [Google Scholar]
- 2.Burtonboy, G., F. Cignoul, N. Delferriere, and P. P. Pastoret. 1979. Canine hemorrhagic enteritis: detection of viral particles by electron microscopy. Arch. Virol. 61:1-11. [DOI] [PubMed] [Google Scholar]
- 3.Carman, P. S., and R. C. Povey. 1983. Comparison of the viral proteins of canine parvovirus-2, mink enteritis virus and feline panleukopenia virus. Vet. Microbiol. 8:423-435. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael, L. E., J. C. Joubert, and R. V. H. Pollock. 1980. Hemagglutination by canine parvovirus: serologic studies and diagnostic applications. Am. J. Vet. Res. 41:784-791. [PubMed] [Google Scholar]
- 5.Carmichael, L. E., J. C. Joubert, and R. V. H. Pollock. 1983. A modified live canine parvovirus vaccine. II. Immune response. Cornell Vet. 73:13-29. [PubMed] [Google Scholar]
- 6.Iida, H., S. Fukuda, N. Kawashima, T. Yamazaki, J. Aoki, K. Tokita, K. Morioka, N. Takarada, and T. Soeda. 1990. Effect of maternally derived antibody levels on antibody responses to canine parvovirus, canine distemper virus and infectious canine hepatitis virus after vaccinations in beagle puppies. Jikken Dobutsu 39:9-19. [DOI] [PubMed] [Google Scholar]
- 7.Kelly, W. R. 1978. An enteric disease of dogs resembling feline panleukopaenia virus. Aust. Vet. J. 54:593. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien, S. E., J. A. Roth, and B. L. Hill. 1986. Response of pups to modified-live canine parvovirus component in a combination vaccine. J. Am. Vet. Med. Assoc. 188:699-701. [PubMed] [Google Scholar]
- 9.Parrish, C. R. 1990. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv. Virus Res. 38:403-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollack, R. V., and L. E. Carmichael. 1982. Maternally derived immunity to canine parvovirus infection: transfer, decline and interference with vaccination. J. Am. Vet. Med. Assoc. 180:37-42. [PubMed] [Google Scholar]
- 11.Pratelli, A., A. Cavalli, V. Martella, M. Tempesta, N. Decaro, L. E. Carmichael, and C. Buonavoglia. 2001. Canine parvovirus (CPV) vaccination: comparison of neutralizing antibody responses in pups after inoculation with CPV2 or CPV2b modified live virus vaccine. Clin. Diagn. Lab. Immunol. 8:612-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratelli, A., A. Cavalli, G. Normanno, M. G. De Palma, G. Pastorelli, C. Buonavoglia, and V. Martella. 2000. Immunization of pups with maternally derived antibodies to canine parvovirus (CPV) using a modified-live variant (CPV-2b). J. Vet. Med. B 47:273-276. [DOI] [PubMed] [Google Scholar]
- 13.Siegl, G. 1984. Canine parvovirus. Origin and significance of a “new” pathogen, p. 363-388. In K. Berns (ed.), The parvoviruses. Plenum, New York, N.Y.
- 14.Siegl, G., R. C. Bates, K. I. Berns, B. J. Carter, D. C. Kelly, E. Kurstak, and P. Tattersall. 1985. Characteristics and taxonomy of Parvoviridae. Intervirology 23:61-73. [DOI] [PubMed] [Google Scholar]
- 15.Vihinen-Ranta, M., E. Lindfors, L. Heiska, P. Veijalainen, and M. Vuento. 1996. Detection of canine parvovirus antigens with antibodies to synthetic peptides. Arch. Virol. 141:1741-1748. [DOI] [PubMed] [Google Scholar]
- 16.Waner, T., S. Mazar, E. Nachmias, E. Keren-Kornblatt, and S. Harrus. 2003. Evaluation of a dot ELISA kit for measuring immunoglobulin M antibodies to canine parvovirus and distemper virus. Vet. Rec. 152:588-591. [DOI] [PubMed] [Google Scholar]
- 17.Waner, T., A. Naveh, I. Wudovsky, and L. E. Carmichael. 1996. Assessment of maternal antibody decay and response to canine parvovirus vaccination using a clinic-based enzyme-linked immunosorbent assay. J. Vet. Diagn. Investig. 8:427-432. [DOI] [PubMed] [Google Scholar]