Abstract
The triggering receptor expressed on myeloid cells (TREM-1) is a recently identified receptor expressed on neutrophils and monocytes. Activation of the receptor induces neutrophils to release the enzyme myeloperoxidase and inflammatory cytokines such as interleukin-8. TREM-1 has an alternatively spliced variant that lacks the transmembrane region, resulting in the receptor being secreted in a soluble form (sTREM-1). Soluble TREM-1 has been detected in plasma during experimental and clinical sepsis and has been advocated as a diagnostic marker of infection for pneumonia and as a prognostic marker for patients with septic shock. We studied TREM-1 surface expression, using flow cytometry, and simultaneously measured sTREM-1 concentrations in culture supernatants of lipopolysaccharide (LPS)-stimulated neutrophils. TREM-1 surface expression was constitutive and was not upregulated upon LPS stimulation. However, sTREM-1 release from neutrophils was significantly upregulated by LPS stimulation (P < 0.0001), an effect that was abrogated by cycloheximide. Soluble TREM-1 is therefore secreted by human neutrophils in response to LPS challenge in a process involving de novo protein synthesis that is not accompanied by an upregulation of the TREM-1 receptor on the surfaces of the cells.
Despite advances in intensive care medicine, sepsis remains a major cause of morbidity and mortality, affecting more than 750,000 patients each year in Europe and the United States, with mortality ranging from 15% for uncomplicated sepsis to 80% for septic shock and multiple organ dysfunction syndrome (1, 15).
Neutrophils are activated as part of the host response to infection, and although protective in nature, this activation could lead to a wide range of pathological effects secondary to the release of inflammatory mediators, toxic substances, and oxidizing agents and through their interaction with endothelial cells (14, 18). The triggering receptor expressed on myeloid cells (TREM-1) is a recently identified receptor expressed on neutrophils and a subset of monocytes. It is a member of the immunoglobulin (Ig) superfamily that contains a single Ig-like extracellular domain and a short cytoplasmic tail with no signaling motif. The transmembrane domain of TREM-1 has been shown to associate with the transmembrane adaptor protein DNAx activation protein 12 (DAP12), which in turn facilitates intracellular signal transduction (5). Triggering of the receptor induces neutrophils to release the enzyme myeloperoxidase and inflammatory cytokines such as interleukin-8 (IL-8). There is some evidence, though not consistent, that TREM-1 is upregulated on neutrophils upon exposure to lipopolysaccharide (LPS) (3). The importance of TREM-1 in the pathogenesis of sepsis was recently highlighted in murine models of sepsis, where the use of a TREM-1 decoy receptor downregulated the inflammatory response and protected mice from lethality (4).
TREM-1 mRNA has an alternatively spliced variant which encodes a protein that lacks the transmembrane region, resulting in the receptor being secreted in a soluble form (sTREM-1). Both the membrane-bound and soluble forms of TREM-1 have the same extracellular domain and, hence, can compete for the same ligand(s). However, the secreted form is incapable of transmitting a signal, suggesting a way for cells to downregulate the TREM-1 pathway (12). In humans, sTREM-1 has been detected in plasmas from volunteers injected with LPS and from patients with sepsis (6, 13). Moreover, sTREM-1 has recently been advocated as a diagnostic marker of infection for patients with pneumonia and as a prognostic marker for patients with septic shock (7, 8).
Despite a potentially crucial role for TREM-1 and its soluble form in the pathogenesis of sepsis, the exact cellular source of sTREM-1 has not been identified. In addition, there are currently no available data relating sTREM-1 secretion to TREM-1 cell surface expression on LPS-activated neutrophils. We hypothesized that LPS stimulation of neutrophils would lead to upregulation of TREM-1 cell surface expression and an increase in sTREM-1 release. We have therefore quantified the changes in cell surface expression of TREM-1 and sTREM-1 secretion in LPS-stimulated neutrophils from healthy volunteers in vitro.
MATERIALS AND METHODS
Power calculation.
A pilot study was conducted (n = 10), and sTREM-1 concentrations were found to be 85.1 ± 43.3 and 135.6 ± 57.8 pg/ml (mean ± standard deviation [SD]) in culture supernatants of unstimulated and LPS-stimulated neutrophils, respectively. A power calculation based on neutrophil sTREM-1 release indicated that 18 subjects were required to achieve a power of 80% and a significance level of <0.05.
Neutrophil isolation.
Following Local Research Ethics Committee approval and informed consent, 30 ml venous blood was collected into sodium heparin-coated tubes, using a sterile closed system, from 18 volunteers (male/female ratio, 10/8; age range, 22 to 52 years). Neutrophils were immediately separated by single-step density gradient centrifugation, using Polymorphprep (Axis-Shield, Oslo, Norway) as previously described (16). The harvested cells were resuspended in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum, 20 mmol/liter l-glutamine, and 1% penicillin-streptomycin. Cell counts were adjusted to 1 × 107 cells/ml. Neutrophil viability was assessed using trypan blue exclusion.
The cell suspension was divided into three tubes: one tube was left unstimulated, whereas LPS was added to the two other tubes to a final concentration of 1 μg/ml in the presence or absence of 10 μg/ml cycloheximide. The cells were then incubated in a humidified incubator at 5% CO2-95% air at 37°C for 16 h. Following incubation, tubes were centrifuged, and culture supernatants were collected and stored at −20°C until further use. Cells were then harvested from culture, washed twice, and resuspended in phosphate-buffered saline. Viability was reassessed, and cell suspensions were readjusted to 1 × 107 cells/ml. Neutrophil viability was consistently above 90%.
Flow cytometry analysis.
Before antibody staining, cells were incubated with 10% normal human serum in phosphate-buffered saline for 20 min at 4°C to block nonspecific Fc binding. Approximately 1 × 106 cells were incubated with a monoclonal anti-human TREM-1 antibody (R&D Systems, Minneapolis, Minn.), followed by an isotype-specific secondary antibody (fluorescein isothiocyanate-conjugated rat anti-mouse IgG1; BD Pharmingen, CA). Cells were subsequently incubated with a phycoerythrin-conjugated mouse anti-human CD16b antibody (BD Pharmingen, CA). Isotype-matched control antibodies were used in order to exclude nonspecific staining. Flow cytometry was carried out on a BD LSR system (Becton Dickinson), and data were analyzed using CellQuest Pro software (Becton Dickinson). The purity of the neutrophil population was initially assessed by the mean fluorescence intensity (MFI) of phycoerythrin-conjugated CD16b antibody staining (Fig. 1a) and was consistently above 95%. Neutrophils were gated according to side scatter and CD16b antibody binding characteristics (Fig. 1b), and cells within this gate were subsequently analyzed for TREM-1 surface expression (Fig. 1c).
FIG. 1.
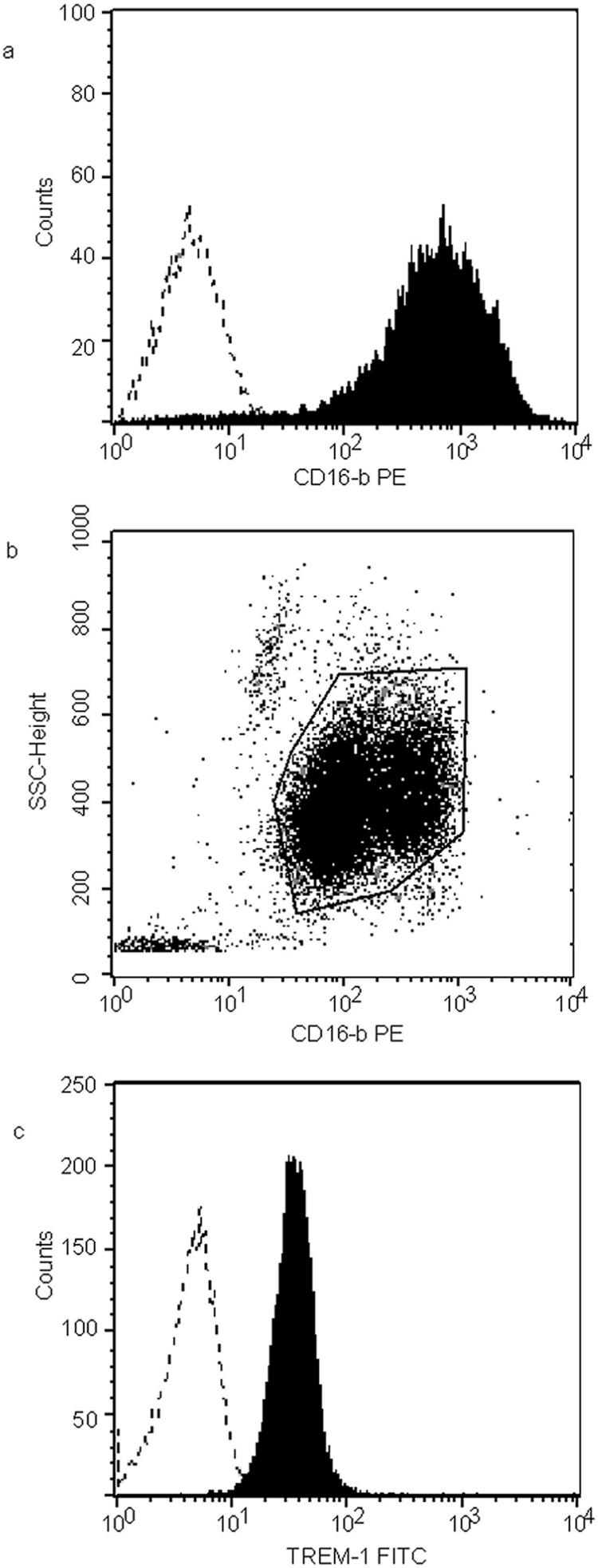
Flow cytometry analysis protocol. (a) Purity of neutrophils assessed using their CD16b binding characteristic. Dotted line, isotype control. (b) Gating on neutrophils using their side scatter (SSC) and CD16b binding characteristics. (c) Using the gate described above, TREM-1 expression was assessed. Dotted line, isotype control.
Enzyme immunoassay.
Soluble TREM-1 was measured in culture supernatants of unstimulated and LPS-stimulated neutrophils by using the commercially available DuoSet enzyme-linked immunosorbent assay development system (R&D Systems). All assays were performed in a single session, with an intra-assay coefficient of variation of 8.7% in our hands (n = 8).
Statistical analysis.
Soluble TREM-1 data were not normally (log normally) distributed and were thus reported as medians (with ranges) and statistically analyzed using the Wilcoxon signed-rank test. TREM-1 expression data obtained from flow cytometry analysis were normally distributed and thus were reported as means ± SD and statistically analyzed using Student's paired t test. Statistical analysis was performed using the Analyze-it software package, and P values of <0.05 were considered significant.
RESULTS
A preliminary dose-response experiment was performed using neutrophils from six volunteers and different concentrations of LPS. All samples were incubated for 16 h. There was a gradual increase in sTREM-1 levels in response to increasing concentrations of LPS up to 1 μg/ml; however, increasing the LPS concentration to 2 μg/ml was not accompanied by further increases in the sTREM-1 concentration (Table 1). In contrast, we did not observe any upregulation in TREM-1 surface expression in response to LPS concentrations ranging from 1 ng to 2 μg (Table 2). Based on the results of this dose-finding experiment, an LPS concentration of 1 μg/ml was used for the current study.
TABLE 1.
Dose-finding study (n = 6)
| Parameter | Unstimulated cellsa | Cells stimulated with LPS for 16 ha
|
||||
|---|---|---|---|---|---|---|
| 1 ng/ml | 10 ng/ml | 100 ng/ml | 1 μg/ml | 2 μg/ml | ||
| sTREM-1 concn (pg/ml) | 91 (61-118) | 108 (92-123) | 141* (131-170) | 207* (159-262) | 346* (260-433) | 353* (292-422) |
Data are medians (ranges). For Friedman analysis of variance P < 0.0001. *, P < 0.05 compared to unstimulated cells, using the Wilcoxon signed-rank test; there was no significant difference between 1 and 2 μg/ml LPS.
TABLE 2.
Dose-finding study (n = 6)
| Parameter | Unstimulated cellsa | Cells stimulated with LPS for 16 ha
|
||||
|---|---|---|---|---|---|---|
| 1 ng/ml | 10 ng/ml | 100 ng/ml | 1 μg/ml | 2 μg/ml | ||
| TREM-1 MFI | 31 ± 9.8 | 29 ± 10.4 | 30 ± 10.0 | 32 ± 7.4 | 29 ± 7.0 | 33 ± 7.8 |
Data are means ± SD.
In our study, following 16 h of incubation, TREM-1 surface expression was similar in both LPS-stimulated and unstimulated neutrophils (P = 0.093), as judged by flow cytometry, and was not affected by coincubation with cycloheximide (Fig. 2). Soluble TREM-1 concentrations were, however, significantly higher (P < 0.0001) in culture supernatants from LPS-stimulated neutrophils (147 [48 to 331] pg/ml) than in those from unstimulated neutrophils (89 [25 to 265] pg/ml). Coincubation of LPS-stimulated neutrophils with cycloheximide abrogated the increase in sTREM-1 (69 [25 to 161] pg/ml), and sTREM-1 release in the presence of cycloheximide was significantly lower (P < 0.001) than that from unstimulated neutrophils (Fig. 3).
FIG. 2.
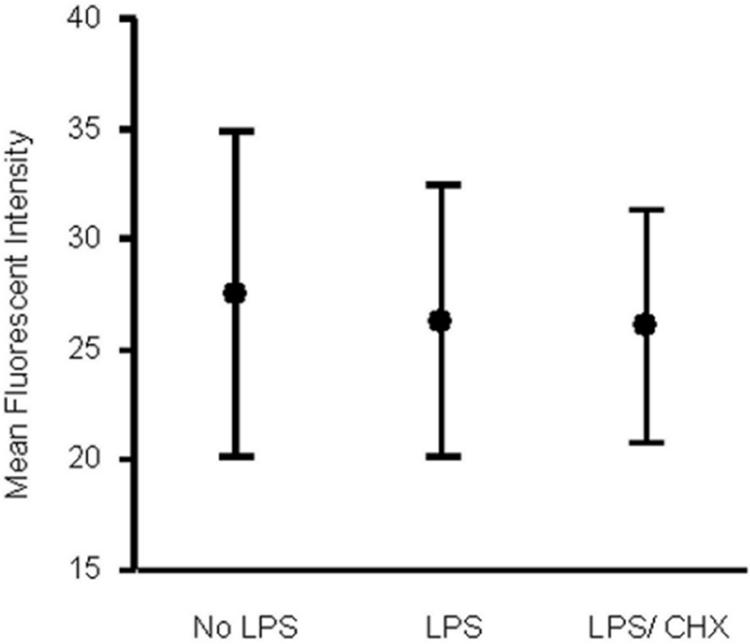
MFI of TREM-1 expression on neutrophils isolated from 18 subjects and incubated ex vivo for 16 h. No LPS, unstimulated cells; LPS, cells stimulated with 1 μg/ml LPS; LPS/CHX, cells stimulated with 1 μg/ml LPS in the presence of 10 μg/ml cycloheximide. The figure shows means (midpoints) and standard deviations (error bars). There was no significant difference between the three groups.
FIG. 3.
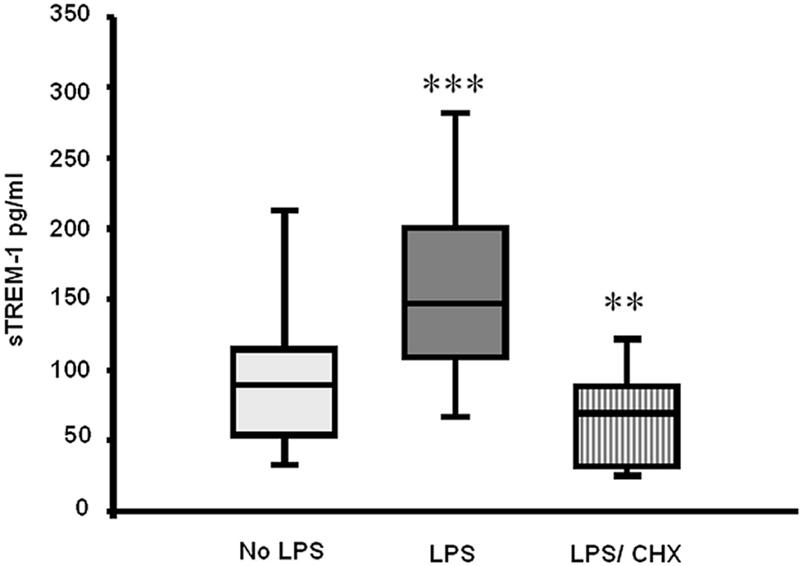
Soluble TREM-1 concentrations in culture supernatants from neutrophils isolated from 18 subjects and incubated ex vivo for 16 h. No LPS, unstimulated cells; LPS, cells stimulated with 1 μg/ml LPS; LPS/CHX, cells stimulated with 1 μg/ml LPS in the presence of 10 μg/ml cycloheximide. Box and whisker plots show medians, 25th and 75th percentiles, and full ranges. **, P < 0.001; ***, P < 0.0001 compared to unstimulated cells.
DISCUSSION
TREM-1 is a transmembrane glycoprotein with a single extracellular Ig-like domain, a transmembrane region, and a short intracellular region. When TREM-1 is engaged by a ligand, it associates with DAP12, triggering several intracellular pathways that lead to intracellular calcium mobilization, actin cytoskeleton rearrangement, and the activation of transcription factors such as nuclear factor κB (9). In murine models, ligation of TREM-1 with monoclonal agonist antibodies stimulates the production of inflammatory cytokines such as IL-8 and leads to rapid neutrophil degranulation and oxidative burst (3, 17). In addition, the activation of TREM-1 in the presence of a Toll-like receptor ligand amplifies the production of proinflammatory cytokines and inhibits the release of IL-10 (2).
TREM-1 mRNA has an alternatively spliced variant which encodes a 30-kDa protein that is specifically recognized by monoclonal antibodies directed against the extracellular domain of TREM-1. The variant lacks the transmembrane region, resulting in the receptor being secreted (sTREM-1). Both membrane-bound and soluble TREM-1 have the same extracellular domain, and they can compete for the same ligand(s). However, the secreted form will be incapable of transmitting a signal, suggesting a way for cells to downregulate the TREM-1 pathway (10, 12). Plasma sTREM-1 levels increased in response to LPS challenge in volunteers (13) and were shown to be higher for patients admitted to an intensive care unit with sepsis than for nonseptic patients (6). In addition, sTREM-1 levels upon admission to the intensive care unit were higher in those patients who went on to survive sepsis than in nonsurvivors, and a progressive decline in plasma sTREM-1 concentration was associated with a favorable clinical outcome of sepsis (8).
The importance of TREM-1/sTREM-1 interaction in the pathogenesis of sepsis was recently shown using murine models of sepsis, where, in two separate experiments, animals were protected from endotoxin lethality by using a TREM-1 decoy receptor (4) or a synthetic peptide mimicking a highly conserved domain of sTREM-1 (10).
In the current study, TREM-1 was constitutively expressed on the surfaces of unstimulated human neutrophils, as previously described (3, 13). However, we did not find an upregulation of this surface expression in response to LPS stimulation. Previous studies have been contradictory, with Bouchon and colleagues reporting an upregulation of surface expression on human neutrophils in response to in vitro LPS stimulation (3) and Knapp and colleagues reporting a downregulation of expression after in vivo administration of LPS in a human experimental endotoxemia model (13). The authors of the latter study explained the unintuitive apparent downregulation of neutrophil TREM-1 expression, which was accompanied by increased monocyte expression, as an artifact of endotoxin-mediated neutrophil sequestration (13). There are experimental differences which may explain the discrepancy between our results and the upregulation previously reported by Bouchon and colleagues (3). Firstly, they used dextran sedimentation followed by single-step density gradient centrifugation designed to optimally separate lymphocytes. Polymorphonuclear leukocytes were then retrieved, along with contaminating erythrocytes, which were removed by hypotonic shock. In contrast, we used a single-step separation system designed and validated solely for the isolation of polymorphonuclear leukocytes, which negates the need for removal of red cells and reduces in vitro activation and, thus, the potential for cell damage. Perhaps more importantly, we measured TREM-1 expression only after selection of CD16b neutrophils, whereas Bouchon and coworkers included all eosinophils and basophils in their analysis (3). Moreover, in support of our findings, a more recent study by Gibot and colleagues found no difference in neutrophil TREM-1 expression in patients with established septic shock, at any time point during the 14-day study period, compared with patients with shock of noninfectious origin or healthy controls (11).
Although there is evidence that sTREM-1 is produced through alternative splicing of the TREM-1 pre-mRNA (12), most studies so far have concentrated on measuring sTREM-1 levels in plasma or body fluids, which limits the availability of information regarding its exact cellular source. In the current study, we have reported for the first time a significant increase in the sTREM-1 concentration in culture supernatants of LPS-stimulated neutrophils, and although this does not exclude its production by other cell types, it does assert that it is produced by neutrophils. Moreover, in the current study, the LPS-stimulated release of sTREM-1 was completely abrogated in the presence of cycloheximide, strongly suggesting that sTREM-1 was being synthesized as a de novo protein by LPS-activated neutrophils. It is also possible, however, that sTREM-1 might have been prestored intracellularly and required the synthesis of another protein in order to be released. We therefore concluded that sTREM-1 was released through a process involving de novo protein synthesis. This finding is in line with the finding of Gingras and colleagues, who reported sTREM-1 as an alternatively spliced variant of TREM-1, not a shed extracellular domain of the cell surface receptor (12).
In summary, we have demonstrated for the first time that sTREM-1 is produced by human neutrophils in response to LPS challenge in a process involving de novo synthesis that is not accompanied by TREM-1 receptor upregulation. This could be seen as a protective mechanism through which neutrophils produce their own TREM-1 decoy receptor as a downregulatory response to limit the effects of persistent activation of the TREM-1/DAP12 pathway. Manipulation of the ratio of expression of sTREM-1 to TREM-1 in a favorable direction may represent a future novel therapeutic approach for the treatment of sepsis.
Acknowledgments
We thank TENOVUS Scotland for funding this study. Jane E. Bruce was on a summer vacation studentship funded by the Anaesthetic Research Society.
REFERENCES
- 1.Angus, D. C., and R. S. Wax. 2001. Epidemiology of sepsis: an update. Crit. Care Med. 29:S109-S116. [DOI] [PubMed] [Google Scholar]
- 2.Bleharski, J. R., V. Kiessler, C. Buonsanti, P. A. Sieling, S. Stenger, M. Colonna, and R. L. Modlin. 2003. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170:3812-3818. [DOI] [PubMed] [Google Scholar]
- 3.Bouchon, A., J. Dietrich, and M. Colonna. 2000. Cutting edge: inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164:4991-4995. [DOI] [PubMed] [Google Scholar]
- 4.Bouchon, A., F. Facchetti, M. A. Weigand, and M. Colonna. 2001. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103-1107. [DOI] [PubMed] [Google Scholar]
- 5.Colonna, M., and F. Facchetti. 2003. TREM-1 (triggering receptor expressed on myeloid cells): a new player in acute inflammatory responses. J. Infect. Dis. 187:S397-S401. [DOI] [PubMed] [Google Scholar]
- 6.Gibot, S., M. N. Kolopp-Sarda, M. C. Bene, A. Cravoisy, B. Levy, G. C. Faure, and P. E. Bollaert. 2004. Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann. Intern. Med. 141:9-15. [DOI] [PubMed] [Google Scholar]
- 7.Gibot, S., A. Cravoisy, B. Levy, M. C. Bene, G. Faure, and P. E. Bollaert. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N. Engl. J. Med. 350:451-458. [DOI] [PubMed]
- 8.Gibot, S., A. Cravoisy, M. N. Kolopp-Sarda, M. C. Bene, G. Faure, P. E. Bollaert, and B. Levy. 2005. Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit. Care Med. 33:792-796. [DOI] [PubMed] [Google Scholar]
- 9.Gibot, S., and B. Levy. 2005. TREM-1: implications during sepsis. Adv. Sepsis 2005:50-55. [Google Scholar]
- 10.Gibot, S., M. N. Kolopp-Sarda, M. C. Bene, P. E. Bollaert, A. Lozniewski, F. Mory, B. Levy, and G. C. Faure. 2004. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibot, S., P. E. Le Renard, P. E. Bollaert, M. N. Kolopp-Sarda, M. C. Bene, G. C. Faure, and B. Levy. 2005. Surface triggering receptor expressed on myeloid cells 1—expression patterns in septic shock. Intensive Care Med. 31:594-597. [DOI] [PubMed] [Google Scholar]
- 12.Gingras, M. C., H. Lapillonne, and J. F. Margolin. 2002. TREM-1, MDL-1, and DAP12 expression is associated with a mature stage of myeloid development. Mol. Immunol. 38:817-824. [DOI] [PubMed] [Google Scholar]
- 13.Knapp, S., S. Gibot, A. de Vos, H. H. Versteeg, M. Colonna, and T. van der Poll. 2004. Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J. Immunol. 173:7131-7134. [DOI] [PubMed] [Google Scholar]
- 14.Nathan, C. 2002. Points of control in inflammation. Nature 420:846-852. [DOI] [PubMed] [Google Scholar]
- 15.Padkin, A., C. Goldfrad, A. R. Brady, D. Young, N. Black, and K. Rowan. 2003. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit. Care Med. 31:2332-2338. [DOI] [PubMed] [Google Scholar]
- 16.Paterson, R. L., H. F. Galley, and N. R. Webster. 2003. The effect of N-acetylcysteine on nuclear factor-κB activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit. Care Med. 31:2574-2578. [DOI] [PubMed] [Google Scholar]
- 17.Radsak, M. P., H. R. Salih, H. G. Rammensee, and H. Schild. 2004. Triggering receptor expressed on myeloid cells-1 in neutrophil inflammatory responses: differential regulation of activation and survival. J. Immunol. 172:4956-4963. [DOI] [PubMed] [Google Scholar]
- 18.Tracey, K. J. 2002. The inflammatory reflex. Nature 420:853-859. [DOI] [PubMed] [Google Scholar]


