Abstract
Bacterial lipoteichoic acid (LTA) shares a structural motif with platelet-activating factor (PAF). Both molecules are strong inflammatory agents and have a glycerol backbone with two lipid chains at the sn-1 and sn-2 positions. PAF is normally inactivated by PAF-acetylhydrolase (PAF-AH), a phospholipase A2 (PLA2), which removes a short acyl group at the sn-2 position. To investigate whether PAF-AH can similarly degrade LTA, we studied the effects of porcine PLA2, bee venom PLA2, and recombinant human PAF-AH on pneumococcal LTA (PnLTA) and staphylococcal LTA (StLTA). After incubation with a porcine or bee venom PLA2, a large fraction of PnLTA lost 264 Da, which corresponds to the mass of the oleic acid group at the sn-2 position. After incubation with recombinant human PAF-AH, PnLTA lost 264 Da; the reduction did not occur when PAF-AH was exposed to Pefabloc SC, an irreversible inhibitor of the PAF-AH active site. Following PAF-AH treatment, PnLTA and StLTA were not able to stimulate mouse RAW 264.7 cells to produce tumor necrosis factor alpha but could stimulate CHO cells expressing human TLR2. This stimulation pattern has been observed with monoacyl PnLTA prepared by mild alkali hydrolysis (22). Taking these data together, we conclude that PAF-AH can remove one acyl chain at the sn-2 position of LTA and produce a monoacyl-LTA that is inactive against mouse cells.
A major component of the cell walls of gram-positive bacteria is lipoteichoic acid (LTA), which is a polyphosphate polymer linked to a glycerol backbone with two acyl chains. The acyl chains anchor the LTA molecule to the bacterial plasma membrane like the acyl chains of lipopolysaccharide (LPS) of gram-negative bacteria. Like LPS, LTA is an amphipathic molecule and an important pathogen-associated molecular pattern that is capable of stimulating innate immunity and that is responsible for gram-positive bacterial sepsis (10, 13, 43). For instance, staphylococcal LTA (StLTA) has been shown to induce septic shock-like changes in rats when peptidoglycan is coadministered (6, 20, 21). Lastly, LTA stimulates Toll-like receptor 2 (TLR2) and induces the production of various inflammatory molecules, including tumor necrosis factor alpha (TNF-α), interleukin-1, and nitric oxide (10, 38, 43), as LPS does via TLR4 (14, 41).
In addition to resembling LPS, LTA has some similarities to platelet-activating factor (PAF), which is a potent intermediate in the host response and which can cause symptoms that replicate many symptoms observed during bacterial sepsis (25). Both the LTA and the PAF molecules share a structural similarity with a glycerol backbone, with acyl groups at the sn-1 and sn-2 positions (24). LTA may stimulate platelet-activating factor receptor (PAFR) either directly or indirectly by inducing production of endogenous PAF (24). In addition to PAFR stimulation, LTA may be able to modulate the degradation of PAF by competitive inhibition of PAF-acetylhydrolase (PAF-AH).
PAF degradation is primarily dependent on plasma PAF-AH, inasmuch as plasma PAF-AH-deficient individuals have a marked inability to clear PAF (19). PAF-AH is a phospholipase A2 (PLA2) and inactivates PAF by removing the acetyl group at the sn-2 position (3, 11, 27, 34, 37). In addition to PAF, PAF-AH can remove acyl chains at the sn-2 position from various glycerolipids, including diacylglycerols, triacylglycerols, and acetylated alkanols (28). However, PAF-AH has not been shown to deacylate a long acyl chain at the sn-2 position, and it is not expected to remove the long acyl chain found in LTA. If PAF-AH can degrade LTA, however, the finding would have a significant impact on our understanding of the inflammatory properties of LTA. We have therefore investigated the ability of PAF-AH to deacylate LTA.
MATERIALS AND METHODS
Reagents.
Recombinant human plasma PAF-AH was kindly provided by ICOS Corporation (Bothell, WA). This enzyme was prepared by expressing the full-length cDNA of human plasma PAF-AH in Escherichia coli (40). The enzyme expressed in this way is as active as the native plasma PAF-AH enzyme and has been used in clinical studies (12, 15). LPS of E. coli O55:B5 was purchased from Sigma-Aldrich (St. Louis, MO), and impurities were removed by further purification, as described previously (14). Phosphatidylcholine, bee venom phospholipase A2, pancreas phospholipase A2, and Pefabloc SC were obtained from Sigma-Aldrich. A synthetic lipoprotein (Pam3CSK4) was obtained from InvivoGen (San Diego, CA).
Cell lines and bacteria.
The mouse macrophage cell line RAW 264.7 (ATCC TIB-71) was obtained from the American Type Culture Collection (Manassas, VA), and the cells were cultured with Dulbecco's modified Eagle's medium (Cellgro Mediatech, Herndon, VA) supplemented with 10% defined fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator. The 3E10-TLR2 cell line, which constitutively expresses human CD14 and human TLR2, was from D. Golenbock (Boston Medical Center, Boston, MA). The cell line expresses CD25 on the cell surface in response to TLR2 ligands. The cells were grown in Ham's F-12 medium (GIBCO-BRL, Rockville, MD) supplemented with 10% defined fetal bovine serum (HyClone), 1 mg/ml of G418 (Calbiochem, La Jolla, CA), and 400 U/ml of hygromycin B (Calbiochem) at 37°C in a 5% CO2 humidified incubator.
Streptococcus pneumoniae strain R36A (ATCC 12214) and Staphylococcus aureus (ATCC 6538) were used for LTA purification. R36A was grown in Todd-Hewitt broth (Becton Dickinson, Franklin Lakes, NJ) supplemented with 0.5% yeast extract (Becton Dickinson, Franklin Lakes, NJ) until late log phase (optical density at 600 nm = 0.6 to 1.0). S. aureus was cultured in tryptic soybean broth (Becton Dickinson, Franklin Lakes, NJ) until late log phase.
Purification of lipoteichoic acid.
Pneumococcal LTA (PnLTA) and StLTA were prepared by using organic solvent extraction, octyl-Sepharose, and an ion-exchange chromatography method, as described previously (1, 22, 30). The structure of PnLTA was characterized by mass spectrometry. Briefly, a mixture of 1 μl of sample and 1 μl of matrix solution (0.5 M 2,5-dihydroxybenzoic acid, 0.1% trifluoroacetic acid in methanol) was applied to a sample plate. After the sample was dried, it was analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Voyager Biospectrometry DE Pro workstation) with a PerSeptive Biosystems (Framingham, MA) mass spectrometer in the Mass Spectrometry Shared Facility at the University of Alabama at Birmingham.
TLC.
Five microliters of phospholipid samples was applied onto a thin-layer chromatography (TLC) plate (5 by 20 cm; Silica Gel 60C; EMDscience, Hawthorne, NY). After the plate was dried, it was placed in a TLC chamber containing a 65:30:5 (vol/vol/vol) mixture of chloroform-methanol-ammonia (25%) at the bottom and the chromatography was performed for 90 min (33, 39). For visualization of phospholipids, the TLC plates were then sprayed with 1.3% molybdenum blue spray reagent (Sigma) (23, 31).
TNF-α production by RAW 264.7 cells.
RAW 264.7 cells were placed in 96-well plates (Costar, Corning, NY) at 2 × 105 cells/well, and the cells were stimulated with 50 μg/ml of PnLTA, 1 μg/ml of StLTA, 0.1 μg/ml of LPS, or 0.2 μg/ml of Pam3CSK4 for 24 h. In some cases, the stimulants were treated with PAF-AH for 2 h at 37°C at the indicated doses, and PAF-AH was inactivated by incubation at 65°C for 2 h or by the addition of 100 μM Pefabloc SC prior to stimulation. The amount of TNF-α in the culture supernatant was determined by a sandwich-type enzyme-linked immunosorbent assay with the Ready-SET-Go kit (eBioscience, San Diego, CA), and the manufacturer's protocol was followed.
CD25 expression by 3E10-TLR2 cells.
3E10-TLR2 cells were placed in six-well plates (Costar) at 5 × 105 cells/well; after 24 h, when the cells were 70% confluent, the cells were stimulated with various molecular preparations. After 16 h, the cells were washed once with phosphate-buffered saline (PBS; pH 7.3) and detached with 2 mM EDTA in PBS. The cells were stained with fluorescein isothiocyanate-conjugated mouse anti-human CD25 (Becton Dickinson, San Diego, CA), and their CD25 expression was determined on a FACSCalibur flow cytometer with CellQuest acquisition analysis software (Becton Dickinson, San Diego, CA).
RESULTS
PAF-AH can monodeacylate PnLTA.
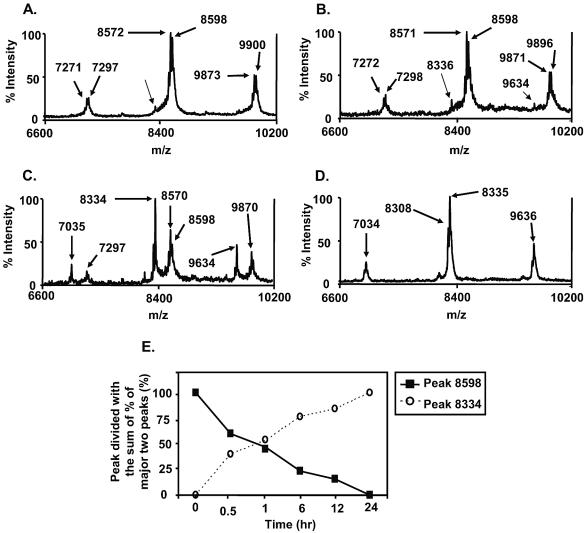
To investigate the effect of PAF-AH on PnLTA, we incubated PnLTA (250 μg/ml in PBS) with various concentrations of PAF-AH (0.5, 5, 10 μg/ml) for 2 h at 37°C and examined the reaction mixture by mass spectrometry. A previous study showed that changes in the mass spectra can be used to monitor the structural alterations of PnLTA (22). Prior to incubation with PAF-AH, PnLTA has distinct mass spectra with three major peaks (7,297, 8,598, and 9,900 m/z), each of which corresponded to LTAs with five, six, and seven repeating units, respectively (Fig. 1A). As observed previously, the major peaks had satellite peaks with a 28-m/z difference (e.g., 8,571 m/z versus 8,598 m/z) (Fig. 1B), consistent with the heterogeneity in the acyl chains (22). When PnLTA was incubated with high PAF-AH concentrations (5 to 10 μg/ml), a new peak at 8,334 m/z became dominant and the original peak at 8,598 m/z decreased significantly (Fig. 1C) or became undetectable (Fig. 1D). The new peak was very small but detectable when PnLTA was incubated with a low concentration (0.5 μg/ml) of PAF-AH (Fig. 1B). This peak was barely detectable even before any PAF-AH treatment (Fig. 1A, arrow), probably as a result of the chemical instability of the second acyl chain (22). The appearance of the new peak was time dependent; about 50% of LTA shifted in 1 h and almost 100% shifted in 6 h in the presence of 1 μg/ml of PAF-AH (Fig. 1E). The peaks at 7,297 or 9,900 m/z also lost 264 m/z in the same patterns.
FIG. 1.
Mass spectra of PnLTA (250 μg/ml) before reaction with PAF-AH (A) and after reaction for 2 h with different concentrations of PAF-AH (0.5, 5, and 10 μg/ml) (B, C, and D, respectively). The peak heights are shown as percent intensity, which indicates the relative percentage of each peak height to the tallest peak height in each spectrum. The positions of various prominent peaks are shown. (E) Heights of the peaks at 8,598 and 8,334 m/z units at various times. The x axis indicates the number of hours that LTA was incubated with PAF-AH at 37°C.
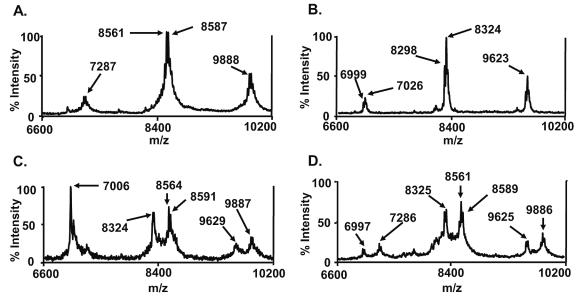
Since PAF-AH is a PLA2, it is likely that the acyl chain at position sn-2 is removed. Indeed, the mass difference between the old and the new peaks (264 m/z) is consistent with the loss of an oleic acid (Fig. 1C). To further confirm that the acyl chain at position sn-2 was removed, we treated PnLTA with two additional PLA2 enzymes (porcine pancreas and bee venom PLA2) and examined the reaction products with a MALDI-TOF mass spectrometer (Fig. 2) (44). Untreated PnLTA showed the three major peaks, as before, although the peak position shifted down by about 10 m/z units compared to that shown in Fig. 1. PnLTA (250 μg/ml) was incubated with PLA2 (50 μg/ml) in a buffer (160 mM HEPES [pH 7.4] and 10 mM CaCl2) for 24 or 48 h (17). Although the enzyme reaction with these PLA2 enzymes was inefficient compared to that with PAF-AH, the two PLA2 enzyme treatments produced new peaks with 264 to 267 mass units less than those of the original peaks (8,591 versus 8,324 m/z in Fig. 2C and 8,589 versus 8,325 m/z in Fig. 2D), as PAF-AH treatment did (Fig. 1B). A peak with 7,006 m/z was found only in porcine-pancreas-treated LTA (Fig. 2C). This peak corresponds to porcine pancreas PLA2 with two charges (2). These findings lend further support to the concept that PAF-AH removed the acyl chain at the sn-2 position of PnLTA.
FIG. 2.
Mass spectra of PnLTA (250 μg/ml) before any reaction (A) and after reaction with 10 μg/ml of PAF-AH for 3 h at 37°C (B), porcine pancreas PLA2 for 48 h at 37°C (C), and bee venom PLA2 for 48 h at room temperature (D). The molecular weight of porcine pancreas PLA2 is about 14,000 (2), and the peak at 7,006 m/z observed in panel C corresponds to the porcine PLA2 with two charge units.
PAF-AH does not deacylate 1,2-dipalmitoyl-phosphatidylcholine (DPPC) or lipoprotein.
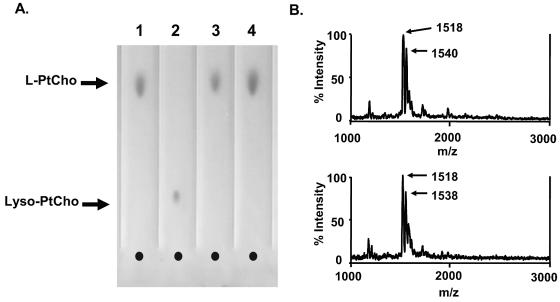
PAF-AH is known to remove only short acyl chains, yet we observed deacylation of a long acyl chain from PnLTA. Therefore, we reexamined our preparation of PAF-AH for its ability to deacylate DPPC. As was done for PnLTA, DPPC (250 μg/ml) was incubated with 10 μg/ml of PAF-AH at RT for 6 h, and the reaction mixture was analyzed by TLC. By TLC, monoacyl phosphatidylcholine (Fig. 3A) can easily be distinguished from diacyl phosphatidylcholine (Fig. 3A) by the differences in their mobilities (Fig. 3A). The reaction mixture did not show any monoacyl phosphatidylcholine. Thus, our preparation of PAF-AH does not deacylate the long acyl chain associated with DPPC.
FIG. 3.
(A) Thin-layer chromatogram of DPPC (lane 1), lyso-DPPC (lane 2), DPPC incubated with PBS (lane 3), and DPPC incubated with PBS containing PAF-AH (10 μg/ml) (lane 4). DPPC and lyso-DPPC are labeled L-PtCho and lyso-PtCho, respectively. The concentration of DPPC or lyso-DPPC was 250 μg/ml, and the incubation time was 6 h. (B) Mass spectra of a synthetic bacterial lipoprotein (Pam3CSK4, 250 μg/ml) before (top panel) and after (bottom panel) a reaction with 10 μg/ml of PAF-AH for 6 h at 37°C. The two major peaks correspond to Pam3CSK4 (at 1,518 m/z) and its sodium salt (at 1,540 m/z) (18).
Bacterial lipoproteins have important pathogen-associated molecular patterns, and they have two acyl chains on a cysteine backbone (4). To investigate whether PAF-AH can deacylate these acyl chains, we incubated a synthetic lipoprotein, Pam3CSK4, with PAF-AH as we did for LTA. We examined the alterations in the molecular structure by mass spectrometry. Two major peaks at 1,518 and 1,540 m/z correspond to Pam3CSK4 and its sodium salt, respectively (18). Unlike what was observed for LTA, we did not observe any changes in the molecular structure following the PAF-AH treatment (Fig. 3B). Thus, PAF-AH does not deacylate Pam3CSK4 and is unlikely able to deacylate bacterial lipoproteins.
PAF-AH can be inactivated by heat or by addition of serine protease inhibitors.
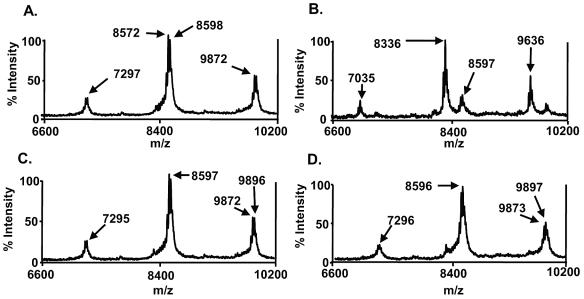
The enzymatically active site of PAF-AH responsible for PAF deacylation can be neutralized by heating at 65°C (7) or by treatment with Pefabloc SC (100 μM) (8), a serine protease inhibitor. To investigate whether the active site of PAF-AH is responsible for deacylating LTA as well as PAF, we investigated whether heating or Pefabloc SC treatment can inactivate PAF-AH's activity on the deacylation of PnLTA. When PnLTA was incubated with PAF-AH at 37°C for 2 h, the major peak shifted from 8,598 m/z (Fig. 4A) to 8,336 m/z (Fig. 4B), as expected. Similar changes were observed for other peaks. However, such a change was not observed when PAF-AH was preincubated at 65°C for 2 h (Fig. 4C) or was incubated with 100 μM Pefabloc SC for 2 h at 37°C (Fig. 4D). Thus, the active site of PAF-AH used for PnLTA is likely identical to the active site used for PAF.
FIG. 4.
Mass spectra of PnLTA before (A) and after reaction with 5 μg/ml of PAF-AH for 2 h (B, C, and D). PAF-AH was inactivated by incubating it at 65°C for 2 h (C) or by incubating it with 100 μM of Pefabloc SC for 2 h at 37°C (D).
PAF-AH treatment alters the functionality of LTA from PnLTA and StLTA.
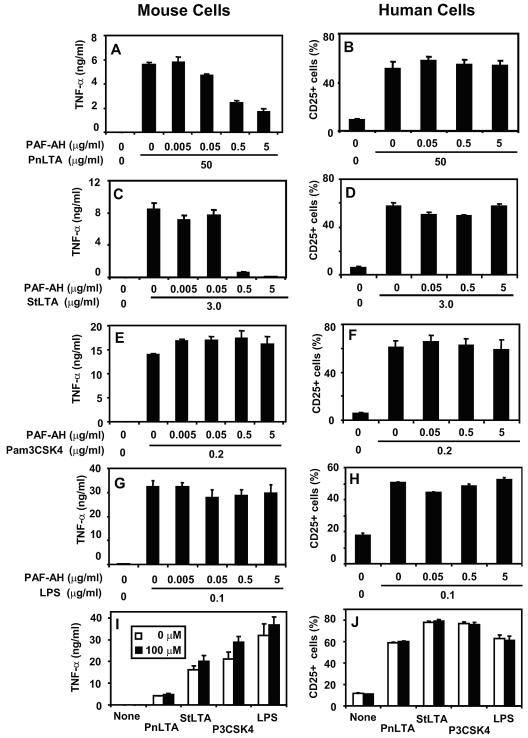
We have previously noted that a controlled mild alkali hydrolysis produces monodeacylated PnLTA that can stimulate human cells but not mouse cells (22). To determine if the PAF-AH-treated PnLTA behaves similarly, PnLTA was incubated with different concentrations of PAF-AH for 2 h at 37°C and treated with Pefabloc SC (100 μM) to stop the PAF-AH activity. Pefabloc SC was not removed from the reaction mixture because it does not affect TNF-α or CD25 production by target cells (Fig. 5I and J). We stimulated mouse RAW 264.7 cells and human 3E10-TLR2 cells with the reaction mixtures for 24 h. PAF-AH-treated PnLTA did not induce RAW 264.7 cells to produce TNF-α (Fig. 5A) but induced CD25 expression by 3E10-TLR2 cells (Fig. 5B). Completely deacylated PnLTA did not stimulate either human or mouse cells (data not shown).
FIG. 5.
TNF-α production by mouse cells (left panels) and CD25 expression by human cells (right panels) in response to 50 μg/ml of PnLTA (A and B), 3 μg/ml of StLTA (C and D), 0.2 μg/ml of Pam3CSK4 (E and F), and 0.1 μg/ml of LPS (G and H). To examine the effect of Pefabloc SC itself, cells were stimulated with various stimulants (I and J): 50 μg/ml of PnLTA, 3 μg/ml of StLTA, 0.2 μg/ml of Pam3CSK4, and 0.1 μg/ml of LPS in the presence (black bars) or absence (white bars) of 100 μΜ of Pefabloc SC. Mouse cells (RAW 264.7) were stimulated for 48 h, and the TNF-α level was determined by enzyme-linked immunosorbent assay. 3E10-TLR2 cells were stimulated for 16 h, and CD25 expression was determined by flow cytometry. The stimulants were treated with PAF-AH for 2 h at 37°C at the indicated doses and were incubated at 65°C for 2 h or with 100 μM of Pefabloc SC before stimulation.
Unlike PnLTA, the StLTA structure cannot be monitored by mass spectrometry. We considered that PAF-AH may monodeacylate StLTA and that the resulting monoacyl StLTA stimulates human cells but not mouse cells like PnLTA does. To investigate whether PAF-AH also monodeacylates StLTA, we incubated highly purified StLTA with PAF-AH and examined the biological properties of the resulting StLTA. As hypothesized, StLTA treated with PAF-AH was able to stimulate human cells (Fig. 5D) but not mouse cells (Fig. 5C), although untreated StLTA stimulated both mouse and human cells (Fig. 5C and D, respectively). Completely deacylated StLTA produced by alkali hydrolysis did not stimulate either human and mouse cells (data not shown). PAF-AH treatment did not alter the capacity of another TLR2 ligand, Pam3CSK4 (200 ng/ml), or a TLR4 agonist, lipopolysaccharide (100 ng/ml), to stimulate human or mouse cells (Fig. 5E to H); and low concentrations of Pam3CSK4 (20 ng/ml) and LPS (10 ng/ml) were also not altered by PAF-AH treatment (data not shown). Considering these data together, deacylation is specific for LTA and PAF-AH can monodeacylate not only PnLTA but also StLTA.
DISCUSSION
Here we demonstrate that PAF-AH can remove an acyl chain from PnLTA in a time- and concentration-dependent manner, apparently using the enzymatic site used for PAF. PAF-AH removed the acyl chain at the sn-2 position, as shown by the size of the mass loss and also because two other PLA2 enzymes produced the same mass loss. Furthermore, PAF-AH appears to be able to deacylate StLTA as well as PnLTA, since PAF-AH treatment endows both LTAs with the characteristic functional pattern of monoacyl LTA (22): activity against human cells but inactivity against mouse cells. The deacylation appears to be specific for LTA, since PAF-AH did not remove acyl chains from DPPC or Pam3CSK4, a model bacterial lipoprotein.
PAF-AH has been shown to remove only short (four- to six-carbon) acyl chains at the sn-2 position (35), although it can remove a slightly longer acyl chain if carboxylic or aldehydic groups are present at the ω site (36). Since LTA has a very long acyl chain (18 carbons) at the sn-2 position and lacks carboxylic or aldehydic groups at the ω end, our finding was therefore totally unexpected. PAF-AH, unlike other PLA2 enzymes, reacts on substrates in solution but not those in micelles (28). Perhaps PAF-AH can deacylate LTA, since a large portion of LTA exists as free molecules in solution due to a large hydrophilic repeating unit at the sn-3 position.
Inactivation of PAF has been considered to be the physiological role of PAF-AH, but its physiological role may now include degradation of LTAs from various gram-positive bacterial species. In support of this idea, we have observed that culture supernatants of several gram-positive bacterial species such as Streptococcus mutans and group B streptococcus become unable to stimulate RAW 264.7 cells to produce TNF-α following PAF-AH treatment (data not shown). Since we used 1 to 10 μg/ml for PAF-AH treatments and normal blood levels of PAF-AH are about 1 μg/ml (32), blood should have enough PAF-AH to inactivate biologically relevant amounts of LTA. Indeed, plasma lipoproteins, which harbor PAF-AH in plasma (28), have been shown to inactivate LTA in the presence of lipopolysaccharide binding protein (9).
The importance of PAF in experimental sepsis is clearly established. Injection of PAF can cause many symptoms associated with sepsis (45). PAF levels are elevated and PAF-AH levels decrease during clinical sepsis (32). PAF-AH was therefore considered a pharmacologic agent for reducing PAF and treating bacterial sepsis. Recent clinical trials of PAF-AH, however, did not show clear clinical benefits (32). We have shown here that PAF-AH monodeacylates LTA, which is shown to be important in an experimental model of gram-positive bacterial sepsis in animals (20). Since about half of the cases of bacterial sepsis are due to gram-positive bacteria (5, 16), one should consider deacylation of LTA to be another pharmacological action of PAF-AH.
PAF-AH appears to use the same enzymatic site to deacylate PAF and LTA. Thus, LTA, if it is present at a high concentration, may interfere with the ability of PAF-AH to deactivate PAF. LTA may be present in body fluids at high concentrations for several reasons. LTA is easily shed from bacteria, and early bacterial cultures in vitro can achieve LTA concentrations of 1 μg/ml (1 μM) (42). Antibiotics such as penicillin can kill a large number of bacteria and release their LTAs as a burst (26, 29). Furthermore, bacteria and their LTAs are confined to a small anatomic area during meningeal or middle-ear infections. It would be interesting to study whether LTA can function as a competitive inhibitor of PAF-AH-inactivating PAF in these clinical situations.
Acknowledgments
We thank W. Welch at ICOS for providing us with PAF-AH.
The work was partially supported by funding from the NIH (AI-30021).
REFERENCES
- 1.Behr, T., W. Fischer, J. Peter-Katalinic, and H. Egge. 1992. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur. J. Biochem. 207:1063-1075. [DOI] [PubMed] [Google Scholar]
- 2.Berg, O. G., M. H. Gelb, M. D. Tsai, and M. K. Jain. 2001. Interfacial enzymology: the secreted phospholipase A(2)-paradigm. Chem. Rev. 101:2613-2654. [DOI] [PubMed] [Google Scholar]
- 3.Blank, M. L., T. Lee, V. Fitzgerald, and F. Snyder. 1981. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid). J. Biol. Chem. 256:175-178. [PubMed] [Google Scholar]
- 4.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2005. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 35:282-289. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J., and E. Abraham. 1999. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J. Infect. Dis. 180:116-121. [DOI] [PubMed] [Google Scholar]
- 6.De Kimpe, S. J., M. Kengatharan, C. Thiemermann, and J. R. Vane. 1995. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc. Natl. Acad. Sci. USA 92:10359-10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr, R. S., M. L. Wardlow, C. P. Cox, K. E. Meng, and D. E. Greene. 1983. Human serum acid-labile factor is an acylhydrolase that inactivates platelet-activating factor. Fed. Proc. 42:3120-3122. [PubMed] [Google Scholar]
- 8.Goyal, J., K. Wang, M. Liu, and P. V. Subbaiah. 1997. Novel function of lecithin-cholesterol acyltransferase. Hydrolysis of oxidized polar phospholipids generated during lipoprotein oxidation. J. Biol. Chem. 272:16231-16239. [DOI] [PubMed] [Google Scholar]
- 9.Grunfeld, C., M. Marshall, J. K. Shigenaga, A. H. Moser, P. Tobias, and K. R. Feingold. 1999. Lipoproteins inhibit macrophage activation by lipoteichoic acid. J. Lipid Res. 40:245-252. [PubMed] [Google Scholar]
- 10.Han, S. H., J. H. Kim, M. Martin, S. M. Michalek, and M. H. Nahm. 2003. Pneumococcal lipoteichoic acid (LTA) is not as potent as staphylococcal LTA in stimulating Toll-like receptor 2. Infect. Immun. 71:5541-5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori, K., M. Hattori, H. Adachi, M. Tsujimoto, H. Arai, and K. Inoue. 1995. Purification and characterization of platelet-activating factor acetylhydrolase II from bovine liver cytosol. J. Biol. Chem. 270:22308-22313. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, W. R., Jr., J. Lu, K. M. Poole, G. N. Dietsch, and E. Y. Chi. 2000. Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J. Immunol. 164:3360-3367. [DOI] [PubMed] [Google Scholar]
- 13.Heumann, D., C. Barras, A. Severin, M. P. Glauser, and A. Tomasz. 1994. Gram-positive cell walls stimulate synthesis of tumor necrosis factor alpha and interleukin-6 by human monocytes. Infect. Immun. 62:2715-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 15.Hofbauer, B., A. K. Saluja, M. Bhatia, J. L. Frossard, H. S. Lee, L. Bhagat, and M. L. Steer. 1998. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology 115:1238-1247. [DOI] [PubMed] [Google Scholar]
- 16.Horn, D. L., D. C. Morrison, S. M. Opal, R. Silverstein, K. Visvanathan, and J. B. Zabriskie. 2000. What are the microbial components implicated in the pathogenesis of sepsis? Report on a symposium. Clin. Infect. Dis. 31:851-858. [DOI] [PubMed] [Google Scholar]
- 17.Janssen, M. J., P. J. Burghout, H. M. Verheij, A. J. Slotboom, and M. R. Egmond. 1999. Introduction of a C-terminal aromatic sequence from snake venom phospholipases A2 into the porcine pancreatic isozyme dramatically changes the interfacial kinetics. Eur. J. Biochem. 263:782-788. [DOI] [PubMed] [Google Scholar]
- 18.Jung-Suk Jang, S.-T. L., and Y.-S. Chang. 1996. Characterization of extremely hydrophobic immunostimulatory lipoidal peptides by matrix assisted laser desorption ionization mass spectrometry. Bull. Korean Chem. Soc. 17:1036-1039. [Google Scholar]
- 19.Karasawa, K., A. Harada, N. Satoh, K. Inoue, and M. Setaka. 2003. Plasma platelet activating factor-acetylhydrolase (PAF-AH). Prog. Lipid Res. 42:93-114. [DOI] [PubMed] [Google Scholar]
- 20.Kengatharan, K. M., S. De Kimpe, C. Robson, S. J. Foster, and C. Thiemermann. 1998. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J. Exp. Med. 188:305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kengatharan, K. M., S. J. De Kimpe, and C. Thiemermann. 1996. Role of nitric oxide in the circulatory failure and organ injury in a rodent model of gram-positive shock. Br. J. Pharmacol. 119:1411-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. H., H. Seo, S. H. Han, J. Lin, M. K. Park, U. B. Sorensen, and M. H. Nahm. 2005. Monoacyl lipoteichoic acid from pneumococci stimulates human cells but not mouse cells. Infect. Immun. 73:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kundu, S. K., S. Chakravarty, N. Bhaduri, and H. K. Saha. 1977. A novel spray reagent for phospholipid detection. J. Lipid Res. 18:128-130. [PubMed] [Google Scholar]
- 24.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 25.Mathiak, G., D. Szewczyk, F. Abdullah, P. Ovadia, and R. Rabinovici. 1997. Platelet-activating factor (PAF) in experimental and clinical sepsis. Shock 7:391-404. [DOI] [PubMed] [Google Scholar]
- 26.Mattie, H., K. Stuertz, R. Nau, and J. T. van Dissel. 2005. Pharmacodynamics of antibiotics with respect to bacterial killing of and release of lipoteichoic acid by Streptococcus pneumoniae. J. Antimicrob. Chemother. 56:154-159. [DOI] [PubMed] [Google Scholar]
- 27.McManus, L. M., and R. N. Pinckard. 2000. PAF, a putative mediator of oral inflammation. Crit. Rev. Oral Biol. Med. 11:240-258. [DOI] [PubMed] [Google Scholar]
- 28.Min, J. H., C. Wilder, J. Aoki, H. Arai, K. Inoue, L. Paul, and M. H. Gelb. 2001. Platelet-activating factor acetylhydrolases: broad substrate specificity and lipoprotein binding does not modulate the catalytic properties of the plasma enzyme. Biochemistry 40:4539-4549. [DOI] [PubMed] [Google Scholar]
- 29.Moore, L. J., A. C. Pridmore, S. K. Dower, and R. C. Read. 2003. Penicillin enhances the toll-like receptor 2-mediated proinflammatory activity of Streptococcus pneumoniae. J. Infect. Dis. 188:1040-1048. [DOI] [PubMed] [Google Scholar]
- 30.Morath, S., A. Geyer, and T. Hartung. 2001. Structure-function relationship of cytokine induction by lipoteichoic acid from Staphylococcus aureus. J. Exp. Med. 193:393-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthing, J., and M. Radloff. 1998. Nanogram detection of phospholipids on thin-layer chromatograms. Anal. Biochem. 257:67-70. [DOI] [PubMed] [Google Scholar]
- 32.Opal, S., P. F. Laterre, E. Abraham, B. Francois, X. Wittebole, S. Lowry, J. F. Dhainaut, B. Warren, T. Dugernier, A. Lopez, M. Sanchez, I. Demeyer, L. Jauregui, J. A. Lorente, W. McGee, K. Reinhart, S. Kljucar, S. Souza, and J. Pribble. 2004. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit. Care Med. 32:332-341. [DOI] [PubMed] [Google Scholar]
- 33.Panasenko, O. M., H. Spalteholz, J. Schiller, and J. Arnhold. 2003. Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines. Free Radic. Biol. Med. 34:553-562. [DOI] [PubMed] [Google Scholar]
- 34.Stafforini, D. M., T. M. McIntyre, G. A. Zimmerman, and S. M. Prescott. 1997. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 272:17895-17898. [DOI] [PubMed] [Google Scholar]
- 35.Stafforini, D. M., S. M. Prescott, and T. M. McIntyre. 1987. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J. Biol. Chem. 262:4223-4230. [PubMed] [Google Scholar]
- 36.Stremler, K. E., D. M. Stafforini, S. M. Prescott, and T. M. McIntyre. 1991. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J. Biol. Chem. 266:11095-11103. [PubMed] [Google Scholar]
- 37.Stremler, K. E., D. M. Stafforini, S. M. Prescott, G. A. Zimmerman, and T. M. McIntyre. 1989. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J. Biol. Chem. 264:5331-5334. [PubMed] [Google Scholar]
- 38.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 39.Tauchi-Sato, K., S. Ozeki, T. Houjou, R. Taguchi, and T. Fujimoto. 2002. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J. Biol. Chem. 277:44507-44512. [DOI] [PubMed] [Google Scholar]
- 40.Tjoelker, L. W., C. Eberhardt, J. Unger, H. L. Trong, G. A. Zimmerman, T. M. McIntyre, D. M. Stafforini, S. M. Prescott, and P. W. Gray. 1995. Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad. J. Biol. Chem. 270:25481-25487. [DOI] [PubMed] [Google Scholar]
- 41.Toshchakov, V., B. W. Jones, P. Y. Perera, K. Thomas, M. J. Cody, S. Zhang, B. R. Williams, J. Major, T. A. Hamilton, M. J. Fenton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 42.van Langevelde, P., J. T. van Dissel, E. Ravensbergen, B. J. Appelmelk, I. A. Schrijver, and P. H. Groeneveld. 1998. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: quantitative measurements and biological reactivities. Antimicrob. Agents Chemother. 42:3073-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang, J. E., M. K. Dahle, M. McDonald, S. J. Foster, A. O. Aasen, and C. Thiemermann. 2003. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 20:402-414. [DOI] [PubMed] [Google Scholar]
- 44.Wittenauer, L. A., K. Shirai, R. L. Jackson, and J. D. Johnson. 1984. Hydrolysis of a fluorescent phospholipid substrate by phospholipase A2 and lipoprotein lipase. Biochem. Biophys. Res. Commun. 118:894-901. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerman, G. A., T. M. McIntyre, S. M. Prescott, and D. M. Stafforini. 2002. The platelet-activating factor signaling system and its regulators in syndromes of inflammation and thrombosis. Crit. Care Med. 30:S294-S301. [DOI] [PubMed] [Google Scholar]