Abstract
A CD4 monoclonal antibody which reacts with CD4+ lymphocytes but does not react significantly with monocytes was generated and used to develop a simple method for CD4 count. In a comparison with standard flow cytometry, high correlation was obtained. The developed method can be an alternative to flow cytometry for resource-limited countries.
The incidence of human immunodeficiency virus (HIV) infection and clinical disease continues to increase rapidly in underdeveloped and developing countries (13). As antiretroviral therapy is becoming more affordable and accessible, the provision of affordable CD4 testing for initiating and monitoring antiretroviral therapy has emerged as a vital issue. Flow cytometry, the existing benchmark method for CD4+ lymphocyte enumeration, is expensive and is not affordable for most developing countries. These factors necessitate the need for simple and inexpensive alternative methods for CD4 count.
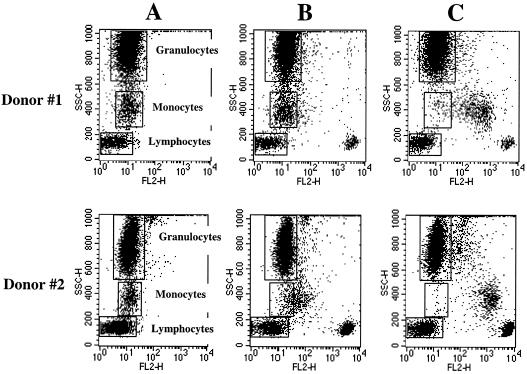
Recently a CD4 monoclonal antibody (MAb), named MT4, was generated in our laboratory (8). When white blood cells (WBC) (n = 4) were stained with MAb MT4, for all tested subjects, CD4+ lymphocytes showed strongly positive reactivity (Fig. 1B, donors 1 and 2). Monocytes from two out of four tested subjects were negative (Fig. 1B, donor 1), whereas the rest of the tested subjects showed very weak reactivity (Fig. 1B, donor 2). Granulocyte population in all tested subjects was negative (Fig. 1B, donors 1 and 2). In contrast, CD4 MAb clone Leu3a (Becton Dickinson) reacted to both CD4+ lymphocytes and monocytes but not to granulocytes in all tested subjects (Fig. 1C, donors 1 and 2). These results indicated that MT4 MAb is a unique CD4 MAb that recognizes CD4 protein on CD4+ lymphocytes but reacts weakly or not at all to CD4 on monocytes. We have no experimental results that bear on the question of why MAb MT4 fails to bind to monocytes. It is possible to speculate that MAb MT4 may be binding to a particular epitope of CD4 molecule which is exposed on lymphocytes but is sterically or conformationally obstructed on monocytes. It also can be that MT4 MAb has low affinity, so it fails to identify the low CD4 expression on monocytes.
FIG. 1.
Immunofluorescence analysis of the reactivities of CD4 MAbs MT4 and Leu3a with peripheral blood leukocytes. Whole-blood samples were stained with either irrelevant negative control MAb (A), CD4 MAb MT4 (B), or CD4 MAb Leu3a (C) purchased from Becton Dickinson and analyzed by lysed whole blood indirect immunofluorescence. Granularity (SSC) and PE fluorescence (FL2) were plotted to show the binding of the MAb to each leukocyte population. The fluorescence intensities of negative control MAb for all cell populations are marked by rectangles. Two subjects (donor #1 and donor #2) are shown as representative of four studied subjects.
Since MAb MT4 strongly reacts with CD4+ lymphocytes but does not react significantly with monocytes, this MAb is advantageous for the development of any method for enumeration of CD4+ lymphocytes. In this study, purified MT4 MAb was coated on latex beads (bead size, 1.1 μm; Sigma). The MT4-coated latex beads were then incubated with whole blood, and a drop of blood/bead mixture was smeared on a glass slide and stained with Wright's stain. Observation of cell morphology under a light microscope revealed that a population of lymphocytes formed rosettes with the MT4-coated beads. As predicted, no rosette-forming monocytes or granulocytes were observed. This is also consistent with results shown in Fig. 1, in which no definite fluorescence labeling of monocytes was observed with MT4.
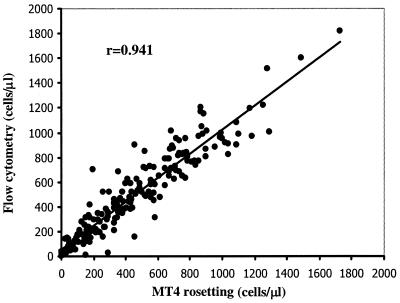
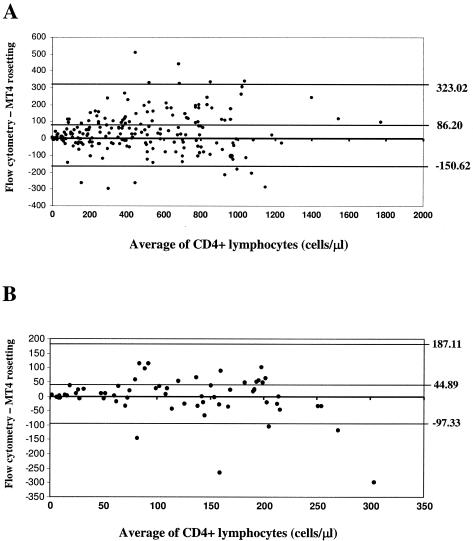
Using the MT4-coated latex beads, a simple non-flow-cytometric method called the MT4 rosetting method was developed for enumeration of CD4+ lymphocytes in whole blood. The MT4 rosetting reagent consists of inert latex particles coated with MAb MT4. The reagent is used to manually enumerate the absolute CD4 count by visible light microscopy. To accomplish this, 15 μl of MT4 rosetting reagent was added to 50 μl of K3EDTA blood in a test tube and mixed gently for 5 min. Then, 15 μl of a blood-latex particle mixture was pipetted into another tube containing 50 μl of Turk's solution (glacial acetic acid, 3%; gentian violet, 0.012%) and mixed gently for 10 to 15 s. The sample was loaded into a hemacytometer chamber and then observed under a light microscope using a ×40 objective. Cells having three or more latex particle attached to them (rosettes) were counted as CD4+ lymphocytes. A total of 300 WBC were counted, and the percentage of bead-rosetted CD4+ lymphocytes in WBC was calculated. The percentage of bead-rosetted CD4+ lymphocytes in WBC was used to calculate the absolute CD4+ cell count by multiplying the percentage of CD4+ lymphocytes by the total WBC count (cells/μl) and dividing by 100. To validate the rosetting method, numbers of CD4+ lymphocytes from 60 healthy and 140 HIV-infected individuals were determined by this method and compared with results obtained with standard flow cytometry. As shown in Fig. 2, a high correlation between the two methods was obtained (r = 0.941). The mean absolute CD4 counts ± standard deviations by the MT4 rosetting method and flow cytometry were 454 ± 336 and 492 ± 348 cells/μl, respectively. The accuracy of the MT4 rosetting method in identifying those individuals with fewer than 200 CD4+ lymphocytes/μl was evaluated, because this count has been incorporated as part of the Centers for Disease Control and Prevention case definition for AIDS to determine clinical levels of HIV disease. The MT4 rosetting method had a sensitivity of 90%, a specificity of 90%, and an accuracy of 90%. The positive and negative predictive values were 75% and 97%, respectively, using flow cytometry as the reference method. By Bland-Altman analysis (2) of all tested samples, the MT4 rosetting method yielded lower CD4+ lymphocyte counts than flow cytometry by a mean of 86 cells/μl, and the limits of agreement at 95% confidence interval were 323 to −151 cells/μl (Fig. 3A). Bland-Altman analysis of samples having CD4+ lymphocyte levels lower than 250 cells/μl indicated a mean difference of 45 cells/μl and limits of agreement at 95% confidence interval of 187 to −97 cells/μl (Fig. 3B).
FIG. 2.
Comparison of absolute numbers of CD4+ lymphocytes by flow cytometry and the MT4 rosetting method (n = 200).
FIG. 3.
Bland-Altman plot of the differences versus the means of CD4+ lymphocyte counts by flow cytometry and the MT4 rosetting method. Upper and lower horizontal lines mark the 95% confidence limits. (A) Bland-Altman analysis of all tested samples. (B) Bland-Altman analysis of samples having CD4+ lymphocytes at levels lower than 250 cells/μl.
The standard flow cytometric method is often too expensive and too sophisticated to be used in resource-limited countries (5, 10). Alternative low-cost and reliable technologies for enumeration of CD4+ T lymphocytes are therefore needed to replace flow cytometry. In recent years, several such technologies have been developed (4-7, 9-12, 14). Of these alternative methods available, the cytosphere assay (Beckman Coulter) is widely regarded as a method of choice (1, 3, 5). In the cytosphere method, inert particles coated with a CD4 MAb are used to identify and manually enumerate the absolute CD4+ lymphocytes by visible light microscopy. Because monocytes also express CD4 molecules, they must be identified and excluded from the CD4+ lymphocytes. A blocking reagent with CD14 MAb-labeled beads was therefore added to the cytosphere assay kit. To do the assay, before the incubation with CD4-coated beads (2.0-μm beads), the blood is incubated with 0.6-μm beads coated with CD14 MAb (blocking reagent), which binds to monocytes. A cell is considered to be a CD4+ lymphocyte if it has three or more of the 2.0-μm beads attached to its surface. Cells with 0.6-μm beads attached are considered to be monocytes and have to be carefully differentiated from the CD4+ lymphocytes. In this study, we developed a reagent for enumeration of CD4+ lymphocytes using the same principle as the cytosphere reagent but in which no blocking of monocytes is necessary. The unique binding property of MAb MT4 makes CD4+ lymphocyte enumeration technically simpler and thereby eliminates a potential source of error.
The developed method is inexpensive, easy to perform, and reliable in identifying those individuals with CD4+ lymphocyte counts fewer than 200 cells/μl and has good correlation to flow cytometry. Therefore, the same CD4+ lymphocyte cutoff number suggested for the flow cytometric method by the Centers for Disease Control and Prevention can also be applied for the developed method. By this method, the only instruments needed to perform the assay are a hemacytometer and a light microscope, making this test especially useful in laboratories with minimal equipment and cost feasible in areas where budgets are very limited.
Acknowledgments
This work was supported by the Commission on Higher Education, the National Center for Genetic Engineering and Biotechnology (BIOTEC), and The Thailand Research Fund.
We are thankful to Sirinporn Intrawut and Umpa Yasamut for their technical assistance. We are also thankful to John McDermed, the Research Institute for Health Sciences, Chiang Mai University, for editorial assistance with the manuscript.
REFERENCES
- 1.Balakrishnan, P., M. Dunne, N. Kumarasamy, S. Crowe, G. Subbulakshmi, A. K. Ganesh, A. J. Cecelia, P. Roth, K. H. Mayer, S. P. Thyagarajan, and S. Solomon. 2004. An inexpensive, simple, and manual method of CD4 T-cell quantitation in HIV-infected individuals for use in developing countries. J. Acquir. Immune Defic. Syndr. 36:1006-1010. [DOI] [PubMed] [Google Scholar]
- 2.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307-310. [PubMed]
- 3.Carella, A. V., M. W. Moss, V. Provost, and T. C. Quinni. 1995. A manual bead assay for the determination of absolute CD41 and CD81 lymphocyte counts in human immunodeficiency virus-infected individuals. Clin. Diagn. Lab. Immunol. 2:623-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carriere, D., J. P. Vendrell, C. Fontaine, A. Jansen, J. Reynes, I. Pages, C. Holzmann, M. Laprade, and B. Pau. 1999. Whole blood Capcellia CD4/CD8 immunoassay for enumeration of CD4+ and CD8+ peripheral T lymphocytes. Clin. Chem. 45:92-97. [PubMed] [Google Scholar]
- 5.Crowe, S., S. Turnbull, R. Oelrichs, and A. Dunne. 2003. Monitoring of human immunodeficiency virus infection in resource-constrained countries. Clin. Infect. Dis. 37(Suppl. l):S25-S35. [DOI] [PubMed] [Google Scholar]
- 6.Denny, T. N., B. D. Jensen, E. I. Gavin, A. G. Louzao, F. A. Vella, J. M. Oleske, and W. Wong. 1995. Determination of CD4 and CD8 lymphocyte subsets by a new alternative fluorescence immunoassay. Clin. Diagn. Lab. Immunol. 2:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Didier, J. M., M. D. Kazatchkine, C. Demouchy, C. Moat, S. Diagbouga, C. Sepulveda, A. M. Di Lonardo, and L. Weiss. 2001. Comparative assessment of five alternative methods for CD4+ T lymphocyte enumeration for implementation in developing countries. J. Acquir. Immune Defic. Syndr. 26:193-195. [DOI] [PubMed] [Google Scholar]
- 8.Kasinrerk, W., N. Tokrasinwit, and P. Naveewongpanit. 1998. Production of monoclonal antibody to CD4 antigen and development of reagent for CD4+ lymphocyte enumeration. J. Med. Assoc. Thailand 81:879-892. [PubMed] [Google Scholar]
- 9.Landay, A., J. L. Ho, D. Hom, T. Russell, R. Zwerner, J. G. Minuty, P. Kataaha, F. Mmiro, and B. Jackson. 1993. A rapid manual method for CD4+ T-cell quantitation for use in developing countries. AIDS 7:1565-1568. [DOI] [PubMed] [Google Scholar]
- 10.Lyamuya, E. F., C. Kagoma, E. C. Mbena, W. K. Urassa, K. Pallangyo, F. S. Mhalu, and G. Biberfeld. 1996. Evaluation of the FAScount, TRAx CD4 and Dynabead methods for CD4 lymphocyte determination. J. Immunol. Methods 195:103-112. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson, J. K. A., W. M. Velleca, S. Jubert, T. A. Green, and L. Bryan. 1994. Evaluation of alternative CD4 technologies for the enumeration of CD4 lymphocytes. J. Immunol. Methods 177:43-54. [DOI] [PubMed] [Google Scholar]
- 12.Saez, M. J., M. de Frutos, P. Martinez, and V. Soriano. 1994. Evaluation of a new fluorescence immunoassay for CD4+ and CD8+ T cell counts in clinical samples. Vox Sang. 67:86-87. [DOI] [PubMed] [Google Scholar]
- 13.UNAIDS/World Health Organization. 2003. AIDS epidemic update, December 2003. UNAIDS/World Health Organization, Geneva, Switzerland.
- 14.World Health Organization. 1992. Report of a WHO workshop on flow cytometry and alternative methodologies for CD4 lymphocyte determinations: applications for developing countries. World Health Organization, Geneva, Switzerland. [PubMed]