Abstract
The collection of maternal placental intervillous blood (IVB), without contamination of fetal blood and with an accurate mononuclear cell profile, is essential for immunological studies of placental malaria and other infectious diseases of the placenta. We have compared five documented methods of IVB collection: perfusion, incision, biopsy, tissue grinding, and puncture (prick) for fetal blood contamination and mononuclear cell profiles using flow cytometry. Twenty-five placentas were obtained from Plasmodium falciparum and human immunodeficiency virus-negative primigravid and secundigravid women delivering at Nyanza Provincial Hospital in Kisumu, western Kenya. Each of the five methods was performed on the same placenta. Fetal red blood cell contamination was significantly lower for the prick and perfusion methods (4.1% and 8.3%, respectively) than for incision (59.5%), biopsy (42.6%), and tissue grinding (19.9%). Significant variation was noted among the five methods in the percentages of monocytes, total T cells, CD4+ and CD8+ T cells, B cells, and NK cells. Further, a pairwise comparison of prick and perfusion, the two methods with low fetal blood contamination, showed that they were not different for fetal red blood cell contamination levels; however, prick yielded significantly higher percentages of CD4 T cells and CD4 memory T cells than perfusion. Collection by prick was determined to be the best method of intervillous blood collection for immunology studies, and perfusion represented the next best method of choice due to high sample volume yield. Overall, in considering the advantages/disadvantages of the two methods with low fetal cell contamination, we conclude that a combination of prick and perfusion is most suitable for immunology studies.
The placenta is a complex, sophisticated organ with several important functions throughout gestation. Its primary purpose is to provide sustenance for the developing fetus; however, the placenta is also a site for pathogen sequestration and/or invasion of the fetus via maternal blood circulation (1, 2, 5, 7, 20). For instance, in regions where malaria is holoendemic, malaria infection in pregnant women leads to parasite accumulation in the placenta, resulting in a condition termed placenta malaria. Placental malaria has serious consequences for maternal health and fetal development, including maternal anemia and low-birth-weight infants (8, 14). Because not all malaria-exposed pregnant women in areas of malaria endemicity suffer from placental malaria and its serious consequences, research is ongoing to elucidate protective immune responses against placental infection and/or immune pathogenesis. However, results from different studies are often discordant. One of the major factors in the discordance is that different techniques of sample collection have been employed in different studies. There is therefore general consensus that studies of placental immune responses require the collection of placental samples by using well-defined and reliable methods.
Various placental tissue sampling and intervillous blood (IVB) collection methods are currently used, but there is a lack of information on the comparative attributes and deficiencies of these methods. In selecting an IVB collection method for studies of maternal immune responses, we need to consider two critical factors. First, IVB must be collected with minimal fetal blood contamination, and second, isolated IVB must have a mononuclear cell profile that most accurately represents maternal IVB. Fetal cells have been reported to modify maternal immune responses, particularly in culture (19, 24), and there is also evidence that excessive manipulation during sampling can alter maternal immune cell profiles and cellular functions (10).
To determine the most appropriate method of placenta IVB collection for placental malaria immunological studies, we compared five documented collection methods for their level of fetal red blood cell contamination and intervillous blood immune cell profiles. The five collection methods compared were placental perfusion (15), placental basal incision (3), placental tissue biopsy (22), placental tissue grinding (9), and placental chorionic plate puncture (prick) (4).
MATERIALS AND METHODS
Study site and subjects.
Placentas were obtained from mothers who delivered at the Nyanza Provincial General Hospital in Kisumu, an area in western Kenya where malaria is holoendemic and human immunodeficiency virus (HIV) is epidemic. After informed consent, mothers in their first and second pregnancies were screened for malaria parasites and HIV. Placentas from mothers negative on a Giemsa-stained thick blood smear for malaria, negative for HIV-1 and HIV-2 antibody, and with no other apparent underlying diseases were processed. Twenty-five placentas were collected immediately upon expulsion into sterile containers with anticoagulant (heparin), carefully examined, and immediately prepared for sample processing if intact and undamaged. A peripheral blood sample from the same mother was collected within 16 h of delivery to serve as a reference for immunophenotyping. Both umbilical cord blood collected by release of the clamp on the cord and an HIV-negative male venous blood sample from the blood bank in Kisumu were included with each set of placenta samples to serve as controls for fetal cell contamination. The study was approved by the Kenya Medical Research Institute Ethical Review Committee and the Institutional Review Board of the Centers for Disease Control and Prevention, Atlanta, GA.
Placenta processing.
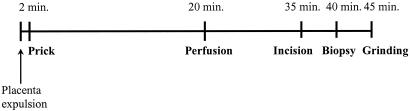
All five methods of IVB collection were performed on each placenta to ensure that differences among methods were comparable. Collection methods were carried out in the following order: prick, perfusion, incision, biopsy, and tissue grinding. The order of collection was determined by the degree of tissue damage resulting from the method of sampling. The placenta was arbitrarily divided into quadrants, with the first four methods performed in separate quadrants. Figure 1 shows a diagrammatic representation of the five sampling methods from the time of placental expulsion to the end of sample processing. Each placenta was processed within a maximum period of 45 min after expulsion.
FIG. 1.
Chronology of sampling events. Within approximately 2 min of placenta expulsion, an intervillous blood sample by the prick method was collected from a region appropriately chosen for the technique. Perfusion was carried out next in a separate quadrant; perfusate was obtained within 20 to 25 min of placental expulsion. Blood collection by incision was carried out next, followed by biopsy within at least 5 min of each other, and finally, approximately 45 min after placental expulsion, blood by tissue grinding was collected. On average, all techniques were carried out within a maximum period of 45 min after placenta expulsion.
(i) Placental prick.
A modification of the method described by Camelo et al. (4) was employed. This method of collection accesses placental intervillous spaces through the chorionic plate. The placenta was carefully placed on a raised sterile wire mesh stand, with the chorionic plate (fetal side) facing down to promote blood accumulation and IVB space accessibility. A large-bore, 14-gauge needle attached to a syringe was directed approximately 0.5 cm deep through the wire mesh into the intervillous space, denoted as dark-purple regions, while we carefully avoided puncture of the surrounding fetal vessels on the surface of the chorionic plate. The syringe was gently pulled to create a vacuum initiating blood flow, followed by withdrawal to allow for dripping blood, about 1 ml, to be collected into microcentrifuge tubes charged with 25 μl of a 1:4 heparin (stock concentration, 1,000 units/ml) dilution in phosphate-buffered saline (PBS).
(ii) Perfusion.
The placenta was then placed on a 20° angled surface, with the maternal side facing up for perfusion as described by Moore et al. (15). A quadrant different from the one used for the prick method was selected. Four umbilical vessel catheters (UVCs) were inserted into the placental tissue to a depth of about 2 to 4 mm, 2 to 4 cm apart. Approximately 50 ml of a 1:150 heparin (stock concentration, 1,000 units/ml) dilution in PBS at a flow rate of 8 ml per minute was delivered into the intervillous spaces by a peristaltic pump (Cole Parmer Instrument Company, Vernon Hills, IL). The 50 ml of PBS-diluted blood flushed out by this action was collected using a blunt 20-ml syringe.
(iii) Incision.
Two to three shallow cuts, approximately 2 mm deep, were made into the maternal surface of the placenta's third quadrant, using a sterile scalpel. The resultant blood, about 2 ml, that seeped into these incisions was collected using a blunt 1-ml syringe and transferred into heparin-charged microcentrifuge tubes (3).
(iv) Biopsy.
A biopsy 2-cm square that was 2 mm deep in size was obtained from the maternal surface, approximately a quarter of the distance from the center of the placenta in the last placental quadrant, and transferred to a heparin-PBS (5 ml)-containing petri dish. Using the flat edge of a scalpel, we gently teased the tissue apart, working from the outer edge inward to release blood into the PBS (21). The resulting cell suspension was placed in a 15-ml centrifuge tube, and the tissue debris was allowed to settle; cells were retained in the supernatant. The supernatant was aspirated and transferred to another 15-ml centrifuge tube.
(v) Tissue grinding.
A pie-shaped, full-thickness block of placental tissue, with a 5-cm radius, was sliced from the placenta, avoiding the quadrant that was perfused, and was fed into a meat grinder. The blood emerging from the grinder before tissue pushed through (approximately 5 ml) was collected into heparin-charged microcentrifuge tubes (8).
Flow cytometry analysis.
The detection of fetal blood contamination was done by single-color flow cytometry using a fluorescein isothiocyanate fluorochrome-labeled antifetal hemoglobin (HbF) monoclonal antibody kit (Caltag Laboratories, Burlingame, CA). For each experiment, a negative (0% fetal red blood cells [RBCs] in adult male blood) and a positive (10% fetal RBCs in adult male blood) control were set up alongside the placental samples according to the manufacturer's instructions (Caltag Laboratories, Burlingame, CA).
Mononuclear cell population and subpopulation phenotypes were determined by using four-color flow cytometry. Immunophenotyping was carried out on blood collected by each method, and a corresponding peripheral sample of maternal blood was used as a reference for the identification of T cells, B cells, NK cells, memory cells, and monocytes using a combination of the fluorochrome-labeled monoclonal antibody reagents listed in Table 1. All the antibody reagents were obtained from Becton Dickinson, San Jose, CA.
TABLE 1.
Fluorochrome-labeled monoclonal antibody panel used for immunophenotypinga
| Tube | Antibody(ies) conjugated by:
|
|||
|---|---|---|---|---|
| FITC | PE | PerCP | APC | |
| 1 | CD3 | CD8 | CD45 | CD4 |
| 2 | CD3 | CD56 and CD16 | CD45 | CD19 |
| 3 | CD45RA | CD8 | CD3 | CD4 |
| 4 | CD45RA | CD14 | ||
| 5 | IgG1 | IgG1 | IgG1 | IgG1 |
| 6 | CD4 | CD8 | CD3 | CD19 |
Tubes 1 to 4 represent the antibody combinations for the identification of various cell subpopulations. Tube 5 represents the isotype control, while tube 6 was used for compensation. The antibodies used are as follows: fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies to CD4 (mouse IgG1 k, RPA-T4), CD3 (mouse IgG1 k, UCHT1), CD45 (mouse IgG1 k, HI30), and CD45RA (mouse IgG1 k, HI100); phycoerythrin (PE)-conjugated antibodies to CD8 (mouse IgG1 k, HIT 8a), CD14 (mouse IgG1 k, M5E2), CD56 (mouse IgG1 k, B159), and CD16 (mouse IgG1 k, 3G8); peridin chlorophyll protein (PerCP)-conjugated antibodies to CD3 (mouse IgG1 k, UCHT1) and CD45 (mouse IgG1 k, TU116); and allophycocyanin (APC)-conjugated antibodies to CD4 (mouse IgG1 k, RPA-T4) and CD19 (mouse IgG1 k, HIB19). In addition, the following isotype controls were used: mouse IgG1 FITC (IgG1 k, MOPC21), mouse IgG1 PE (IgG1 k, MOPC21), mouse IgG1 PerCP (IgG1 k, MOPC21), and mouse IgG1 APC (IgG1 k, MOPC21).
Fetal RBC and maternal mononuclear cell population/subpopulation enumeration was performed on a FACSCalibur (BD Biosciences, California) flow cytometer using BD CellQuest Pro software (BD Biosciences, California). For determination of fetal blood cell contamination, red blood cells were gated by forward scatter-side scatter (FSC-SSC) and a total of 50,000 events were acquired. Contamination levels were determined by the subtraction of the negative control value from the tested sample value, and results were reported as percentages of gated red blood cells. For immunophenotyping, compensation was performed for each experimental setup using maternal peripheral blood. Lymphocytes were identified and gated by FSC-SSC. A minimum of 5,000 lymphocytes within the lymphocyte gate were acquired and were used as the guide for total event acquisition. Isotype controls were included for each placental sample and were used for the subtraction of background. Results for total T cells and T-cell subpopulations, B cells, and NK cells were reported as percentages of the lymphocyte gate events, while results of CD14+ monocytes were reported as percentages of total CD45+ cells.
Statistical analysis.
Data analysis compared the five different methods of placenta collection for fetal RBC contamination levels and immune cell population/subpopulation profiles. Since five methods were performed on the same placenta, to account for the correlation between measurements from the same placenta, a random intercept model was fit with the collection method as the (fixed) predictor of interest and a random effect for each placenta. The model was fit with an unstructured covariance structure on the random effect (12). When necessary, the outcome data were transformed (natural log or square root transformation) to obtain an approximate normal distribution. An overall F test was used to test whether the mean percentages of all methods were the same. Pairwise differences are reported for each pairwise comparison between the mean percentage estimates. Confidence limits (CLs) based on empirical standard error estimates are reported. CLs and P values of the differences are adjusted using the Tukey method to account for the 10 pairwise comparisons being made. In pairwise comparisons, a significance level of 0.01 was set a priori. A nonparametric sign test was used to compare whether the difference in prick versus peripheral cell percentages was different from 0. The data were analyzed using SPSS (SPSS version 11.0.1; Chicago, IL) and SAS (SAS version 8.0.2; Cary, NC) software packages.
RESULTS
Fetal hemoglobin contamination levels.
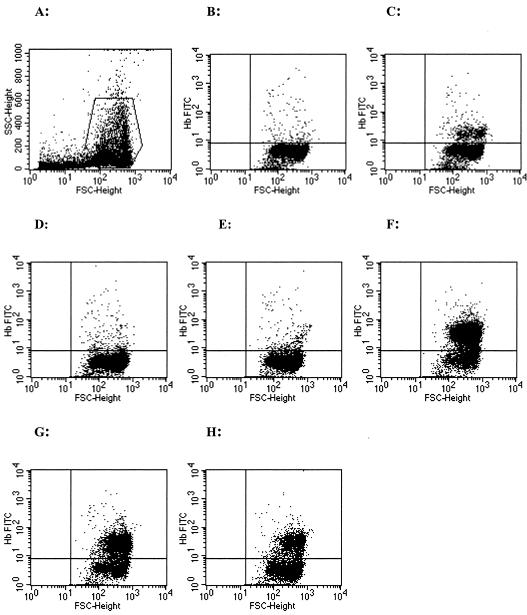
Figure 2 shows an example of the fetal RBC staining dot plot analysis of samples collected from a representative placenta. Gating for RBCs was performed on the FSC-SSC plot (Fig. 2A), and quadrant markers were set according to the 0% and 10% controls (Fig. 2B and C). The analysis plots for each method of collection for this representative sample are shown in Fig. 2D to H. The lowest percentage of positive staining for HbF was observed for the prick method of collection, whereas the highest percentage of positive staining was obtained from the incision method of collection. Mean levels of percent positive HbF staining (means ± standard deviations) from all 25 placentas for each method of collection were as follows: prick, 4.1 ± 3.7; perfusion, 8.3 ± 9.2; incision, 59.5 ± 26.0; biopsy, 42.6 ± 26.3; and tissue grinding, 19.9 ± 12.5. The differences in fetal RBC contamination among the five methods of maternal IVB collection were statistically significant (P < 0.0001). Further pairwise comparisons between the different methods for fetal RBC contamination levels revealed that the prick and perfusion methods of collection were similar (P = 0.27), as were incision and biopsy (P = 0.3) (Table 2).
FIG. 2.
Fetal hemoglobin staining dot plot analysis for a representative placenta sample. (A) FSC-SSC gating of red blood cells; (B and C) 0 and 10% controls, respectively; (D) placental prick (5.5%); (E) perfusion (12%); (F) incision (71.6%); (G) biopsy (50.5%); and (H) tissue grinding (24.1%).
TABLE 2.
Paired comparisons of levels of fetal red blood cell contamination between methods
| Paired method | Mean estimate (%) | Lower CL (%)a | Upper CL (%)a | P value |
|---|---|---|---|---|
| Prick and perfusion | −0.63 | −1.70 | 0.43 | 0.27 |
| Prick and incision | −2.60 | −3.55 | −1.65 | <0.0001 |
| Prick and biopsy | −2.25 | −3.20 | −1.30 | <0.0001 |
| Prick and tissue grinding | −1.64 | −2.45 | −0.83 | <0.0001 |
| Perfusion and incision | −1.96 | −2.97 | −0.96 | <0.0001 |
| Perfusion and biopsy | −1.61 | −2.55 | −0.67 | <0.0001 |
| Perfusion and tissue grinding | −1.01 | −1.75 | −0.27 | 0.0002 |
| Incision and biopsy | 0.35 | −0.25 | 0.95 | 0.3 |
| Incision and tissue grinding | 0.96 | 0.43 | 1.48 | <0.0001 |
| Biopsy and grinding | 0.61 | 0.18 | 1.04 | <0.0001 |
99% confidence limit.
Mononuclear cell profiles.
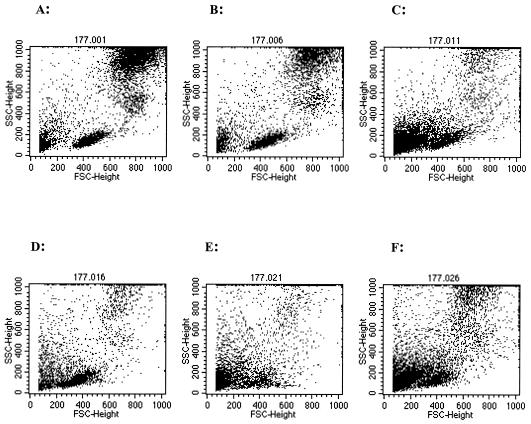
Figure 3 shows the FSC-SSC analysis dot plots of mononuclear cell profiles from a representative placental sample by the five methods of collection. Peripheral blood leukocytes, shown for reference purposes, separated into three distinct cell subpopulations: lymphocytes, monocytes, and granulocytes (Fig. 3A). Mononuclear cells by the prick method of collection exhibited a distribution of cells similar to that of peripheral blood cells, with separation into three discrete populations (Fig. 3B). Incision and tissue grinding displayed some degree of separation (Fig. 3D and F), while samples collected by perfusion and biopsy did not separate well into distinct cell subpopulations (Fig. 3C and E).
FIG. 3.
Immunophenotyping FSC-SSC dot plot analysis for a representative placenta sample. Peripheral blood is used as a reference (A), and IVB was obtained by prick (B), perfusion (C), incision (D), biopsy (E), and tissue grinding (F).
Table 3 shows mean percentages of cell populations (monocytes and lymphocytes) and cell subpopulations (CD4 and CD8 T cells, memory T cells, B cells, and NK cells) obtained from 25 placentas. Mean percentages for monocytes and lymphocytes were different among the five methods of collection (P < 0.0001). Differences in T-cell yields by the method of collection were observed, whether assessed as total T cells (CD3+ T cells) or independently as CD4+ and CD8+ T cells (P < 0.0001). For these cell types, yields were highest with the prick collection method. An analysis of T cells for CD45RA marker distribution, the absence of which was used as an indication of T-cell memory, showed differences for both CD8 memory T-cell (P < 0.001) and CD4 memory T-cell (P = 0.002) percentages. Yields of B and NK cells were also different among the five methods of collection (P = 0.004 and P < 0.0001, respectively). Further pairwise comparisons of the above cell phenotypes between prick and perfusion, the two methods with the lowest fetal red cell contamination, revealed that sample collection by prick (i) yielded significantly higher percentages of CD4 T and CD4 memory T cells (P < 0.0001 and P = 0.009, respectively) and (ii) had marginally higher total T-cell and B-cell percentages than perfusion (P = 0.04 and P = 0.03, respectively) (Table 4).
TABLE 3.
Summary of percentages of cells by the five methods of maternal IVB collection
| Cell type | % of cells (mean ± SD) by methoda
|
P valueb | |||||
|---|---|---|---|---|---|---|---|
| Peripheral blood | Prick | Perfusion | Incision | Biopsy | Tissue grinding | ||
| Monocytes | 7.1 (3.2) | 9.5 (5.3) | 8.1 (6.7) | 11.9 (5.8) | 7.3 (5.2) | 15.6 (8.3) | <0.0001 |
| Lymphocytes | 80.4 (9.5) | 73.9 (9.5) | 57.6 (20) | 53.8 (17) | 45.4 (16) | 52.5 (19) | <0.0001 |
| Total T cells | 69.1 (10.2) | 58.9 (12.2) | 45.8 (16.4) | 43.3 (15.0) | 38.0 (14.2) | 41.4 (15.1) | <0.0001*** |
| CD4 T cells | 36.1 (8.1) | 28.2 (8.9) | 19.0 (8.7) | 28.2 (11.3) | 18.1 (8.5) | 19.6 (7.6) | <0.0001*** |
| CD8 T cells | 29.3 (8.6) | 27.1 (10.0) | 20.0 (7.8) | 16.3 (7.8) | 15.0 (6.9) | 19.1 (7.8) | <0.0001 |
| Memory CD4 T cells | 29.4 (9.1) | 21.1 (10.2) | 17.6 (8.0) | 15.5 (10.7) | 16.9 (9.6) | 15.6 (8.3) | 0.002*** |
| Memory CD8 T cells | 16.3 (7.5) | 16.3 (8.8) | 15.4 (9.1) | 8.6 (7.9) | 8.6 (10.3) | 13.2 (6.8) | <0.0001 |
| B cells | 11.1 (5.5) | 15.1 (11.7) | 10.8 (10.8) | 9.6 (6.7) | 8.2 (7.7) | 11.0 (10.8) | 0.004 |
| NK cells | 11.9 (6.9) | 13.7 (8.3) | 15.3 (9.6) | 16.2 (7.8) | 16.6 (7.9) | 13.9 (7.1) | <0.0001 |
Peripheral blood was used only as a reference. Results for monocytes were obtained from CD45/CD14 staining. Lymphocyte numbers were obtained from the FCS-SSC plot. All other results were obtained from lymphocyte gating.
P values derived from (F test) comparison of all five methods of IVB collection. ***, P < 0.05 for comparisons (sign test) between peripheral and prick cell numbers (data not shown).
TABLE 4.
Paired comparisons of immune cell yields between prick and perfusion
| Cell type | Mean estimate (%) | Lower CL (%) | Upper CL (%) | P value |
|---|---|---|---|---|
| Monocytes | 0.31 | −0.50 | 1.11 | 0.70 |
| Total T cells | 10.84 | −1.67 | 23.34 | 0.04 |
| CD4 T cells | 10.36 | 4.99 | 15.73 | <0.0001 |
| CD8 T cells | 5.14 | −3.93 | 14.21 | 0.32 |
| Memory CD4 T cells | 5.11 | 0.03 | 10.19 | 0.009 |
| Memory CD8 T cells | −0.18 | −1.19 | 0.82 | 0.97 |
| B cells | 0.53 | −0.06 | 1.13 | 0.03 |
| NK cells | −1.26 | −5.39 | 2.86 | 0.84 |
Paired comparison results for various cell subpopulations between only prick and perfusion are reported here, as these two methods showed similar low levels of fetal cell contamination (see Table 2).
DISCUSSION
Five methods of placental IVB collection (prick, perfusion, incision, biopsy, and tissue grinding) were compared for fetal blood contamination and maternal mononuclear cell profiling. The data from this study provide comprehensive information for the determination of an appropriate method of intervillous blood collection for immunological studies.
Fetal blood contamination levels in maternal intervillous blood were determined by a sensitive fluorochrome-labeled monoclonal antibody directed against HbF, which is able to detect contamination levels as low as 1 fetal RBC per 100,000 maternal red cells. The prick method of collection had the lowest level of contamination. This result is not entirely unexpected. Entry into IVB spaces without puncture of fetal vessels is better facilitated from the chorionic plate than the basal plate, due to the absence of minute fetal vessels in this region. Perfusion accesses IVB spaces via UVCs, through the basal plate on the maternal side that contains a dense lattice of fetal capillaries. Capillaries are likely to be broken upon insertion of the UVCs and may explain the slightly higher, albeit statistically insignificant, levels of fetal blood contamination observed. Sample collection by incision, biopsy, and tissue grinding demonstrated unacceptably high levels of fetal blood contamination. Over 50% of the blood sample obtained by incision was fetal in origin. It is likely that both the incision and biopsy methods of IVB sampling cut directly into the villous tree, with the blood that wells into the incision being largely fetal blood and not maternal IVB.
Immune cell profiles in IVB collected by five different methods were determined by flow cytometry. Significant variation was observed among the five methods in the levels of monocytes, total T cells, CD4+ and CD8+ T cells, B cells, and NK cells. It is easy to envision that the degree of fetal blood contamination in each of these five methods is a major cause for the varied immune cell profiles seen in this study. Numerous studies have shown that immune cell levels in fetal blood are significantly different from those in the peripheral blood of adults (6). Cell release from tissue, due to physical trauma to placenta caused by the sampling method may also contribute to differences observed in immune cell profiles. A disruption of maternal tissue, such as decidua, known to have high levels of macrophages (monocytes) and NK cells, may release cells into the IVB and thus alter cell profiles. The placental surface sampled, basal or chorionic, may be critical to disruptions in the anatomical structure of placental tissue. The prick method of collection, with minimal tissue trauma, accesses IVB spaces from the chorionic surface and delivers relatively pure IVB. Perfusion, on the other hand, involves accessing IVB spaces via the basal plate; this coupled with the fact that perfusion requires external pressure to flush out IVB blood, which results in some tissue trauma from turbulence during the procedure, may account for the results observed. The methods of incision, biopsy, and tissue grinding also sampled the maternal basal surface; each was highly invasive, resulting in high levels of tissue trauma that may subsequently release cells from maternal tissue.
Monocyte levels are generally increased in the placenta during pregnancy as part of the innate immune response necessary for protection against infection (18). A recent study conducted by us that used perfusion to obtain placental blood mononuclear cells reported mean levels of IVB monocyte cells that were 49% higher than peripheral blood monocytes (17). In the current study, however, relatively lower levels of monocytes by perfusion were observed. In both studies, perfusion was performed in essentially the same manner, with the only difference being the choice of anticoagulant. Whereas the present study used heparin for placenta sample collection, EDTA was used in the previous study. The difference in use of anticoagulant by the two studies may offer an explanation for the discordant results. EDTA, known as a chelating agent for calcium, may have facilitated the release of immune cells, adhering via cell surface adhesion molecules with trophoblast epithelial cells of the placenta or with each other (25, 26), thereby resulting in the higher numbers of monocyte cells reported. Histopathological examinations of placenta perfused with EDTA show that essentially all IVB mononuclear cells are flushed out of the perfused area (J. M. Moore et al., unpublished data). It is noteworthy that the forward and side scatter image of IVB obtained by perfusion with EDTA is much more similar to that observed with peripheral blood in our previous study (16) than that observed with heparin perfusion in the current study (Fig. 3C). However, heparin is the anticoagulant of choice for many functional studies due to the impact of EDTA on calcium flux in immune cells.
T lymphocytes are crucial to the immune process and are often the focus of immune response investigations; thus, the collection of blood samples with accurate lymphocyte proportions is imperative. In this study, pairwise comparisons of T-cell yields between prick and perfusion, the two methods with low fetal blood contamination, revealed that prick obtained significantly higher percentages of both CD4 T and CD4 memory T cells than perfusion. Cord blood has been reported to exhibit a greater proportion of naive cells than memory cells (11, 21). In view of the fact that the prick method had the lowest levels of contamination, sample collection by prick may be a true reflection of IVB composition. In this study, we also observed that T-cell and T-cell subpopulation levels were lower in IVB by the prick method than in peripheral blood. The lower levels of T lymphocytes in placenta than in peripheral blood could be attributable to the fact that the placenta would not have appreciable T-cell responses, particularly in first and second pregnancies (16).
B-cell levels revealed borderline differences between prick and perfusion, with prick yielding higher levels of B cells. Given that the prick method of collection obtained minimal fetal blood contamination, the yield of B cells by this method may reflect the true humoral immune environment necessary for a good pregnancy outcome. Immunology studies have shown that asymmetric immunoglobulin G (IgG) synthesized by placental B cells plays an important role in the degeneration of paternal antigens for fetal protection (13).
There was no difference in NK cell percentages between prick and perfusion methods, but these two methods showed higher levels of NK cells in IVB than in peripheral blood. These results are in agreement with our previous study using the perfusion method, which reported IVB NK cell levels threefold higher than peripheral blood levels (17). In contrast, a study in Tanzania showed a total absence of NK cells in the intervillous spaces in selected samples from women investigated for cell-mediated inflammatory responses, regardless of placenta malaria status (18). The basis for this significant disparity may be attributed to differences in the techniques used. Whereas the current study used highly sensitive immunophenotyping by flow cytometry, using anti-CD56 and anti-CD16 monoclonal antibodies to identify NK cells, the Tanzanian study used a relatively less-sensitive technique, immunohistochemical analysis of formalin-fixed, paraffin-embedded tissue, which also failed to detect CD4+ T cells (18). We speculate that IVB cells may have been lost in the process of tissue fixation or that antigen recovery was insufficient in that study, thus resulting in the underestimation of NK cell levels. In addition, immunohistochemical analysis by morphology is not adequate to distinguish NK cells. Nonetheless, NK cells in IVB were detected in the present study, and additional investigations have also shown an association between placental NK cells and protection from placenta malaria (C. Othoro et al., unpublished data). A more recent study has also reported that NK cells in placenta may be among the mechanisms involved in poor birth outcome associated with placenta malaria infection (23). Taken together, accumulation of NK cells in the placenta is part of the nonspecific immune responses necessary for protection against or pathogenesis of placental infection.
In conclusion, among the five methods of IVB collection, the placenta prick method offered the best technique for immunology studies. Prick had the lowest fetal RBC contamination as well as yielded the highest percentages of various cell subpopulations. Plasma derived from prick sampling is not diluted and can therefore be used for assays requiring plasma. Furthermore, prick is simple and easy to perform, involves minimal placental manipulation, and does not require sophisticated equipment for collection, making it easily adaptable for field studies. It presents the most natural method of placental blood collection, much in the same way that finger prick blood is collected. However, one limitation to this method of collection is the low volume obtained. Perfusion represents the next best method of choice, due to the relatively low levels of fetal blood contamination and high volume yield. The use of electric power to flush out maternal cells, however, results in high placental manipulation that may render perfused blood inappropriate for assays requiring minimum manipulation, such as RNA and activation assays. In addition, perfusion highly dilutes plasma, making it inappropriate for antibody measurements and intracellular staining assays requiring autologous plasma. Perfusion also requires special equipment that may limit its appeal for field-based studies. Although both prick and perfusion are not significantly different in fetal blood contamination levels, differences in CD4 T and CD4 memory T cells are evident. Given these advantages and disadvantages, we propose that a combination of prick and perfusion for IVB collection performed on the same placenta is ideal for the immunological study of placental infection. Incision, biopsy, and tissue grinding methods of collection display high levels of fetal blood contamination and varied immune cell profiles. Cord blood T cells have been reported to exert high suppressor activity on maternal lymphocytes (19); thus, blood collection by any one of these three methods would skew data of maternal immune responses. These limitations notwithstanding, collection by incision, biopsy, and tissue grinding may still be used for immunological investigations in which the study correlate is an adverse pregnancy outcome related to the fetus, such as low birth weight. Immune responses generated by both maternal and fetal cells at the placental level may contribute to pregnancy outcome.
Acknowledgments
This study was supported by TDR/WHO training grant identification number 980427 and by grant number AOT0483-PH1-2171, HRN-A-00-04-00010-02 from the United States Agency for International Development. J.M.M. is supported by the NIH, grant number RO1 AI 50240.
We are grateful to the mothers who participated in this study. We also thank the field and laboratory staff of the CDC/Kenya Medical Research Institute (KEMRI) who facilitated collection of data and processing of samples. We thank Davy Koech, Director of KEMRI, for his support and his approval with regard to publication of this paper. We also thank Venkatachalam Udhayakumar of DPD/NCID/CDC for helpful comments on the manuscript.
REFERENCES
- 1.Abbasi, M., K. Kowalewska-Grochowska, M. A. Bahar, R. T. Kilani, B. Winkler-Lowen, and L. J. Guilbert. 2003. Infection of placental trophoblasts by Toxoplasma gondii. J. Infect. Dis. 188:608-616. [DOI] [PubMed] [Google Scholar]
- 2.Barragan, A., and L. D. Sibley. 2003. Migration of Toxoplasma gondii across biological barriers. Trends Microbiol. 11:426-430. [DOI] [PubMed] [Google Scholar]
- 3.Bulmer, J. N., F. N. Rasheed, N. Francis, L. Morrison, and B. M. Greenwood. 1993. Placental malaria. I. Pathological classification. Histopathology 22:211-218. [DOI] [PubMed] [Google Scholar]
- 4.Camelo, J. S. J., F. E. Martinez, S. M. Jorge, and M. Matheus de la Sala. 1995. A new method for sampling maternal blood in the intervillous space. Fetal Diagn. Ther. 10:322-325. [DOI] [PubMed] [Google Scholar]
- 5.Crocker, I. P., O. M. Tanner, J. E. Myers, J. N. Bulmer, G. Walraven, and P. N. Baker. 2004. Syncytiotrophoblast degradation and the pathophysiology of the malaria-infected placenta. Placenta 25:273-282. [DOI] [PubMed] [Google Scholar]
- 6.D'Arena, G., P. Musto, N. Cascavilla, G. Di Giorgio, S. Fusilli, F. Zendoli, and M. Carotenuto. 1998. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica 83:197-203. [PubMed] [Google Scholar]
- 7.Diana, A., M. Epiney, M. Ecoffey, and R. E. Pfister. 2004. “White dots on the placenta and red dots on the baby”: congential cutaneous candidiasis-a rare disease of the neonate. Acta Paediatr. 93:996-999. [DOI] [PubMed] [Google Scholar]
- 8.Duffy, P. E., and R. S. Desowitz. 2002. Pregnancy malaria throughout history: dangerous labours, p. 1-25. In P. E. Duffy and M. Fried (ed.), Malaria in pregnancy: deadly parasite, susceptible host. Taylor and Francis, New York, N.Y.
- 9.Fried, M., R. O. Muga, A. O. Misore, and P. E. Duffy. 1998. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J. Immunol. 160:2523-2530. [PubMed] [Google Scholar]
- 10.Hartel, C., G. Bein, M. Muller-Steinhardt, and H. Kluter. 2001. Ex vivo induction of cytokine mRNA expression in human blood samples. J. Immunol. Methods 249:63-71. [DOI] [PubMed] [Google Scholar]
- 11.Juretic, E., B. Uzarevic, M. Petrovecki, and A. Juretic. 2000. Two-color flow cytometric analysis of preterm and term newborn lymphocytes. Immunobiology 202:421-428. [DOI] [PubMed] [Google Scholar]
- 12.Little, R. C., G. A. Milliken, W. W. Stroup, and R. D. Wolfinger. 1996. SAS system for mixed models. SAS Institute Inc., Cary, N.C.
- 13.Malan Borel, I., T. Gentile, J. Angelucci, J. Pividori, M. C. Guala, R. A. Binaghi, and R. A. Margni. 1991. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 20:129-140. [DOI] [PubMed] [Google Scholar]
- 14.Menendez, C. 1995. Malaria during pregnancy: a priority area of malaria research and control. Parasitol. Today 11:178-183. [DOI] [PubMed] [Google Scholar]
- 15.Moore, J. M., B. Nahlen, A. V. Ofulla, J. Caba, J. Ayisi, A. Oloo, A. Misore, A. J. Nahmias, A. A. Lal, and V. Udhayakumar. 1997. A simple perfusion technique for isolation of maternal intervillous blood mononuclear cells from human placentae. J. Immunol. Methods 209:93-104. [DOI] [PubMed] [Google Scholar]
- 16.Moore, J. M., B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2000. Immunologic memory in the placenta: a lymphocyte recirculation hypothesis. Med. Hypotheses 54:505-510. [DOI] [PubMed] [Google Scholar]
- 17.Moore, J. M., Y. P. Shi, C. Othoro, B. L. Nahlen, A. A. Lal, and V. Udhayakumar. 2003. Comparative flow cytometric analysis of term placental intervillous and peripheral blood from immediate postpartum women in western Kenya. Placenta 24:779-785. [DOI] [PubMed] [Google Scholar]
- 18.Ordi, J., C. Menendez, M. R. Ismail, P. J. Ventura, A. Palacin, E. Kahigwa, B. Ferrer, A. Cardesa, and P. L. Alonso. 2001. Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J. Infect. Dis. 183:1100-1107. [DOI] [PubMed] [Google Scholar]
- 19.Papadogiannakis, N., S. A. Johnsen, and L. B. Olding. 1985. Human fetal/neonatal suppressor activity: relation between OKT phenotypes and sensitivity to prostaglandin E2 in maternal and neonatal lymphocytes. Am. J. Reprod. Immunol. Microbiol. 9:105-110. [DOI] [PubMed] [Google Scholar]
- 20.Pusztai, R., A. Lukacsi, and I. Kovacs. 2004. Mother-to-fetus transmission of cytomegalovirus. A review. Acta Microbiol. Immunol. Hung. 51:385-401. [DOI] [PubMed] [Google Scholar]
- 21.Rabian-Herzog, C., S. Lesage, E. Gluckman, and D. Charron. 1993. Characterization of lymphocyte subpopulations in cord blood. J. Hematother. 2:255-257. [DOI] [PubMed] [Google Scholar]
- 22.Rasheed, F. N., J. N. Bulmer, L. Morrison, M. F. Jawla, M. Hassan-King, E. M. Riley, and B. M. Greenwood. 1992. Isolation of maternal mononuclear cells from placentas for use in in vitro functional assays. J. Immunol. Methods 146:185-193. [DOI] [PubMed] [Google Scholar]
- 23.Sartelet, H., D. Schleiermacher, J. Y. Le-Hesran, O. Graesslin, D. Gaillard, M. Fe, C. Lechki, A. Gaye, P. Le Bouteiller, and P. Birembaut. 2005. Less HLA-G expression in Plasmodium falciparum-infected third trimester placentas is associated with more natural killer cells. Placenta 26:505-511. [DOI] [PubMed] [Google Scholar]
- 24.Shohat, B., M. Hirsch, O. Jardena, J. Henry, I. Levy, and N. Trainin. 1986. Cellular immune aspects of the human fetal-maternal relationship. Am. J. Reprod. Immunol. Microbiol. 11:125-129. [DOI] [PubMed] [Google Scholar]
- 25.van Kooyk, Y., P. Weder, K. Heije, and C. G. Figdor. 1994. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J. Cell Biol. 124:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Kooyk, Y., P. Weder, F. Hogervorst, A. J. Verhoeven, G. van Seventer, A. A. te Velde, J. Borst, G. D. Keizer, and C. G. Figdor. 1991. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J. Cell Biol. 112:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]