Abstract
An immunochromatographic test for the simultaneous detection of Babesia caballi- and B. equi-specific antibodies (BceICT) was developed using a recombinant B. caballi 48-kDa rhoptry protein (rBc48) and a recombinant truncated B. equi merozoite antigen 2 (rEMA-2t). An evaluation of the ability of the BceICT to detect antibodies in sera from uninfected horses and experimentally infected horses showed high sensitivities and specificities of 83.3% (10/12 sera) and 92.9% (52/56 sera), respectively, for the anti-B. caballi antibody and 94.1% (16/17 sera) and 88.2% (45/51 sera), respectively, for the anti-B. equi antibody. Results from the detection of antibodies in field-collected sera indicated that the BceICT results corresponded with those of enzyme-linked immunosorbent assays (ELISA), showing 91.8% correspondence (67/73 sera) for B. caballi and 95.9% correspondence (70/73 sera) for B. equi, and that the BceICT results also corresponded with the ICT for B. caballi and for B. equi, both of which were 98.2% (55/56 sera). The comparable results of the ICT and ELISA and the simplicity and rapidity of the performance of the ICT suggest that the BceICT would be a feasible test for the simultaneous serodiagnosis of both agents of equine babesiosis in the field.
Equine piroplasmosis, caused by Babesia caballi and Babesia equi, is an important protozoan disease worldwide from both veterinary and economic viewpoints (2). Various serodiagnostic tests have been developed for the disease, such as the complement fixation test (1, 11, 12), the indirect immunofluorescent antibody test (1, 11, 12), the enzyme-linked immunosorbent assay (ELISA) (1, 3, 5, 7, 8, 9, 10, 13, 14), the competitive-inhibition ELISA (9), and the immunochromatographic test (ICT) (6). In our previous studies, ELISAs for the serodiagnoses of B. caballi and B. equi infections demonstrated many advantages, such as higher sensitivity and specificity, lower cost of materials, and greater objectivity in the determination of results (5, 8), over the complement fixation test, indirect immunofluorescent antibody test, and competitive-inhibition ELISA. Compared with ELISA, however, the ICT is relatively simple, can be performed quickly, and has the listed advantages of ELISA (6).
Babesia caballi and B. equi have overlapping geographical distributions (4). In such areas, an individual horse may be infected by both species. Therefore, a test capable of detecting the antibodies induced by both types of parasites would be desirable. Here, we report an ICT for the simultaneous detection of B. caballi- and B. equi-specific antibodies (BceICT) that uses the recombinant B. caballi 48-kDa rhoptry protein (rBc48) and the recombinant truncated B. equi merozoite antigen 2 (rEMA-2t) as antigens for the simultaneous serodiagnosis of infections caused by two Babesia spp. in horses.
MATERIALS AND METHODS
rEMA-2t.
rEMA-2 was expressed in Escherichia coli as a fusion protein with glutathione S-transferase, as described previously (5). The fusion protein was purified using glutathione Sepharose 4B (Amersham Pharmacia Biotech, Uppsala, Sweden). The leader protein, glutathione S-transferase, was cleaved by thrombin protease.
rBc48.
rBc48 was prepared as described previously, with some modification (7, 8). Briefly, the Bc48 gene inserted into pBluescript SK(+) vectors was subcloned into pGEX-4T (Amersham) of the bacterial expression vector after digestion with EcoRI and XhoI. The E. coli (BL21 strain) colony transformed with pGEX-4T/Bc48 was cultured on a small scale overnight in Luria-Bertani (LB) medium (1% Bacto tryptone, 0.5% yeast extract, 1% NaCl, and 0.1% 5 N NaOH) with 50 μg/ml of ampicillin sodium at 37°C. The overnight culture was then diluted to 1:100 in an LB medium for a large-scale culture at 25°C. When the optical density at 600 nm (OD600) reached 0.50, E. coli was induced to express the rBc48 protein by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside and incubation for another 4 h at 25°C. The purification procedure for rBc48 was the same as that for rEMA-2t.
Conjugates.
After dialysis in a 5 mM phosphate buffer at the proper pH (6.5 for rEMA-2t and 8.0 for rBc48), rEMA-2t and rBc48 were diluted to their optimal concentrations, 200 μg/ml and 125 μg/ml, respectively, and mixed gently with gold colloid particles (British BioCell International, SDX, United Kingdom) at the optimal pH. The ratio of volumes was 1:10. The mixtures were incubated at room temperature for 10 min without disturbance. Then, 0.05% polyethylene glycol 20,000 (PEG) and 1% bovine serum albumin (BSA) were added to stabilize and block the conjugate particles. After centrifugation at 18,000 × g for 20 min, 90% of the supernatants were discarded, and the pellets were resuspended in the remaining supernatants by sonication and then washed with phosphate-buffered saline containing 0.5% BSA and 0.05% PEG. Following the second centrifugation, the pellets were resuspended in phosphate-buffered saline with 0.5% BSA and 0.05% PEG until the OD520 reached 5. After the two conjugates were mixed and diluted in 10 mM Tris-HCl (pH 8.2) with 5% sucrose, the mixture was sprayed onto glass fiber (Schleicher & Schuell, NH) and dried in a vacuum overnight.
Rabbit anti-rEMA-2t IgG.
A rabbit was immunized with 1 ml of rEMA-2t (2 mg/ml) mixed with 1 ml of complete Freund's adjuvant (Difco, Detroit, MI) by multiple intradermal injections into its dorsum. Two booster injections were given in a 2-week interval, with the same dose of antigen mixed with incomplete Freund's adjuvant (Difco). The rabbit was bled 10 days after the last booster. The immunoglobulin G (IgG) fraction was purified from blood serum with an Econo-Pac protein A kit (Bio-Rad, CA) according to the manufacturer's instructions and used as the control for the ICT.
Immobilization of rEMA-2t, rBc48, and rabbit anti-rEMA-2t IgG on nitrocellulose (NC) membrane.
rEMA-2t (500 μg/ml), rBc48 (125 μg/ml), and rabbit anti-rEMA-2t IgG (1,500 μg/ml) were linearly jetted onto an NC membrane with a plastic backing (Schleicher & Schuell, NH) using a BioDot Biojet 3050 quanti-dispenser (BioDot, Inc., CA). The positions of the three lines are shown in Fig. 1. The membrane was dried at 50°C for 30 min and blocked with 0.5% casein in a 50 mM boric acid buffer (pH 8.5) for 30 min. After a wash with 50 mM Tris-HCl (pH 7.4) containing 0.5% sucrose and 0.05% sodium cholate, the membrane was air dried overnight.
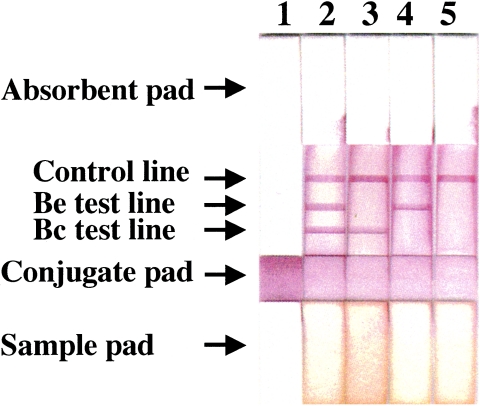
FIG. 1.
Pretest (lane 1) and posttests (lanes 2 to 5) of BceICT strips. Bc test line: rBc48 was immobilized on the nitrocellulose membrane for the detection of antibody to B. caballi. Be test line: rEMA-2t was immobilized on the nitrocellulose membrane for the detection of antibody to B. equi. Lanes: 1, pretest; 2, antibodies to both B. caballi and B. equi are positive; 3, only the antibody to B. caballi is positive; 4, only the antibody to B. equi is positive; 5, the antibodies to both B. caballi and B. equi are negative.
Assemblage of test strips and detection of specific antibodies in horse serum.
The NC membrane containing antigens and antibodies was assembled on an adhesive card (Schleicher & Schuell) with other components, such as an absorbent pad, a conjugate pad, and a sample pad, and cut into 6-mm-wide strips using a BioDot cutter (BioDot, Inc.), as shown in Fig. 1 (lane 1). Detection was performed by pipetting 100 μl of serum onto the sample pad. In the preliminary test, color in the control line took a maximum of 7 min to develop; color in the test lanes took a maximum of 15 min to develop, and the results did not change when the sample pad was read later than 15 min. Therefore, results were determined 15 min after the application of serum samples and recorded as (i) positive for both equine babesiosis species (Fig. 1, lane 2); (ii) positive for B. caballi and negative for B. equi (Fig. 1, lane 3); (iii) negative for B. caballi and positive for B. equi (Fig. 1, lane 4); and (iv) negative for both B. caballi and B. equi (Fig. 1, lane 5).
Sera.
Thirty-nine uninfected sera were from race horses in Japan, a country assumed to be free of equine babesiosis. Twelve B. caballi- and 17 B. equi-infected sera were from horses infected experimentally with the parasites. Of the 73 field-collected sera, 56 were from horses in Jilin province, China, and 17 were from horses imported to Japan from different countries suspected of harboring Babesia infections.
RESULTS
Detection of specific antibodies against B. caballi and B. equi in sera from experimentally infected horses.
The results of experiments for the detection of specific antibodies are summarized in Tables 1 and 2. The sensitivities and specificities of the BceICT were 83.3% (10/12 sera) and 92.9% (52/56 sera), respectively, for the detection of the antibody against B. caballi and 94.1% (16/17 sera) and 88.2% (45/51 sera), respectively, for the detection of the antibody against B. equi infection. The sensitivity of the BceICT for detecting antibodies to B. caballi (83.3%) and B. equi (94.1%) were equal to those of B. caballi ELISA (BcELISA) and B. equi ELISA (BeELISA). On the other hand, the specificity of the BceICT for detecting antibodies to B. caballi (92.9%) and B. equi (88.2%) were slightly lower than those of BcELISA (100%) and BeELISA (100%).
TABLE 1.
Comparison of BceICT with BcELISA in the detection of specific antibodies against B. caballi in equine seraa
| BcELISA result | No. of sera with indicated BceICT result from:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected sera (n = 39)
|
B. equi-infected sera (n = 17)
|
B. caballi-infected sera (n = 12)
|
Field sera (n = 73)
|
|||||
| + | − | + | − | + | − | + | − | |
| + | 0 | 0 | 0 | 0 | 10 | 0 | 19 | 5 |
| − | 1 | 38 | 3 | 14 | 0 | 2 | 1 | 48 |
| Total | 1 | 38 | 3 | 14 | 10 | 2 | 20 | 53 |
The sensitivity of both BcELISA and BceICT for detecting antibody to B. caballi was 83.3% (10/12), and the specificities of BcELISA and BceICT were 100% (56/56) and 92.9% (52/56), respectively.
TABLE 2.
Comparison of BceICT with BeELISA in the detection of specific antibodies against B. equi in equine seraa
| BeELISA result | No. of sera with indicated BceICT result from:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected sera (n = 39)
|
B. equi-infected sera (n = 17)
|
B. caballi-infected sera (n = 12)
|
Field sera (n = 73)
|
|||||
| + | − | + | − | + | − | + | − | |
| + | 0 | 0 | 16 | 0 | 0 | 0 | 39 | 1 |
| − | 2 | 37 | 0 | 1 | 4 | 8 | 2 | 31 |
| Total | 2 | 37 | 16 | 1 | 4 | 8 | 41 | 32 |
The sensitivity of both BeELISA and BceICT for detecting antibody to B. equi was 94.1% (16/17), and the specificities of BeELISA and BceICT were 100% (51/51) and 88.2% (45/51), respectively.
Detection of specific antibodies against B. caballi and B. equi in sera from horses in an area of endemicity.
Comparisons between the BceICT and ELISAs in the detection of field-collected sera are shown in the last columns of Tables 1 and 2. The comparisons between the BceICT and BcICT and between the BceICT and BeICT are summarized in Table 3. The corresponding results were 91.8% (67/73) between BceICT and BcELISA, 95.9% (70/73) between BceICT and BeELISA, 98.2% (55/56) between BceICT and BcICT, and 98.2% (55/56) between BceICT and BeICT.
TABLE 3.
Comparison of BceICT with BcICT and BeICT in the detection of specific antibodies against B. caballi and B. equi infections in field sera
| BcICT or BeICT detection result | BceICT detection of antibodies specific fora:
|
|||
|---|---|---|---|---|
| Anti-B. caballi antibody
|
Anti-B. equi antibody
|
|||
| No. of positive sera (%) | No. of negative sera (%) | No. of positive sera (%) | No. of negative sera (%) | |
| BcICT | ||||
| + | 18 (32.1) | 1 (1.8) | ||
| − | 0 | 37 (66.1) | ||
| BeICT | ||||
| + | 26 (46.4) | 0 | ||
| − | 1 (1.8) | 29 (51.8) | ||
| Total (n = 56) | 18 (32.1) | 38 (67.9) | 27 (48.2) | 29 (51.8) |
The percentage of results that corresponded with those of BceICT was 98.2% for both BcICT and BeICT.
DISCUSSION
The ICT is a nitrocellulose membrane-based immunoassay that relies on the migration of a liquid across the surface of the membrane by the capillary mechanism and the capture of the antibodies in the sample using the antigens in the mobile phase, which are conjugated with gold particles, and antigens and antibodies in the immobile phase. The captured antigen and antibody complex then develops a colored line. As soon as the test strip is available, the performance is as simple as loading the sample onto the strip, and the result can be determined in a few minutes with the naked eye, according to the colored lines. No equipment or testing skills are required. Therefore, this test is more practical to use in the field than any other test. In our previous studies, the BcICT and BeICT were developed for the detection of antibodies to B. caballi (unpublished data) and B. equi (6). Both of the tests showed results that were comparable with those of ELISAs. To combine the two ICTs into one test, we developed a BceICT for the simultaneous detection of antibodies against infection by two species of Babesia. Using this test, some materials used for the preparation of test strips, sera, manpower, and time required could be reduced by one-half.
Detection results of the specific antibodies in the known B. caballi- and B. equi-infected and uninfected horses indicate that the sensitivity and specificity of the BceICT was 83.3% and 92.9%, respectively, for anti-B. caballi antibody and 94.1% and 88.2%, respectively, for anti-B. equi antibody. No significant differences were observed in sensitivity between BceICT and BcELISA and between BceICT and BeELISA. However, the specificity of the BceICT was less than those of BcELISA and BeELISA. The nonspecific reaction in the BceICT for the detection of B. equi infection was observed mainly in sera from B. caballi-infected horses, in reverse, and that for the detection of B. caballi infection was observed mainly in sera from B. equi-infected horses. Therefore, these nonspecific reactions may be due to an antigen or antibody cross-reaction rather than the effect of some physical or chemical factors. The reaction may occur when the two conjugates are mixed. Other possibilities are related to the storage of the sera, for example, the length of the storage period, the quantity of preservative added, or the conditions for the preparation of the test strips. If further discrimination between the two species is necessary, ELISAs could be carried out to examine the BceICT-positive sera.
The high correspondence of BceICT results with ICT or ELISA results were also found for B. caballi and B. equi infections, respectively, in sera collected from horses in the field. The correspondence of the BceICT with BcELISA, BeELISA, BcICT, and BeICT were 91.8%, 95.9%, 98.2%, and 98.2%, respectively (Tables 1, 2, and 3). These results for B. equi infection were very comparable with those in previous studies (6).
In conclusion, the present study indicates that the BceICT employing antigen bound to nitrocellulose membranes has a high specificity and sensitivity for detecting antibodies to both B. caballi and B. equi. The results of the BceICT are easily obtained and comparable with those from ELISA. Therefore, the BceICT is a feasible field test for the simultaneous serodiagnosis of both types of equine babesiosis, even though some improvements of the BceICT and an evaluation on a larger scale are necessary.
Acknowledgments
This study was supported by grants from the 21st Century COE Program (A-1) and Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Bruning, A. 1996. Equine piroplasmosis: an update on diagnosis, treatment, and prevention. Br. Vet. J. 152:139-151. [DOI] [PubMed] [Google Scholar]
- 2.de Waal, D. T. 2000. Global importance of piroplasmosis. J. Protozool. Res. 10:106-127. [Google Scholar]
- 3.Hirata, H., H. Ikadai, N. Yokoyama, X. Xuan, K. Fujisaki, N. Suzuki, T. Mikami, and I. Igarashi. 2002. Cloning of a truncated Babesia equi gene encoding an 82-kilodalton protein and its potential use in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 40:1470-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holbrook, A. A. 1969. Biology of equine piroplasmosis. J. Am. Vet. Med. Assoc. 155:453-454. [PubMed] [Google Scholar]
- 5.Huang, X., X. Xuan, N. Yokoyama, L. Xu, H. Suzuki, C. Sugimoto, H. Nagasawa, K. Fujisaki, and I. Igarashi. 2003. High-level expression and purification of a truncated merozoite antigen-2 of Babesia equi in Escherichia coli and its potential for immunodiagnosis. J. Clin. Microbiol. 41:1147-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang, X., X. Xuan, L. Xu, S. Zhang, N. Yokoyama, N. Suzuki, and I. Igarashi. 2004. Development of an immunochromatographic test with recombinant EMA-2 for the rapid detection of antibodies against Babesia equi in horses. J. Clin. Microbiol. 42:359-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikadai, H., X. Xuan, I. Igarashi, S. Tanaka, T. Kanemaru, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 1999. Cloning and expression of a 48-kilodalton Babesia caballi merozoite rhoptry protein and potential use of the recombinant antigen in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37:3475-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikadai, H., C. R. Osorio, X. Xuan, I. Igarashi, H. Nagasawa, K. Fujisaki, N. Suzuki, and T. Mikami. 2000. Detection of Babesia caballi infection by enzyme-linked immunosorbent assay using recombinant 48-kDa merozoite rhoptry protein. Int. J. Parasitol. 30:633-635. [DOI] [PubMed] [Google Scholar]
- 9.Knowles, D. P., Jr., L. E. Perryman, L. S. Kappmeyer, and S. G. Hennager. 1991. Detection of equine antibody to Babesia equi merozoite proteins by a monoclonal antibody-based competitive inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 29:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka, T., X. Xuan, H. Ikadai, I. Igarashi, H. Nagasawa, K. Fujisaki, T. Mikami, and N. Suzuki. 1999. Expression of Babesia equi merozoite antigen-2 by recombinant baculovirus and its use in the ELISA. Int. J. Parasitol. 29:1803-1808. [DOI] [PubMed] [Google Scholar]
- 11.Tenter, A. M., and K. T. Friedhoff. 1986. Serodiagnosis of experimental and natural Babesia equi and B. caballi infections. Vet. Parasitol. 20:49-61. [DOI] [PubMed] [Google Scholar]
- 12.Weiland, G. 1986. Species-specific serodiagnosis of equine piroplasma infections by means of complement fixation test, immunofluorescence, and enzyme-linked immunosorbent assay. Vet. Parasitol. 20:43-48. [DOI] [PubMed] [Google Scholar]
- 13.Xuan, X., A. Larsen, H. Ikadai, T. Tanaka, I. Igarashi, H. Nagasawa, K. Fujisaki, Y. Toyoda, N. Suzuki, and T. Mikami. 2001. Expression of Babesia equi merozoite antigen 1 in insect cells by recombinant baculovirus and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 39:705-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xuan, X., A. Nagai, B. Battsetseg, S. Fukumoto, L. H. Makala, N. Inoue, I. Igarashi, T. Mikami, and K. Fujisaki. 2001. Diagnosis of equine piroplasmosis in Brazil by serodiagnostic methods with recombinant antigens. J. Vet. Med. Sci. 63:1159-1160. [DOI] [PubMed] [Google Scholar]