Abstract
Evidence from animals suggests that anti-anthrax protective antigen (PA) immunoglobulin G (IgG) from vaccination with anthrax vaccine adsorbed (AVA) is protective against Bacillus anthracis infection. Measurement of anti-PA IgG in human sera can be performed using either enzyme-linked immunosorbent assay or fluorescent covalent microsphere immunoassay (ELISA) (R. E. Biagini, D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder, Clin. Diagn. Lab. Immunol. 11:50-55, 2004). Both these methods are laboratory based. We describe the development of a rapid lateral-flow immunochromatographic assay (LFIA) test kit for the measurement of anti-PA IgG in serum or whole-blood samples (30-μl samples) using colloidal gold nanoparticles as the detection reagent and an internal control. Using sera from 19 anthrax AVA vaccinees (anti-PA IgG range, 2.4 to 340 μg/ml) and 10 controls and PA-supplemented whole-blood samples, we demonstrated that the LFIA had a sensitivity of approximately 3 μg/ml anti-PA IgG in serum and ∼14 μg/ml anti-PA IgG in whole blood. Preabsorption of sera with PA yielded negative anti-PA LFIAs. The diagnostic sensitivity and specificity of the assay were 100% using ELISA-measured anti-PA IgG as the standard. This kit has utility in determining anti-PA antibody reactivity in the sera of individuals vaccinated with AVA or individuals with clinical anthrax.
Bacillus anthracis is the causative agent of anthrax, a disease that in the inhalation form can have a fatal outcome when it is not treated in humans (30). The principal virulence factors of B. anthracis are encoded on two plasmids, one involved in the synthesis of a γ-linked polyglutamic acid capsule that inhibits phagocytosis of vegetative forms and the other bearing the genes for the synthesis of the exotoxins, edema toxin and lethal toxin (LTx), that it secretes (7). The exotoxins are binary, composed of a B (binding) protein that is necessary for entry into the host cell and an A (enzymatically active) protein. The B component is known as the protective antigen (PA) and is common to both toxins. The protective antigen component of the anthrax toxins is an essential virulence factor of B. anthracis and is the major protective immunogen (6). The A component of edema toxin is the edema factor, a calmodulin-dependent adenylate cyclase that is responsible for the prominent edema at the sites of infection, inhibition of neutrophil function, and hindrance of the production by monocytes of tumor necrosis factor and interleukin-6 (10). The A component of the LTx is lethal factor, a zinc metalloprotease that inactivates members of the mitogen-activated protein kinase kinase family, leading to the inhibition of intracellular signaling. LTx stimulates the release by macrophages of tumor necrosis factor alpha and interleukin-1β, a mechanism that appears to contribute to sudden death (27). LTx activity has also been shown to down-regulate the immune response of the host and thus promotes the massive bacteremia that is characteristic of systemic anthrax (1, 9).
The licensed U.S. anthrax vaccine, anthrax vaccine adsorbed (AVA), is a cell-free culture filtrate of B. anthracis V770-NPI-R, an avirulent, nonencapsulated, nonproteolytic variation of a 1951 bovine isolate from Florida (23). Studies of animals have shown AVA to be completely protective against an aerosol challenge of anthrax spores (28). Anti-PA immunoglobulin G (IgG) levels have been shown to be potential human correlates of immunity in animal models (11, 13, 18, 22) treated with AVA.
Measurement of anti-PA IgG in human sera can be performed using either enzyme-linked immunosorbent assay (ELISA) (21) or fluorescent covalent microsphere immunoassay (2, 3). Both of these methods are laboratory based. In the present work we describe the development and evaluation of a prototype rapid lateral-flow immunochromatographic assay (LFIA) test kit for the measurement of anti-PA IgG in serum or whole blood using colloidal gold nanoparticles as the detection reagent and internal control.
MATERIALS AND METHODS
Human serum and blood samples.
Twenty-nine serum samples, 19 having anti-PA IgG concentrations ranging from nondetectable to 340 μg/ml measured by ELISA (2) and 10 sera randomly selected from 534 samples collected from the residual sera in tubes from volunteer blood donors (Indiana Blood Center, Indianapolis, IN) were used in this study. A heparinized whole-blood sample was also used. An anti-anthrax vaccine adsorbed (anti-AVA) standard human reference serum pool, AVR414, was used to standardize the assay (24). AVR414 was prepared by pooling equal volumes of serum from each of three healthy adult CDC volunteers who had received a minimum of four subcutaneous injections of AVA (BioThrax; BioPort Corp., Lansing, MI) with the licensed regimen (at 0, 2, and 4 weeks and 6, 12, and 18 months with two yearly boosters). The use of all human serum samples was approved by the CDC Human Subjects Review Board.
Lateral-flow immunochromatographic device.
The LFIA device for the detection of anti-PA IgG was manufactured under contract with Arista Biologicals, Inc. (Allentown, PA) as a single-antigen direct sandwich assay. Some of the details in the preparation of the device are proprietary. The device consists of a plastic support to which a nitrocellulose membrane (thickness, 205 ± 1 μm) is mounted. Purified anthrax recombinant PA (rPA) (List Biological Laboratories, Inc., Campbell, CA) was striped in the “test line” position (2 mg/ml), while a biotinylated bovine serum albumin (BSA) conjugate was striped in the “control line” position (2 mg/ml). Gold particles (40 nm), individually conjugated to PA and streptavidin, were prepared and mixed. The PA- and streptavidin-colloidal gold conjugate mixture was dispensed onto a conjugate pad. The conjugate pad was then affixed to the test strip by overlapping the nitrocellulose membrane at its proximal end; the addition of a sample pad completed the assembly by overlapping onto the conjugate pad (Fig. 1). Devices that could be used to analyze ∼30 μl of serum or whole blood (with the addition of a blood separation sample pad) were produced. Assay (chase) buffers (either 50 mM phosphate buffer containing 0.1% Igepal CA-720, 0.03% sodium dodecyl sulfate, 1% BSA, and 0.1% sodium azide or the same buffer containing 0.85% NaCl [for whole blood]) were included with the sealed foil packaged tests, which also contained desiccant. The wicking speed of the device was 4 cm in 100 to 140 seconds with normal saline. Recent experiments performed by Arista Biologicals, Inc., indicated that the LFIA device described in the present report had essentially equivalent performance 23 months after first manufactured when stored at room temperature.
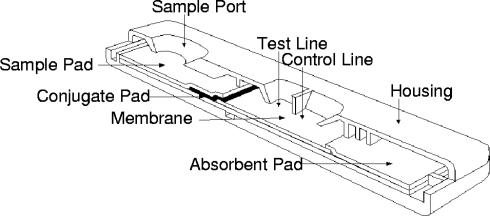
FIG. 1.
Diagram of lateral-flow immunochromatographic assay cartridge.
Direct antibody sandwich format.
Serum or blood samples (30 μl) are placed in the “sample port” at “S” on the device. If the sample contains antibodies specific to PA, they bind the gold-conjugated PA on the conjugate pad. After the addition of chase buffer (3 drops) into the “sample port,” the anti-PA-gold conjugate and -streptavidin conjugate migrate down the nitrocellulose membrane by capillary action. At the test “T” line, the anti-PA-gold conjugate binds to immobilized PA, immobilizing the gold conjugate-PA-anti-PA conjugate complex. If the concentration of the antibody sandwich is sufficient, the gold can be visualized as a red line.
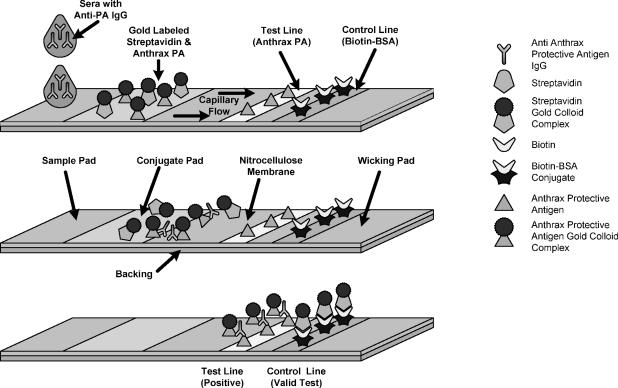
During development of the assay, the amount of gold-conjugated PA, the number of conjugated colloidal gold particles used in the device, and the amount of antigen on the colloidal gold were empirically titrated to yield a distinct line at the test position with a serum sample containing a relatively high concentration of anti-PA IgG. The ratio of serum anti-PA IgG to antigen is such that monodentate binding of anti-PA to epitope is favored on the basis of steric conditions and other conditions. Subsequent bidentate antibody binding is favored at the “test line” due to the extremely high concentration of PA antigen (2 mg/ml). This process, once optimized, would be independent of the concentration of serum anti-PA IgG. At the control line position, gold-conjugated streptavidin conjugate binds to immobilized biotin-BSA, forming a red line (Fig. 2). The color formation for both reactions is complete after ∼5 to 10 min.
FIG. 2.
Diagram of the mechanism of a direct sandwich assay.
Determination of the visual limit of detection and diagnostic sensitivity/specificity of the LFIA.
Human anti-anthrax vaccine standard AVR414 was diluted with phosphate-buffered saline (PBS), yielding anti-PA IgG concentrations ranging from 0.14 to 141.2 μg/ml. A control serum, previously shown to have no detectable anti-PA IgG (2), was diluted in a similar manner. Standard serum AVR414 (in PBS) was added to heparinized whole blood (dilution, 1:10), yielding a final concentration of 14.1 μg/ml anti-PA IgG. Another aliquot of heparinized whole blood was diluted 1:10 with PBS (pH 7.4) (Sigma, St. Louis, MO) as a control. All diluted serum and blood samples were run in the LFIA, and the lowest concentration yielding a positive test was defined as the visual limit of detection (VLD). The assay diagnostic performance was computed by using the following definitions (with TP defined as a true-positive diagnostic test result, TN defined as a true-negative diagnostic test result, FN defined as a false-negative diagnostic test result, and FP defined as a false-positive diagnostic test result). Sensitivity {[TP/(TP + FN)] × 100} was defined as the percentage of positive test responses in sera with detectable anti-PA IgG (2), while specificity {[TN/(FP + TN)] × 100} was defined as the percentage of negative tests in sera with values below the VLD of the LFIA. Positive or negative results from the LFIA were scored by at least two individuals numerous times.
Competitive inhibition LFIA.
To determine the specificity of measurements performed by LFIA, serum AVR414 was diluted 1:10 and 1:50 with PBS. Aliquots of these diluted sera were preincubated with 100 μg PA, and all sera were incubated overnight at 4°C. The sera were then centrifuged, and the supernatants were analyzed by LFIA as outlined above.
Comparison of LFIA results with known anti-PA IgG values measured by ELISA.
The 19 serum samples from above whose anti-anthrax PA IgG had been previously measured (2, 21) and 10 blood donor sera were run in the LFIA device, and the values were compared to ELISA-derived anti-PA IgG concentrations. Results from the LFIA and ELISA were dichotomized on the basis of being above or below the lower limit of quantification of the ELISA (3.0 μg/ml anti-PA IgG) or above or below the VLD of the LFIA. These binary results were evaluated using the Mann-Whitney rank sum test (SigmaStat for Windows version 3.1; Systat Software, Inc., Point Richmond, CA).
RESULTS
The lateral-flow immunochromatographic device described in the present effort yields visual results for the determination of anti-PA IgG in serum or whole blood. Control experiments with buffer alone or control serum yields a clear distinct red line at the control area (“C” line) on the device with no red line at the test line. When diluted human anti-anthrax vaccine standard serum AVR414 was used in the device, two distinct red lines were observable at the “C” line and the test area (“T” line) identified on the device, indicating a positive test (Fig. 3). Whole blood, diluted with AVR414, yielding a concentration of 14.1 μg/ml anti-PA IgG gives similar results (Fig. 4). Control experiments with whole blood alone and whole blood diluted 1:9 with PBS yield distinct red lines at the control area (“C” line) on the device with no evidence of red lines at the test area (“T” line). The results of completed tests, while having a slight loss of staining intensity, are stable after drying.
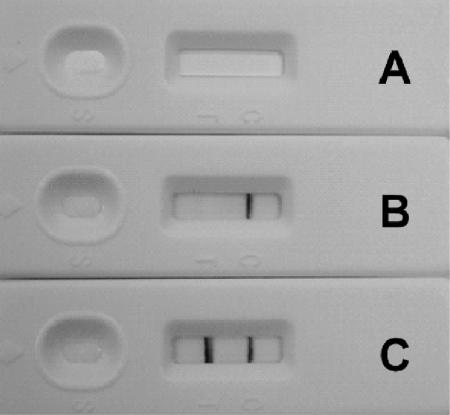
FIG. 3.
Photograph of representative results of LFIA with serum. Results with no addition (A) and with control serum (B) and human anti-PA IgG-positive serum (C) are shown.
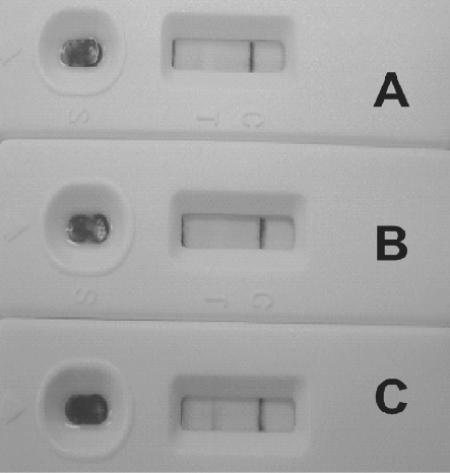
FIG. 4.
Photograph of representative results of LFIA with whole blood. Results with whole blood (A), whole blood-PBS (diluted 1:9) (B), and whole blood-PBS (diluted 1:9) with 14.1 μg/ml human anti-PA IgG (C) are shown.
Experiments designed to detect the VLD of the device for anti-PA IgG in serum are outlined in Table 1. As can be seen, the lateral-flow immunochromatographic assay yielded a positive result at 2.8 μg/ml anti-PA IgG, while an equivocal result was obtained at 1.4 μg/ml anti-PA IgG. A true negative result was observed at 0.6 μg/ml anti-PA IgG in serum. On the basis of these findings, the VLD was determined to be 2.8 μg/ml anti-PA IgG.
TABLE 1.
Determination of the visual cutoff point for a positive human anti-PA IgG lateral-flow immunochromatographic assay result
| AVR414 dilution | Result | Human anti-PA IgG concn (μg/ml) |
|---|---|---|
| None (neat) | Positive | 141.2 |
| 1/5 | Positive | 28.2 |
| 1/10 | Positive | 14.1 |
| 1/25 | Positive | 5.6 |
| 1/50 | Positive | 2.8 |
| 1/100 | Positive or negative | 1.4 |
| 1/250 | Negative | 0.6 |
| 1/500 | Negative | 0.3 |
| 1/1,000 | Negative | 0.1 |
When the results of the lateral-flow immunochromatographic assay were compared to known anti-PA IgG concentrations measured by an ELISA (21), extremely good agreement was observed. Results of the Mann-Whitney rank sum testing indicated no statistically significant difference between the methods (P = 0.993). The diagnostic sensitivity and specificity of the lateral-flow immunochromatographic device were 100% with no false-positive or false-negative results observed compared to ELISA results (21) (Table 2).
TABLE 2.
Evaluation of human anti-PA IgG lateral-flow immunochromatographic assay in known positive and negative sera (test group) and sera from blood donor controls
| Sample | LFIA result | Human anti-PA IgG concn (μg/ml)a |
|---|---|---|
| Test groups | ||
| QC1 | Positive | 150.2 |
| QC2 | Positive | 121.5 |
| QC3 | Positive | 72.1 |
| S1 | Positive | 96.5 |
| S2 | Positive | 250.4 |
| S3 | Negative | NDb |
| S4 | Positive | 95.9 |
| S5 | Positive | 180.1 |
| S6 | Positive | 51.8 |
| S7 | Positive | 86.3 |
| S8 | Positive | 340.0 |
| S9 | Positive | 280.8 |
| S10 | Positive | 137.9 |
| S11 | Positive | 127.6 |
| S12 | Positive | 262.2 |
| S13 | Positive | 169.6 |
| S14 | Positive | 105.1 |
| S15 | Positive | 149.0 |
| S16 | Positive | 139.5 |
| Blood donor controls | ||
| 12 | Negative | NMc |
| 98 | Negative | NM |
| 73 | Negative | NM |
| 236 | Negative | NM |
| 268 | Negative | NM |
| 272 | Negative | NM |
| 307 | Negative | NM |
| 323 | Negative | NM |
| 358 | Negative | NM |
Human anti-PA IgG concentrations for the test group were measured by an ELISA (21).
ND, not detectable. The result was below the anti-PA IgG minimum detectable concentration of the ELISA (3.0 μg/ml human anti-PA IgG).
NM, not measured. Human anti-PA IgG concentrations in serum samples from blood donor controls were not measured but assumed to be negative.
When diluted AVR414 serum (1:10 and 1:50) was preincubated with 100 μg/ml PA (inhibition experiment) and the resultant serum was evaluated in the lateral-flow immunochromatographic device, positive red lines were observed only at the “C” position. Diluted AVR414 preincubated with PBS (no PA) yielded distinct red lines at both the “C” and “T” positions for both dilutions tested (data not shown).
DISCUSSION
Spores of B. anthracis can be used as a military or terrorist weapon, are stable for years under harsh conditions (26), can be produced in large quantities with relatively basic technology, and can be converted to powders capable of aerosol dissemination (8). The Department of Defense estimates that among individuals exposed to B. anthracis spores, the lethal dose (50% lethal dose) of B. anthracis is between 8,000 and 10,000 spores (4). Because of this, the Department of Defense developed the Anthrax Vaccine Immunization Program to protect active-duty and reserve members of the U.S. military forces, as well as emergency-essential civilians assigned to areas deemed to be at high risk for anthrax attack (16). More than 500,000 personnel received approximately 2 million doses of AVA over the 4-year period from 1998 through 2001 (25). The licensed AVA vaccination regimen consists of three subcutaneous injections at 0, 2, and 4 weeks and three booster vaccinations at 6, 12, and 18 months (5). Virtually all individuals seroconvert using this regimen, with peak anti-PA IgG levels occurring at 6 weeks after the first injection (19). To maintain immunity, the manufacturer recommends an annual booster injection (5). A report by the National Academy of Science, Institute of Medicine indicated either nonexistent or low levels of anti-PA IgG antibodies 2 years after initial vaccinations with AVA (17). We have shown that sera from individuals (3) vaccinated with AVA ∼18 months prior to sampling without boosting contained a mean value of 16.9 μg/ml anti-PA IgG.
The anthrax attacks of 2001 resulted in 11 cases of inhalational anthrax, 5 of who died. The median period from the presumed time of exposure to the onset of symptoms was 4 days (range, 4 to 6 days). The mean incubation period for cutaneous anthrax cases diagnosed from the anthrax attacks of 2001 was 5 days, with a range of 1 to 10 days for the 11 cases of cutaneous anthrax (12). Eleven days after the onset of symptoms (15 days after likely exposure), anti-PA IgG was detected in 16 of 17 patients with confirmed or suspected clinical anthrax who were tested (20). Serology was particularly important in diagnosing cases of cutaneous anthrax after the 2001 bioterror attacks, as for 3 of the 10 cutaneous anthrax cases, serology was the only laboratory test that produced a positive result (14). Anti-PA IgG was detectable 8 to 16 months after the onset of symptoms in all 6 survivors of inhalation anthrax and in 7 of 11 survivors of cutaneous anthrax who were tested (14).
There is recent evidence that anti-PA antibodies bind to the B. anthracis spore surface and inhibit germination and that inactivated B. anthracis spores can contribute to protective immune responses in animals (6, 29). This suggests that anti-PA IgG antibodies may be produced from nonlethal infections and, as such, may be useful as indicators of previously undiagnosed anthrax. Newer anthrax vaccines, based on rPA, have been shown to be as effective as AVA and the United Kingdom vaccine (anthrax vaccine precipitated [AVP]) in producing anti-PA IgG in nonhuman primates (30). These findings argue that anti-PA IgG measurements can be used to determine immune status after vaccination and possibly, under correct conditions, may be indicative of nonlethal infection. For example, if sera were obtained from an individual who had been vaccinated with an anthrax vaccine, a positive result would indicate measurable anti-PA IgG from vaccination, especially if there are no symptoms of anthrax infection. A negative serum result in a vaccinated individual would indicate nonmeasurable anti-PA IgG. Longitudinal surveillance of anti-PA IgG levels in vaccinated individuals could indicate loss of protective immunity. Measurement of anti-PA IgG levels in nonvaccinated individuals could also indicate noninfective levels of exposure through environmental, occupational, or biological warfare means.
There are commercial (QuickELISA Anthrax-PA kit; Immunetics, Inc., Boston MA) and other assays (2, 15, 19, 21) for the measurement of anthrax anti-PA IgG. One ELISA (21) has a reactivity threshold of 3 μg/ml anti-PA IgG, while another fluorescence covalent microbead immunosorbent assay has a minimum detectable concentration of 1.5 μg/ml anti-PA IgG. A LFIA for anti-PA IgG which has a detection threshold of ∼3 μg/ml anti-PA IgG and 75% specificity and 80% sensitivity compared with ELISA data has also been reported (15).
In the present work we describe a LFIA which has a clear limit of detection at 2.8 μg/ml anti-PA IgG, which is more sensitive than the validated anti-PA (21) ELISA and is specific in that preincubation with PA can inhibit the assay's response. The assay can be used with small volumes (∼30 μl) of whole blood or serum, an amount that can be obtained from finger-stick sampling. The assay was shown to have 100% diagnostic sensitivity and specificity for the detection of anti-PA IgG. Existing ELISA and other assays for anti-PA IgG tests are laboratory based, require separation of serum from whole blood, and are relatively slow compared to the LFIA described here. In addition, highly trained laboratory personnel and relatively sophisticated equipment are also necessary for laboratory-based assays, whereas the LFIA is rapid (<10 min), easy to use, and highly portable.
In conclusion, in the present work we describe a LFIA suitable for determining anti-PA IgG concentrations in sera with a diagnostic sensitivity and specificity comparable to those of anti-PA IgG ELISAs. In addition, the LFIA can also be used with small volumes of whole blood as could be obtained from finger-stick sampling. The device is portable, yields results in 5 to 10 min, and can be used in a nonlaboratory environment. Its use would be a valuable adjunct to laboratory serologic testing, especially when there is a need to rapidly test high numbers of samples of serum or whole blood from U.S., British, or rPA vaccinees and/or clinical anthrax patients, a scenario which could rapidly overwhelm the diagnostic capacities of whole countries.
Acknowledgments
Mention of a product or company name does not constitute endorsement by NIOSH. The content and conclusions of this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
This work was supported in part by an interagency agreement between NIOSH and NIEHS (Y1-ES-0001; Clinical Immunotoxicity).
REFERENCES
- 1.Agrawal, A., J. Lingappa, S. H. Leppla, S. Agrawal, A. Jabbar, C. Quinn, and B. Pulendran. 2003. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature 424:329-334. [DOI] [PubMed] [Google Scholar]
- 2.Biagini, R. E., D. L. Sammons, J. P. Smith, B. A. MacKenzie, C. A. Striley, V. Semenova, E. Steward-Clark, K. Stamey, A. E. Freeman, C. P. Quinn, and J. E. Snawder. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin. Diagn. Lab. Immunol. 11:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagini, R. E., D. L. Sammons, J. P. Smith, E. H. Page, J. E. Snawder, C. A. Striley, and B. A. MacKenzie. 2004. Determination of serum IgG antibodies to Bacillus anthracis protective antigen in environmental sampling workers using a fluorescent covalent microsphere immunoassay. Occup. Environ. Med. 61:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block, S. 2001. The growing threat of biological weapons. Am. Sci. 89:28-37. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Use of anthrax vaccine in the United States. Morb. Mortal. Wkly. Rep. 49:1-20. [Google Scholar]
- 6.Cote, C. K., C. A. Rossi, A. S. Kang, P. R. Morrow, J. S. Lee, and S. L. Welkos. 2005. The detection of protective antigen (PA) associated with spores of Bacillus anthracis and the effects of anti-PA antibodies on spore germination and macrophage interactions. Microb. Pathog. 38:209-225. [DOI] [PubMed] [Google Scholar]
- 7.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 8.Dizer, U., L. Kenar, M. Ortatatlý, and T. Karayýlanoðlu. 2004. How to weaponize anthrax? East. J. Med. 9:13-16. [Google Scholar]
- 9.During, R. L., W. Li, B. Hao, J. M. Koenig, D. S. Stephens, C. P. Quinn, and F. S. Southwick. 2005. Anthrax lethal toxin paralyzes neutrophil actin-based motility. J. Infect. Dis. 192:837-845. [DOI] [PubMed] [Google Scholar]
- 10.Friedlander, A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335-349. [PubMed] [Google Scholar]
- 11.Iacono-Connors, L. C., S. L. Welkos, B. E. Ivins, and J. M. Dalrymple. 1991. Protection against anthrax with recombinant virus-expressed protective antigen in experimental animals. Infect. Immun. 59:1961-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 13.Ivins, B. E., S. L. Welkos, G. B. Knudson, and S. F. Little. 1990. Immunization against anthrax with aromatic compound-dependent (Aro−) mutants of Bacillus anthracis and with recombinant strains of Bacillus subtilis that produce anthrax protective antigen. Infect. Immun. 58:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan, D. B., P. L. Raghunathan, B. P. Bell, R. Brechner, E. A. Bresnitz, J. C. Butler, M. Cetron, M. Cohen, T. Doyle, M. Fischer, C. Greene, K. S. Griffith, J. Guarner, J. L. Hadler, J. A. Hayslett, R. Meyer, L. R. Petersen, M. Phillips, R. Pinner, T. Popovic, C. P. Quinn, J. Reefhuis, D. Reissman, N. Rosenstein, A. Schuchat, W. J. Shieh, L. Siegal, D. L. Swerdlow, F. C. Tenover, M. Traeger, J. W. Ward, I. Weisfuse, S. Wiersma, K. Yeskey, S. Zaki, D. A. Ashford, B. A. Perkins, S. Ostroff, J. Hughes, D. Fleming, J. P. Koplan, and J. L. Gerberding. 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lininger, L. A., D. R. Bienek, L. G. Simonson, J. C. Ragain, V. A. Semenova, A. E. Freeman, P. P. Wilkins, and C. P. Quinn. 2003. Presented at the 5th International Conference on Anthrax, 30 March to 3 April 2003, Nice, France.
- 16.Mazzuchi, J. F., R. G. Claypool, K. C. Hyams, D. Trump, J. Riddle, R. E. Patterson, and S. Bailey. 2000. Protecting the health of U.S. military forces: a national obligation. Aviat. Space Environ. Med. 71:260-265. [PubMed] [Google Scholar]
- 17.National Academy of Sciences. 2002. The anthrax vaccine: is it safe? Does it work? National Academies Press, Washington, D.C. [PubMed]
- 18.Pitt, M. L., S. F. Little, B. E. Ivins, P. Fellows, J. Barth, J. Hewetson, P. Gibbs, M. Dertzbaugh, and A. M. Friedlander. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768-4773. [DOI] [PubMed] [Google Scholar]
- 19.Pittman, P. R., G. Kim-Ahn, D. Y. Pifat, K. Coonan, P. Gibbs, S. Little, J. G. Pace-Templeton, R. Myers, G. W. Parker, and A. M. Friedlander. 2002. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine 20:1412-1420. [DOI] [PubMed] [Google Scholar]
- 20.Quinn, C. P., P. M. Dull, V. Semenova, H. Li, S. Crotty, T. H. Taylor, E. Steward-Clark, K. L. Stamey, D. S. Schmidt, K. W. Stinson, A. E. Freeman, C. M. Elie, S. K. Martin, C. Greene, R. D. Aubert, J. Glidewell, B. A. Perkins, R. Ahmed, and D. S. Stephens. 2004. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J. Infect. Dis. 190:1228-1236. [DOI] [PubMed] [Google Scholar]
- 21.Quinn, C. P., V. A. Semenova, C. M. Elie, S. Romero-Steiner, C. Greene, H. Li, K. Stamey, E. Steward-Clark, D. S. Schmidt, E. Mothershed, J. Pruckler, S. Schwartz, R. F. Benson, L. O. Helsel, P. F. Holder, S. E. Johnson, M. Kellum, T. Messmer, W. L. Thacker, L. Besser, B. D. Plikaytis, T. H. Taylor, Jr., A. E. Freeman, K. J. Wallace, P. Dull, J. Sejvar, E. Bruce, R. Moreno, A. Schuchat, J. R. Lingappa, S. K. Martin, J. Walls, M. Bronsdon, G. M. Carlone, M. Bajani-Ari, D. A. Ashford, D. S. Stephens, and B. A. Perkins. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reuveny, S., M. D. White, Y. Y. Adar, Y. Kafri, Z. Altboum, Y. Gozes, D. Kobiler, A. Shafferman, and B. Velan. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riedel, S. 2005. Anthrax: a continuing concern in the era of bioterrorism. Proc. (Bayl. Univ. Med. Cent.) 18:234-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semenova, V. A., E. Steward-Clark, K. L. Stamey, T. H. Taylor, Jr., D. S. Schmidt, S. K. Martin, N. Marano, and C. P. Quinn. 2004. Mass value assignment of total and subclass immunoglobulin G in a human standard anthrax reference serum. Clin. Diagn. Lab. Immunol. 11:919-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever, J. L., A. I. Brenner, A. D. Gale, J. M. Lyle, L. H. Moulton, B. J. Ward, and D. J. West. 2004. Safety of anthrax vaccine: an expanded review and evaluation of adverse events reported to the Vaccine Adverse Event Reporting System (VAERS). Pharmacoepidemiol. Drug Saf. 13:825-840. [DOI] [PubMed] [Google Scholar]
- 26.Shafazand, S., R. Doyle, S. Ruoss, A. Weinacker, and T. A. Raffin. 1999. Inhalational anthrax: epidemiology, diagnosis, and management. Chest 116:1369-1376. [DOI] [PubMed] [Google Scholar]
- 27.Swartz, M. N. 2001. Recognition and management of anthrax—an update. N. Engl. J. Med. 345:1621-1626. [DOI] [PubMed] [Google Scholar]
- 28.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533-539. [DOI] [PubMed] [Google Scholar]
- 29.Welkos, S., S. Little, A. Friedlander, D. Fritz, and P. Fellows. 2001. The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores. Microbiology 147:1677-1685. [DOI] [PubMed] [Google Scholar]
- 30.Williamson, E. D., I. Hodgson, N. J. Walker, A. W. Topping, M. G. Duchars, J. M. Mott, J. Estep, C. Lebutt, H. C. Flick-Smith, H. E. Jones, H. Li, and C. P. Quinn. 2005. Immunogenicity of recombinant protective antigen and efficacy against aerosol challenge with anthrax. Infect. Immun. 73:5978-5987. [DOI] [PMC free article] [PubMed] [Google Scholar]