Abstract
Eosinophil activity in vivo and in vitro was studied in relation to infection intensities and plasma cytokine profiles of 51 Schistosoma mansoni-infected Ugandan fishermen before treatment and 24 h and 3 weeks posttreatment. Blood eosinophil numbers significantly declined 24 h posttreatment, but significant eosinophilia had developed by 3 weeks posttreatment. Cellular eosinophil cationic protein (ECP) content increased significantly during the transient eosinopenia but was significantly reduced 3 weeks later. No similar reduction in cellular eosinophil protein X (EPX) content was seen. Before treatment, S. mansoni infection intensity was positively correlated with 24-h boosts in plasma interleukin-5 (IL-5) and IL-6 levels, which were in turn negatively correlated with the posttreatment fall in eosinophil numbers. Significant correlations were observed between pretreatment infection intensities and plasma IL-10 and eotaxin levels. Treatment induced significant fluctuations in plasma IL-5, IL-6, IL-10, tumor necrosis factor alpha (TNF-α), and eotaxin levels. Optimal relative release of ECP and EPX in vitro was detected in S. mansoni soluble egg antigen-stimulated cultures during transient eosinopenia. Our data suggest that blood eosinophils are activated during S. mansoni infection and that treatment induces a burst in released antigens, causing increased production of IL-5, IL-6, IL-10, and eotaxin; a drop in TNF-α levels; and a transient sequestration of eosinophils, which leaves fewer degranulated eosinophils in the circulation 24 h posttreatment, followed by the development of eosinophilia 3 weeks later. During these events, it appears that preferential release of ECP occurs in vivo. Moreover, it is possible that infection intensity-dependent levels of plasma IL-10 may be involved in the prevention of treatment-induced anaphylactic reactions.
The association between helminth infections and eosinophilia in the blood and tissues of the host has been known for more than a century and is well documented (8, 14, 60). Helminth-induced eosinophilia appears to be most pronounced during acute infections with tissue-migrating larvae or following the sudden release of antigens from parasites dying either spontaneously or following chemotherapy (8). The exact role of eosinophils in host protection is still debated, but they seem primarily to be potent effector cells involved in the defense against infective larval stages of parasitic helminths, whereas intact adult worms appear to be resistant to eosinophil attack (44). Human protective immunity to schistosome infection is acquired very slowly and is associated with a Th2-skewed immune response with high levels of worm-specific immunoglobulin E (IgE) and eosinophilia (22, 28, 29, 30). In infected populations living in areas where schistosomiasis is endemic, the intensity of infection peaks among older children and declines towards lower levels in adults (38), even in situations where adults have greater exposure to infection than their children (32). The slow development of acquired immunity may be associated with the intensity of parasite transmission (69) or age-dependent immune competence (67), or it may be induced only by antigens that are not exposed to the immune system until the long-living adult worms die (72). This is supported by previous studies of Zimbabwean children and Kenyan car washers that indicated that treatment of Schistosoma mansoni infections may speed up the process of worm antigen exposure and may thus have a “vaccine or immunizing effect” that renders people more resistant to reinfection (33, 46). This is supported by data showing that treatment increases the level of worm-specific IgE (22, 70, 71).
One mechanism by which adult schistosomes avoid the immune recognition system is by coating their outer tegument surface with host antigens such as immunoglobulins, complement components, blood- and tissue-type antigens, and β2 microglobulin (13, 25, 59, 63). As the worm dies, either naturally or following chemotherapy, this defense mechanism breaks down as cells of the immune system, eosinophils in particular, rapidly adhere to the parasite and begin to degrade it (45). Eosinophils kill the parasite's schistosomula larval stage in vitro by complement- and antibody-dependent cytotoxicity (7), and eosinophils from S. mansoni-infected donors are more efficient in damaging schistosomula than normal nonactivated eosinophils (66). From cell-free in vitro experiments, it is known that the two eosinophil-derived cationic granule proteins, major basic protein and eosinophil cationic protein (ECP), are particularly involved in this cytotoxic activity (9, 43).
Treatment of human schistosomiasis rapidly induces eosinophilia (36, 37, 48) and therefore provides an opportunity to study the development of eosinophilia in relation to other immunological events induced by the release of worm antigens after treatment and the mechanisms that prevent systemic posttreatment hypersensitivity reactions, which might be expected to occur in the presence of high levels of circulating worm-specific IgE.
We previously reported that treatment induced significant changes in circulating eosinophil numbers in S. mansoni-infected Ugandan fishermen (24). In the present study, we examine in detail the eosinophil activity before treatment and 24 h and 3 weeks posttreatment in vivo and in vitro in a subgroup of these fishermen in relation to plasma cytokine levels and infection intensities. In vivo eosinophil activity was estimated from blood eosinophil counts and from cellular content and plasma levels of the two granule proteins, ECP and eosinophil protein X (EPX)/eosinophil-derived neurotoxin. Eosinophil activity in vitro was estimated by ECP and EPX release in antigen-stimulated whole blood cultures. We also examined the role of plasma cytokines in treatment-induced eosinophil responses and protection against hypersensitivity reactions.
MATERIALS AND METHODS
Study population and study design.
The study was conducted in Bugoigo, a fishing community on the eastern shore of Lake Albert, Masindi District, in north western Uganda. The population consisted of approximately 3,000 inhabitants, with the majority belonging to the Alur and Mugungu tribes. Fishing was the only economic activity, and the men in particular were heavily exposed to S. mansoni infection.
A study cohort of 69 S. mansoni-infected adult males (mean age, 34.5 years; range, 18 to 45 years) who had lived in Bugoigo for at least 3 years was randomly selected after initial parasitological screening of the entire population. Parasitological examination was based on three stool samples per individual, with two 50-mg Kato thick smears per sample (34). The mean pretreatment egg count for the selected cohort was 225 eggs per gram of feces (epg) (range, 3 to 1,340 epg). All members of the selected cohort gave informed consent to participate in the study. We focus on 51 members of the cohort who donated blood at all three examination time points.
Blood collection and treatment with praziquantel.
Blood samples (30 ml) were collected using heparinized syringes (10 U/ml heparin Na salt; Sigma, United Kingdom) by venipuncture on three occasions, designated bleed A (at baseline, immediately before treatment with praziquantel [40 mg/kg body weight]), bleed B (24 h after treatment), and, finally, bleed C (3 weeks after treatment). One-milliliter aliquots of blood were immediately transferred into EDTA-containing tubes to prevent Ca2+-dependent activation and granule protein release from eosinophils (55) and processed for eosinophil counts, whole blood extracts of ECP and EPX, and plasma ECP and EPX measurements within 1 h.
Blood eosinophil count.
Blood eosinophil counts were performed using single-use counting chambers (Fast Read; ISL Immune System Ltd., Paignton, United Kingdom) after dilution of the blood 10 times in eosin counting fluid (0.1% eosin Y, 0.3% sodium citrate, and 19% acetone in distilled H2O).
Extraction of ECP and EPX from whole blood.
Whole blood was diluted 1:10 with extraction buffer (1% N-cetyl-N,N,N-trimethylammonium bromide) in 0.15 M NaCl, mixed vigorously, left at room temperature for 1 h, mixed again, and frozen at −20°C. Following thawing, the cell extracts were centrifuged (3,000 × g for 10 min), and the supernatants containing the extracted proteins were used for ECP and EPX determinations. The remaining EDTA-treated heparinized blood was centrifuged (2,000 × g, 10 min), and the plasma was recovered and frozen.
Whole blood cultures.
Whole blood cultures were set up using heparinized blood diluted 1:6 (final dilution) in RPMI 1640 medium (Sigma, United Kingdom) supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), and l-glutamine (2 mM). Diluted blood was dispensed into 48-well plates (flat-bottomed wells; Costar, United Kingdom) and stimulated with soluble worm antigen (SWA) or soluble egg antigen (SEA) (final concentration, 10 μg/ml) or incubated with medium alone. Antigens were prepared as previously described (24, 31). All cultures were set up in duplicate wells, and the plates were incubated at 37°C in sealed boxes after the addition of a “gas-generating kit” (Oxoid Ltd., United Kingdom), providing a 5% CO2 atmosphere. Culture supernatants were harvested after 4, 24, and 96 h of incubation and immediately frozen at −20°C. The supernatants were virally inactivated by incubation for 2 h in the presence of 0.3% tri-(n-butyl)-phosphate (Sigma, United Kingdom) and 1% Tween 80 (Sigma, United Kingdom) before being either assayed for cytokines and eosinophil-derived granule proteins or stored at −70°C until used for protein determination. The assays were not affected by the viral inactivation procedure (data not shown).
Measurements of ECP and EPX.
Levels of ECP and EPX in whole blood extracts, plasma, and culture supernatants were measured by two enzyme-linked immunosorbent assay (ELISA) techniques described in detail previously (54, 56). Both assays are based on a polyclonal sandwich-type ELISA with a biotin-avidin-peroxidase amplification step and measure ECP and EPX in the ranges of 15 to 1,000 pg/ml and 30 to 2,000 pg/ml, respectively. ECP and EPX purified from extracts of human blood eosinophils were used as standards. Before measurement, the standards and test samples were diluted in sample buffer (0.1% Tween 20, 0.1% N-cetyl-N,N,N-trimethylammonium bromide, 20 mM EDTA, 0.2% human serum albumin, and 0.1% NaN3 in phosphate-buffered saline [pH 7.4]).
Cytokine assays.
Interleukin-4 (IL-4), IL-5, IL-6, eotaxin, RANTES, tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), IL-10, IL-13, and gamma interferon (IFN-γ) were measured using an in-house sandwich ELISA as previously described in detail (24, 31). The standard operating procedure for the IL-10 assay, however, was slightly modified compared to the previously reported standard operating procedure, as subsequent studies and retesting of plasma from the same study population indicated that heterophilic antibody activity in a small number of subjects was a source of inaccuracy in the IL-10 determinations. To block this activity, we preincubated the plasma samples with 10% complement-inactivated animal serum consisting of an equal mixture of mouse, rat, goat, and fetal bovine sera. The paired monoclonal antibodies (Abs) (capture Ab and biotinylated detection Ab) used for the assays were as follows: IL-4 (8D4-8 and MP4-25D2; Pharmingen), IL-5 (TRFK5 and JES1-5A10; Pharmingen), IL-6 (MQ2-13AS and MQ2/39C3; Pharmingen), eotaxin (43911.11 and polyclonal goat [catalog no. BAF320]; R&D Systems), RANTES (polyclonal rabbit [catalog no. 551343] and polyclonal rabbit [catalog no. 554678]; Pharmingen), TNF-α (Mab1 and Mab11; Pharmingen), IL-13 (JES10-5A2 and B69-2; Pharmingen), IFN-γ (N1B42 and 4S.B3; Pharmingen), and IL-10 (JES3-9D7 and JES3-12G8). In the TGF-β assay, Ab clone 9016 (R&D Systems) was used for capture, and a polyclonal chicken Ab was used for detection.
Standards.
Recombinant IL-4, IL-5, IL-6, RANTES, and IL-10 were obtained from Pharmingen. Recombinant eotaxin and IL-13 were obtained from R&D Systems. Recombinant IFN-γ and TGF-β were obtained from Genzyme.
Statistical analysis.
Nonparametric statistical tests were used. The Wilcoxon rank-sum test and the Friedman test were used for paired observations, and the Mann-Whitney rank-sum test was used for unpaired observations. The coefficient of correlation was calculated as Spearman's ρ. All calculations were carried out using SPSS package software (Jan Dell Scientific, San Rafael, CA). A P value of <0.05 was considered significant.
Ethical considerations.
At the completion of this study, the entire Bugoigo community was treated with 40 mg/kg body weight praziquantel. Informed consent was obtained from all those who participated in this study, in line with the national guidelines of the Ugandan Ministry of Health, whose ethical review committees approved all the protocols used.
RESULTS
Blood eosinophil counts and cellular content of ECP and EPX.
Blood eosinophil counts differed significantly between the three time points (P = 1.003 × 10−9; n = 51). Before treatment, the median blood eosinophil count was 0.37 × 106 eosinophils/ml (range, 0.05 × 106 to 1.13 × 106 eosinophils/ml). Twenty-four hours posttreatment, there was a small but significant decline in eosinophil counts from pretreatment levels to 0.34 × 106 eosinophils/ml (range, 0.05 × 106 to 1.47 × 106 eosinophils/ml) (P = 0.04; n = 51), and this was followed by a highly significant increase to 0.75 × 106 eosinophils/ml (range, 0.04 × 106 to 4.49 × 106 eosinophils/ml) 3 weeks posttreatment (P = 1.5 × 10−6) (Fig. 1A). Although the eosinophil counts fluctuated significantly, they intercorrelated between the three time points (for pretreatment eosinophil counts versus eosinophil counts at 24 h and 3 weeks posttreatment, ρ = 0.65 and 0.45, respectively [P ≤ 5 × 10−7 for both]; for eosinophil counts at 24 h posttreatment versus 3 weeks posttreatment, ρ = 0.51 [P = 1.0 × 10−6]).
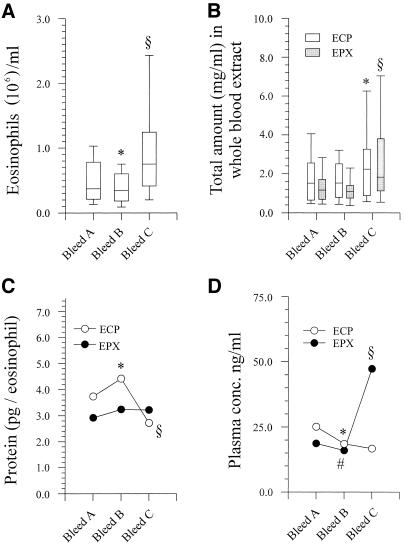
FIG. 1.
Pre- and posttreatment blood eosinophil counts, total ECP counts, and total EPX levels in whole blood extracts; cellular content of ECP and EPX; and plasma ECP and EPX levels. The boxes in panels A and B represent the 25th, 50th, and 75th percentile ranges, and the error bars show the ranges of 10th and 90th percentiles. (A) Blood eosinophil counts. *, P = 0.04; §, P = 1.5 × 10−6. (B) Total ECP and EPX counts in whole blood extracts. *, P ≤ 1.0 × 10−12; §, P = 1.0 × 10−8. (C) Cellular content of ECP and EPX (medians). *, P = 0.005; §, P = 0.002. No significant fluctuations in the cellular content of EPX were seen. (D) Plasma ECP and EPX levels (medians). *, P = 0.001; #, P = 0.019; §, P ≤ 0.027.
The total amounts of ECP and EPX extracted from whole blood also increased significantly 3 weeks posttreatment, with a P value of ≤1 × 10−12 for total ECP and a P value of 1 × 10−8 for total EPX (Fig. 1B). There was no change in total amounts of the two granule proteins 24 h posttreatment, despite a significant decline in eosinophil levels in blood. Consequently, the amounts of ECP and EPX per eosinophilic granulocyte differed between the three time points. Twenty-four hours posttreatment, the cellular ECP content increased from 3.74 pg/eosinophil to 4.40 pg/eosinophil (P = 0.005; n = 51), and by 3 weeks posttreatment, the ECP content had declined to 2.72 pg/eosinophil, which differed significantly from the cellular content in samples collected both pretreatment and 24 h posttreatment (P ≤ 0.002 for both). There was a small, nonsignificant increase in cellular EPX content in the samples from 24 h and 3 weeks posttreatment (P = 0.12) (Fig. 1C).
Plasma levels of ECP and EPX changed significantly during the study period but not in parallel. Between pretreatment and 24 h posttreatment, both plasma ECP and plasma EPX levels dropped significantly (P = 0.001 and P = 0.019, respectively). No further changes were seen in plasma ECP levels at 3 weeks; in contrast, there was a significant increase in plasma EPX levels compared with those at both pretreatment and 24 h posttreatment (P ≤ 0.027) (Fig. 1D). Plasma ECP and plasma EPX levels correlated with the corresponding blood eosinophil counts at all time points (0.496 ≤ ρ ≤ 0.734 [P ≤ 0.006 for all comparisons]). Despite the differences in cellular contents of granule proteins at the three time points, there were significant correlations between eosinophil counts and extracted granule proteins at all time points (data not shown).
Inverse relationship between eosinophil counts and cellular ECP and EPX content.
Negative interindividual correlations between eosinophil counts and cellular content of both ECP and EPX were seen at all study points (for eosinophil counts versus ECP/eosinophil and EPX/eosinophil pretreatment samples, ρ = −0.333/−0.459 [P = 0.017/0.001]; for samples obtained 24 h posttreatment, ρ = −0.496/−636 [P = 0.0001/0.0001]; for samples obtained 3 weeks posttreatment, ρ = −0.307/−0.458 [P = 0.028/0.001]). This negative association was also seen for individual donors when the changes in eosinophil counts between time points were correlated to the changes in cellular ECP and EPX content (data not shown).
ECP and EPX release in vitro following stimulation with SEA, SWA, or medium.
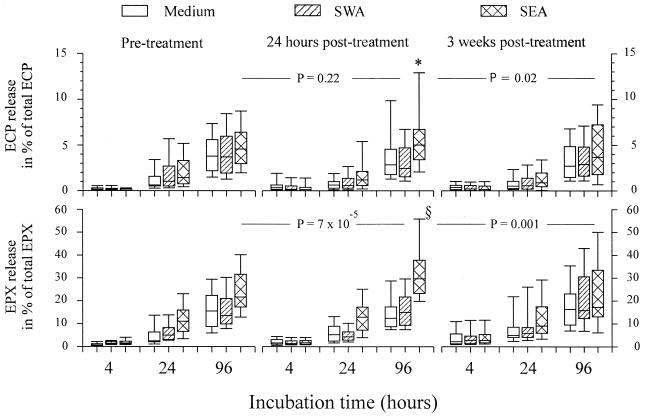
Release of ECP and EPX in whole blood cultures stimulated with SEA, SWA, or medium alone was monitored. A significant time-dependent release of ECP and EPX was seen in cultured pretreatment blood samples and in posttreatment sample cultures both with and without antigen stimulation (P ≤ 2.1 × 10−15 for all).
The highest levels of EPX were released in the 96-h cultures of the samples obtained 3 weeks posttreatment, as could be expected from the higher number of eosinophils and the larger amount of total EPX in blood samples at this time point. However, comparable amounts of ECP were released by cultured blood samples from the various pre- and posttreatment time points despite the observed lower eosinophil numbers and total blood ECP levels at the earlier time points than at 3 weeks posttreatment, suggesting that the release of ECP from samples obtained 3 weeks posttreatment was impaired. Normalization of the data, i.e., expressing the amounts of ECP and EPX released as percentages of total blood ECP and EPX levels, modified this overall release pattern between study time points and showed that the greatest relative release of both ECP and EPX was from SEA-stimulated 96-h cultures of blood samples obtained 24 h posttreatment (Fig. 2). SEA stimulation of these samples obtained 24 h posttreatment released 5.00% of the total ECP compared to 2.83% when the samples were stimulated with medium only and 2.4% when the samples were stimulated with SWA (P = 2.1 × 10−7; Friedman test) and compared to 4.5% and 3.6% with SEA stimulation of 96-h cultures from pretreatment samples and samples obtained 3 weeks posttreatment (P = 0.0095). EPX release reached 29.7% in SEA-stimulated cultures compared to 12.4% and 14.49% with medium only and SWA-stimulated cultures (P = 4.1 × 10−16; Friedman test) and compared to 21.6% and 17.2% in SEA-stimulated 96-h cultures from pretreatment samples and samples obtained 3 weeks posttreatment (P = 9.02 × 10−5). In general, no significant differences between levels of release in cultures stimulated with either SWA or medium only were seen.
FIG. 2.
Relative release in vitro of ECP and EPX in whole blood cultures stimulated with SWA, SEA, or medium alone. The relative release is calculated as the released amount of ECP and EPX as a percentage of the total content of ECP or EPX extracted from whole blood before the cultures were set up. The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars illustrate the ranges of the 10th and 90th percentiles. Significant time-dependent release of ECP and EPX was seen in all cultures (P ≤ 2.2 × 10−15 for all). Optimal release of both ECP and EPX was seen in SEA-stimulated cultures from bleed B after 96 h of incubation. For optimal ECP release, the P values were 3.8 × 10−6 and 1.6 × 10−7 (*), respectively, compared to 96-h cultures stimulated with SWA or medium alone. For optimal EPX release, P values were 7.34 × 10−10 and 5.46 × 10−10 (§), compared to 96-h cultures from bleed B stimulated with SWA or medium alone. Levels of significance compared to SEA-stimulated 96-h cultures from bleed A and bleed C are indicated.
Associations between blood eosinophil counts, pretreatment intensity of infection, and plasma cytokines.
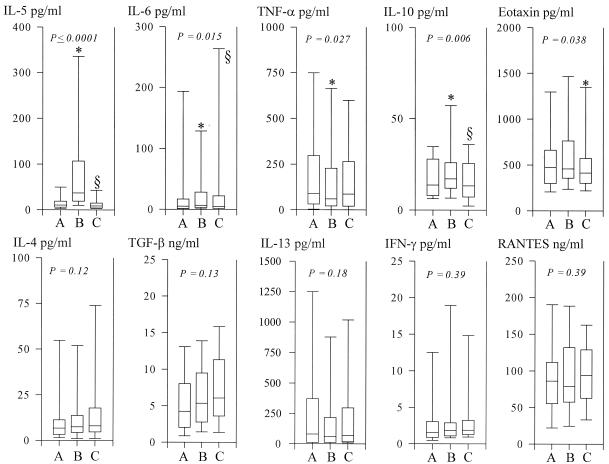
Treatment induced significant fluctuations in both eosinophil numbers and plasma cytokine levels. No associations between pretreatment intensities of infection and eosinophil counts in blood were observed. However, a clear trend between intensity of infection and the change in eosinophil count 24 h posttreatment was seen (epg versus eosinophil count for bleed B/eosinophil count for bleed A) (ρ = −0.276; P = 0.052). Significant posttreatment fluctuations were observed in plasma IL-5, IL-6, IL-10, eotaxin-1, and TNF-α levels. Plasma levels of IL-4 and TGF-β increased slowly after treatment, but the increase did not reach significance. No trend or significant fluctuations were seen in plasma levels of IL-13, IFN-γ, and RANTES (Fig. 3).
FIG. 3.
Plasma cytokine levels before treatment (bleed A) and 24 h (bleed B) and 3 weeks (bleed C) after treatment. The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars illustrate the ranges of the 10th and 90th percentiles. Significant fluctuations as tested by the Friedman ρ test (levels of significance are indicated in the figures) were seen in IL-5, IL-6, TNF-α, IL-10, and eotaxin-1 levels. No significant fluctuations were seen in IL-4, TGF-β, IL-13, IFN-γ, or RANTES levels. For IL-5, a significant increase (bleed A versus bleed B) followed by a significant decline (bleed B versus bleed C) (* and §, P ≤ 5.0 × 10−6) was seen; in addition, a significant decline (P = 0.02) was seen when bleed A was compared with bleed C. For IL-6, a nonsignificant increase (*, P = 0.063), followed by a significant decline (bleed B versus bleed C) (§, P = 0.016), was seen. For TNF-α, a significant decline (*, P = 0.001) was seen. For IL-10, a significant increase (*, P = 0.025) followed by a significant decline (bleed B versus bleed C) (§, P = 0.033) was seen. For eotaxin, a significant decline (bleed B versus bleed C) (*, P = 0.026) was seen.
No direct association between eosinophil counts and cytokine levels in the corresponding plasma samples was found, but the eosinophil counts 24 h posttreatment showed significant negative correlations with the relative change in plasma levels of IL-5, IL-6, and eotaxin-1 from pretreatment to 24 h posttreatment (ρ = −0.344 and P = 0.015, ρ = −0.338 and P = 0.021, and ρ = −0.282 and P = 0.045, respectively). These associations became stronger if the relative changes in eosinophil count, instead of actual counts, were considered and compared to the relative change ratio in the cytokines (for IL-5, ρ = −0.549 and P < 0.0001; for IL-6, ρ = −0.412 and P = 0.004; for eotaxin-1, ρ = −0.314 and P = 0.025).
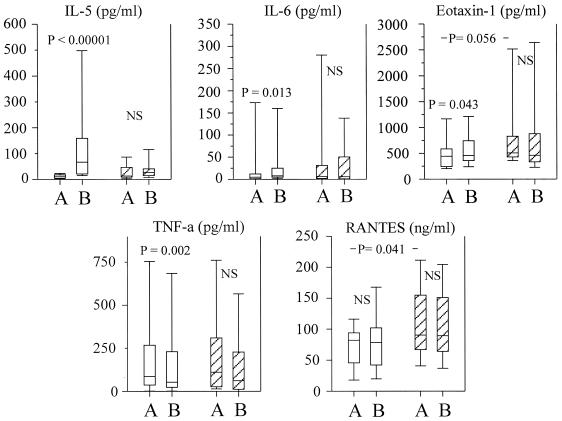
A simple partition of the cohort based on the eosinophil response after treatment was made. Those patients who responded to treatment with a significant decline in eosinophil counts were designated “high-eosinophil responders,” and those who showed nothing or a minor increase were designated “low-eosinophil responders.” Pretreatment eosinophil counts differed significantly between the two groups. The high-eosinophil responders had median eosinophil counts of 0.55 × 106 eosinophils/ml (range, 0.07 × 106 to 1.09 × 106 eosinophils/ml), and the low-eosinophil responders had eosinophil counts of 0.23 × 106 eosinophils/ml (range, 0.05 × 106 to 1.113 × 106 eosinophils/ml) (P = 0.016). There were no significant differences in either intensity of infection or plasma cytokine levels between the two groups before treatment, except for RANTES, for which there was a small but significantly higher level in the low-responder group (P = 0.041) and a nonsignificant but increased level of eotaxin (P = 0.056) (Fig. 4). However, at 24 h posttreatment, there were statistically significant changes in the high responders, in which the decline in blood eosinophil numbers was associated with significant increases in plasma levels of IL-5, eotaxin, and IL-6 and a decline in TNF (Fig. 4). The levels of these cytokines did not significantly change in the low responders, and no significant change in RANTES was seen in any of the groups. The small but significant posttreatment increase in IL-10 in the whole cohort was not significant in either subgroup.
FIG. 4.
Plasma cytokine levels before treatment (bleed A) and 24 h posttreatment (bleed B) in high-eosinophil responders (n = 33; open boxes) and low-eosinophil responders (n = 18; hatched boxes). The boxes represent the 25th, 50th, and 75th percentile ranges, and the error bars show the ranges of the 10th and 90th percentiles.
The plasma IL-10 levels were related to the pretreatment intensity of infection both before treatment (ρ = 0.431; P = 0.002) and 24 h posttreatment after a small (ρ = 0.304; P = 0.032) but significant increase in IL-10. Significant correlations between infection intensities and plasma eotaxin levels were found at all three time points (ρ = 0.315 and P = 0.029, ρ = 0.366 and P = 0.009, and ρ = 0.314 and P = 0.034, respectively). There was no significant association between the infection intensity and IL-10 levels at 3 weeks posttreatment, when a significant decline in plasma IL-10 levels had occurred. The correlation with eotaxin was still significant at 3 weeks posttreatment, despite a significant decline in levels of eotaxin in plasma.
IL-5 and IL-6 levels in plasma appeared to be related to the treatment-induced, infection intensity-dependent antigen release after treatment, as pretreatment intensities of infection correlated significantly with both plasma IL-5 and IL-6 levels 24 h posttreatment (ρ = 0.631 and P < 0.0001 and ρ = 0.318 and P = 0.024, respectively) and the transient relative increase in levels of IL-5 and IL-6 in plasma 24 h posttreatment, compared to the pretreatment levels of these cytokines (for IL-5, ρ = 0.392 and P = 0.005; for IL-6, ρ = 0.317 and P = 0.034).
DISCUSSION
The relationship between eosinophil activity, plasma cytokines, and infection intensities during treatment was examined in S. mansoni-infected Ugandans. The in vivo eosinophil activity was estimated from blood eosinophil counts, cellular ECP and EPX content, and plasma ECP and EPX levels. In vitro eosinophil activity was measured by ECP and EPX release in whole blood cultures. Whole blood assays are feasible under field conditions and allow for direct and indirect interactions between different cell populations. Thus, these assays possibly reflect in vivo activity more closely than assays based on purified cell populations.
Twenty-four hours after treatment, a significant and infection intensity-dependent decline in eosinophil number was followed by significant eosinophilia by 3 weeks posttreatment. A transient infection intensity-dependent decline in peripheral blood eosinophil numbers shortly after treatment suggests that this immediate eosinophil response is dependent on the dose of worm antigen released by chemotherapy. Similar treatment-induced eosinophil responses have previously been reported for filarial worm infections (1, 16, 18, 26, 41), but this is the first report of a reduction in circulating eosinophils in response to treatment of schistosomiasis. In contrast, the development of posttreatment eosinophilia has been reported for S. mansoni (36, 37, 48), Wuchereria bancrofti, and Onchocerca volvolus infections (16, 18, 26, 27, 42, 48). Interestingly, the eosinophil response to bronchial allergen challenge (5, 15, 20) is very similar to the response induced by treatment of worm infections described above and thus appears to be a ubiquitous eosinophil response when sensitized individuals with Th2-skewed immune responses are challenged with specific antigens.
Eosinophil counts correlated significantly with extracted ECP and EPX at each time point, but significant fluctuations in ECP/eosinophil levels were seen. A significant increase in ECP/cell levels occurred during the transient eosinopenia, followed by a significant decline during eosinophilia 3 weeks posttreatment. EPX/cell levels also tended to increase at 24 h posttreatment, but no decrease in EPX/cell levels occurred at 3 weeks posttreatment. Plasma ECP and EPX levels correlated with their corresponding eosinophil counts at all time points but fluctuated differently. The decline in eosinophil counts was paralleled by reduced plasma ECP and EPX levels. However, eosinophilia at 3 weeks posttreatment was accompanied by an increase in plasma EPX levels only, as plasma ECP and cellular ECP content declined. These differences in cellular contents of ECP and EPX in vivo and in changing plasma ECP and EPX levels support previous reports of selective or preferential release of these proteins in vitro (35, 52, 57). Blood eosinophil counts, cellular EPX content, and serum or plasma EPX levels correlated strongly in this study and in other studies. It has been reported previously that neutrophils may produce minute amounts of EPX (62); thus, the contribution of a small amount of neutrophil-derived EPX to these data cannot be ruled out completely. The negative relationships between eosinophil counts, changes in eosinophil counts, and cellular ECP and EPX content are similar to previous observations of diseases associated with eosinophilia and increased eosinophil activation, such as hypereosinophil syndrome and asthma (11, 40).
Eosinophils from patients with blood eosinophilia, including those with schistosomiasis, are characterized as being hypodense, with reduced ECP and EPX content; as being significantly more helminthotoxic or cytotoxic; or as having a higher relative level of ECP and EPX release than eosinophils from normal individuals (11, 21, 53, 64, 65). Antigen provocation of sensitized individuals induces both an increased number of eosinophils and a further reduction in cellular density (39). In S. mansoni-infected patients, enhanced eosinophil activity correlated with the infection intensity (64, 65), and in patients who are allergic to pollen, eosinophils released relatively more ECP and EPX in vitro when stimulated during the pollen season than they did out of season (10). This implies that eosinophilia involves an increase in eosinophil numbers and the up-regulation of functional capacities. Our data support the observation that eosinophils are activated during chronic infections, as there was little or no difference in ECP and EPX release in vitro with SWA stimulation compared to medium-only culture, suggesting that further activation does not occur in vitro, despite the fact that SWA stimulates the in vitro release of cytokines with potent eosinophil-activating activities, including IL-3, granulocyte-macrophage colony-stimulating factor, IL-5, and RANTES (24; unpublished observations). The highest relative release was seen in SEA-stimulated cultures. This can probably be ascribed to a direct secretagogue effect of egg-derived, eosinophil-activating factors described previously for S. mansoni and Schistosoma japonicum eggs (49). Surprisingly, the optimal relative release of ECP and EPX was seen 24 h posttreatment, during transient eosinopenia, with a relatively low level of release accompanying the later eosinophilia. Thus, in this case, the association between eosinophilia and up-regulation of functional activity is not seen. This disagreement could be due to the timing of the posttreatment sampling. Following bronchial allergen provocation, peripheral eosinophil counts decline rapidly, reaching nadir within 4 to 5 h (20), and in those who develop a late asthmatic response, blood eosinophilia develops after 24 h (20, 23, 51). In filarial infections, a decline in circulating eosinophil numbers occurs within a few hours, reaching nadir at 8 h posttreatment, followed by peak blood eosinophilia between 6 and 14 days posttreatment (1, 26, 41, 42). Considering the rapid action of praziquantel (12), the 24-h posttreatment time point may have missed nadir by at least 12 h. Therefore, if eosinophil counts in blood were in fact increasing when sampled, the eosinophil population could have been dominated by fewer degranulated cells newly released from the bone marrow. Similarly, at 3 weeks posttreatment, the peak in the blood eosinophil count may have passed, and the circulating eosinophil population may have been dominated by “exhausted,” degranulated eosinophils, as eosinophil counts returned to normal. The data on cellular ECP content, plasma ECP levels, and in vitro ECP release all support this notion. Thus, it is important to discriminate between functional capabilities of eosinophils collected during steady-state eosinophilia, with a constant turnover of eosinophils, and those collected during eosinophilia development or resolution. The idea that eosinophils in a resolving eosinophilia are exhausted may explain the observation made previously by Kimani and colleagues (36), who noted that by 3 weeks after treatment of S. mansoni infection, the in vitro cytotoxic capacity of eosinophils was significantly reduced, despite pronounced eosinophilia.
Pre- and posttreatment plasma IL-10 and eotaxin levels were significantly correlated to infection intensities before treatment, whereas IL-5 and IL-6 levels were related to the intensity-dependent antigen release after treatment. The link between infection intensity and the eosinophil response 24 h posttreatment appears to be the treatment-induced, intensity-dependent boost in IL-5 levels. In addition to its eosinophilopoetic activity, IL-5 has an eosinophil-activating capacity that includes the functional up-regulation of β2 integrins (57). Therefore, IL-5 may cause eosinophil sequestration and give a transient negative correlation between eosinophil counts and the boost in plasma IL-5 levels. A close association between plasma IL-5 levels and eosinophils following treatment of onchocerciasis and lymphatic filariasis (16, 41, 42), in which increased levels of IL-5 in plasma precede the peak eosinophilia, indicating the importance of IL-5 in production, maturation, and release of eosinophils from the bone marrow, has also been described (16, 26, 27, 41, 42). No significant associations between eosinophils and plasma IL-4, TNF, IFN-γ, IL-10, TGF-β, or IL-13 levels were found, despite some increases in posttreatment IL-4 and TGF-β levels.
Significant correlations were also seen between eosinophil counts and the 24-h boosts in eotaxin and IL-6 levels. Previously, Gopinath and colleagues (26) did not find similar correlations following filariasis treatment, and the eosinophil response may not be directly linked to the IL-6 boost, which may reflect a general systemic inflammatory reaction induced by released parasite antigens. However, the inverse eosinophil association with the eotaxin boost may be directly related to the eosinophil sequestration, as eotaxin is important in the activation and attraction/migration of eosinophils, and up-regulation of eotaxin has been seen in dermal vascular endothelial cells and mononuclear cells in O. volvolus-infected patients 24 h posttreatment (17, 50).
There were no differences in pretreatment plasma cytokine levels between the high- and low-eosinophil responders, except with regard to RANTES. However, only the high-eosinophil responder group had significant posttreatment increases in IL-5 and IL-6 and a decrease in TNF-α levels. In addition, a significant posttreatment increase in plasma eotaxin levels was observed when this subgroup was tested alone. The low-eosinophil responders, with no posttreatment decrease in blood eosinophils, had significantly higher pretreatment plasma RANTES levels than the high-eosinophil responders, suggesting an association between RANTES and the development of eosinophilia. Cooper et al. (17) have also previously reported evidence suggesting that high levels of RANTES in plasma were able to down-regulate eosinophil sequestration.
Severe allergic reactions to praziquantel treatment of schistosomiasis are not as frequent as they are following treatment of filariasis, in which such reactions are significantly associated with microfilaria density and fluctuations in circulating eosinophil numbers (4, 16, 61). Bronchial allergen provocation studies have also shown a close association between eosinophil counts and postchallenge eosinophil fluctuations and the development and magnitude of early and late asthmatic responses (15, 20, 68). It is not known why schistosomiasis treatment only rarely causes anaphylactic reactions, when parasite antigens are released into the bloodstream in the presence of high levels of specific IgE. Adverse posttreatment reactions did not occur in this study, despite the similarities between the eosinophil responses reported here and those reported after filariasis treatment or bronchial allergen challenge. This may imply that adverse reactions to filariasis treatment are not necessarily directly associated, as previously suggested, to the eosinophil response (1, 16) but may be due to other, coinciding mechanisms, perhaps involving mast cells (19).
IL-10 is a major anti-inflammatory and regulatory cytokine (3, 47). It inhibits in vitro mast cell degranulation (58), which can cause adverse reactions and allergic inflammation in vivo. In vivo expression of IL-10 can be low in allergic individuals, and increases in IL-10 levels after successful immunotherapy have been reported (2, 6). In the present study, high infection intensity-dependent pretreatment plasma IL-10 levels and the IL-10 boost at 24 h posttreatment are consistent with the hypothesis that severe adverse systemic reactions may be prevented by an anti-inflammatory network that includes IL-10. In addition, the small but significant decline in proinflammatory TNF and even the nonsignificant increase in TGF-β could be part of an anti-inflammatory response. However, any regulatory effect of IL-10 may not be directly acting on the eosinophil response.
The results from this study are in line with previous reports of the pre- and posttreatment eosinophil fluctuations and activity in filarial infections and have remarkable similarities with reports of events occurring 24 to 48 h after bronchoalveolar allergen challenge of patients with atopic asthma. Population-based treatment studies of worm infections provide an excellent model of in vivo regulation and activity of human eosinophils as well as other cell populations, including lymphocytes, neutrophils, and basophils, which are of importance in immunity to, and the pathogenesis of, helminth infections. Knowledge of the mechanisms regulating these cells may provide important information about immune reactions in parasite infection and may assist in vaccine development but may also be of the utmost importance in the battle against allergic and autoimmune diseases.
Acknowledgments
This work was supported by the British Medical Research Council, the Wellcome Trust, and the Commission of the European Community's Science and Technology for Development Programme (INCO-DC contract IC18 CT97-0237 and INCO-DEV contract ICA4-CT-1999-10003).
We gratefully acknowledge Timothy Kamau for carefully performing all of the phlebotomies, and we thank Susanne Kronborg, Maureen Laidlaw, and Karen Plant for their skillful technical assistance.
REFERENCES
- 1.Ackerman, S. J., G. M. Kephart, F. H. Francis, K. Awadzi, G. J. Geich, and E. A. Ottesen. 1990. Eosinophil degranulation. An immunologic determinant in the pathogenesis of the Mazzotti reaction in human onchocerciasis. J. Immunol. 144:3961-3969. [PubMed] [Google Scholar]
- 2.Akdis, C. A., T. Blesken, M. Akdis, B. Wuthrich, and K. Blaser. 1998. Role of interleukin 10 in specific immunotherapy. J. Clin. Investig. 102:98-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asadullah, K., W. Sterry, and H. D. Volk. 2003. Interleukin-10 therapy—review of a new approach. Pharmacol. Rev. 55:241-269. [DOI] [PubMed] [Google Scholar]
- 4.Berhe, N., S. G. Gundersen, F. Abebe, H. Birrie, G. Medhin, and T. Gemetchu. 1999. Praziquantel side effects and efficacy related to Schistosoma mansoni egg loads and morbidity in primary school children in north-east Ethiopia. Acta Trop. 72:53-63. [DOI] [PubMed] [Google Scholar]
- 5.Booij-Noord, H., K. de Vries, H. J. Sluiter, and N. G. M. Orie. 1972. Late bronchial obstructive reaction to experimental inhalation of house dust extract. Clin. Allergy 2:43-61. [DOI] [PubMed] [Google Scholar]
- 6.Borish, L., A. Aarons, J. Rumbyrt, P. Cvietusa, J. Negri, and S. Wenzel. 1996. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 97:1288-1296. [DOI] [PubMed] [Google Scholar]
- 7.Butterworth, A. E., R. Sturrock, V. Houba, A. A. F. Mahmoud, A. Sher, and P. H. Rees. 1975. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature 256:272-279. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth, A. E., and K. J. I. Thorne. 1993. Eosinophils and parasitic diseases, p. 119-150. In H. Smith and R. M. Cook (ed.), Immunopharmacology of eosinophils. Academic Press, London, United Kingdom.
- 9.Butterworth, A. E., M. A. Vadas, D. L. Wassom, A. Dessein, M. Hogan, B. Sherry, G. J. Gleich, and J. R. David. 1979. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible eosinophil adherence. J. Exp. Med. 150:1456-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson, M., L. Håkanson, M. Kämpe, G. Stålenheim, C. Peterson, and P. Venge. 1992. Degranulation of eosinophils from pollen-atopic patients with asthma is increased during pollen season. J. Allergy Clin. Immunol. 89:131-139. [DOI] [PubMed] [Google Scholar]
- 11.Carlson, M., G. Oberg, C. Peterson, and P. Venge. 1994. Releaseability of human hypereosinophilic eosinophils is related to the density of the cells. Br. J. Haematol. 86:41-47. [DOI] [PubMed] [Google Scholar]
- 12.Cioli, D., and L. Pica-Mattoccia. 2003. Praziquantel. Parasitol. Res. 90(Suppl. 1):3-9. [DOI] [PubMed] [Google Scholar]
- 13.Clegg, J. A., S. R. Smithers, and R. J. Terry. 1971. Acquisition of human antigens by Schistosoma mansoni during cultivation in vitro. Nature 232:653-654. [DOI] [PubMed] [Google Scholar]
- 14.Conrad, M. E. 1971. Hematologic manifestations of parasitic infections. Semin. Hematol. 8:267-303. [PubMed] [Google Scholar]
- 15.Cookson, W. O., C. F. Craddock, M. K. Benson, and S. R. Durham. 1989. Falls in peripheral eosinophil counts parallel the late asthmatic response. Am. Rev. Respir. Dis. 139:458-462. [DOI] [PubMed] [Google Scholar]
- 16.Cooper, P. J., K. Awadzi, E. A. Ottesen, D. Remick, and T. B. Nutman. 1999. Eosinophil sequestration and activation are associated with the onset and severity of systemic adverse reactions following the treatment of onchocerciasis with ivermectin. J. Infect. Dis. 179:738-742. [DOI] [PubMed] [Google Scholar]
- 17.Cooper, P. J., L. A. Beck, I. Espinel, N. M. Deyampert, A. Hartnell, P. J. Jos, W. Rardes, R. H. Guderian, and T. B. Nutman. 2000. Eotaxin and RANTES expression by dermal endothelium is associated with eosinophil infiltration after ivermectin treatment of onchocerciasis. Clin. Immunol. 95:51-61. [DOI] [PubMed] [Google Scholar]
- 18.Cooper, P. J., R. H. Guderian, D. Prakash, D. G. Remick, I. Espinel, T. B. Nutman, D. W. Taylor, and G. E. Griffin. 1996. RANTES in onchocerciasis: changes with ivermectin treatment. Clin. Exp. Immunol. 106:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper, P. J., L. B. Schwartz, A. M. Irani, K. Awadzi, R. H. Guderian, and T. B. Nutman. 2002. Association of transient dermal mastocytosis and development of adverse reactions after treatment of onchocerciasis with ivermectin. J. Infect. Dis. 186:1307-1313. [DOI] [PubMed] [Google Scholar]
- 20.Dahl, R., P. Venge, and I. Olsson. 1978. Variations of blood eosinophils and eosinophil cationic protein in serum in patients with bronchial asthma. Studies during inhalation challenge test. Allergy 33:211-215. [DOI] [PubMed] [Google Scholar]
- 21.David, J. R., M. A. Vadas, A. E. Butterworth, P. A. de Brito, E. M. Carvalho, R. A. David, J. C. Bina, and Z. A. Andrade. 1980. Enhanced helminthotoxic capacity of eosinophils from patients with eosinophilia. N. Engl. J. Med. 303:1147-1152. [DOI] [PubMed] [Google Scholar]
- 22.Dunne, D. W., A. E. Butterworth, A. J. C. Fulford, H. C. Kariuki, J. G. Langley, J. H. Ouma, A. Capron, R. J. Pierce, and R. F. Sturrock. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483-1494. [DOI] [PubMed] [Google Scholar]
- 23.Durham, S. R., and A. B. Kay. 1985. Eosinophils, bronchial hyperreactivity and late-phase asthmatic reactions. Clin. Allergy 15:411-418. [DOI] [PubMed] [Google Scholar]
- 24.Fitzsimmons, C. M., S. Joseph, F. M. Jones, C. M. Reimert, K. F. Hoffmann, F. Kazibwe, G. Kimani, J. K. Mwatha, J. H. Ouma, E. M. Tukahebwa, H. C. Kariuki, B. J. Vennervald, N. B. Kabatereine, and D. W. Dunne. 2004. Schistosomiasis chemotherapy in Ugandan fishermen: treatment can cause a rapid increase in plasma interleukin-5 levels but decreased levels of eosinophilia and worm-specific immunoglobulin E. Infect. Immun. 72:4023-4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldring, O. L., J. A. Clegg, S. R. Smithers, and R. J. Terry. 1976. Acquisition of human blood group antigens by Schistosoma mansoni. Clin. Exp. Immunol. 26:181-187. [PMC free article] [PubMed] [Google Scholar]
- 26.Gopinath, R., L. E. Hanna, V. Kumaraswami, V. Perumal, V. Kavitha, V. Vijayasekaran, and T. B. Nutman. 2000. Perturbations in eosinophil homeostasis following treatment of lymphatic filariasis. Infect. Immun. 68:93-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagan, J. B., K. R. Bartemes, H. Kita, E. A. Ottesen, K. Awadzi, T. B. Nutman, and G. J. Gleich. 1996. Elevations in granulocyte-macrophage colony-stimulating factor and interleukin-5 levels precede post-treatment eosinophilia in onchocerciasis. J. Infect. Dis. 173:1277-1280. [DOI] [PubMed] [Google Scholar]
- 28.Hagan, P., U. J. Blumenthal, M. Chaudri, B. M. Greenwood, R. J. Hayes, J. Hodgson, C. Kelly, M. Knight, A. J. G. Simpson, S. R. Smithers, and H. A. Wilkins. 1987. Resistance to reinfection with Schistosoma haematobium in Gambian children: analysis of their immune responses. Trans. R. Soc. Trop. Med. Hyg. 81:938-946. [DOI] [PubMed] [Google Scholar]
- 29.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 30.Hagan, P., H. A. Wilkins, U. J. Blumenthal, R. J. Hayes, and B. M. Greenwood. 1985. Eosinophilia and resistance to Schistosoma haematobium in man. Parasite Immunol. 7:625-632. [DOI] [PubMed] [Google Scholar]
- 31.Joseph, S., F. M. Jones, G. Kimani, J. K. Mwatha, T. Kamau, F. Kazibwe, J. Kemijumbi, N. B. Kabatereine, M. Booth, H. C. Kariuki, J. H. Ouma, B. J. Vennervald, and D. W. Dunne. 2004. Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect. Immun. 72:728-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kabatereine, N. B., B. J. Vennervald, J. H. Ouma, J. Kemijumbi, A. E. Butterworth, D. W. Dunne, and A. J. Fulford. 1999. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology 118:101-105. [DOI] [PubMed] [Google Scholar]
- 33.Karanja, D. M., A. W. Hightower, D. G. Colley, P. N. Mwinzi, K. Galil, J. Andove, and W. E. Secor. 2002. Resistance to reinfection with Schistosoma mansoni in occupationally exposed adults and effect of HIV-1 co-infection on susceptibility to schistosomiasis: a longitudinal study. Lancet 360:592-596. [DOI] [PubMed] [Google Scholar]
- 34.Katz, N. A., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. São Paulo 14:397-400. [PubMed] [Google Scholar]
- 35.Khalife, J., M. Capron, J. Y. Cesbron, P.-C. Tai, H. L. Taelman, and A. Capron. 1986. Role of specific IgE antibodies in peroxidase (EPO) release from human eosinophils. J. Immunol. 137:1659-1664. [PubMed] [Google Scholar]
- 36.Kimani, G., C. N. Chunge, A. E. Butterworth, T. Kamau, J. Bwayo, G. Gachihi, B. Mungai, and M. Mugambi. 1991. Eosinophilia and eosinophil helminthotoxicity in patients treated for Schistosoma mansoni infections. Trans. R. Soc. Trop. Med. Hyg. 85:489-492. [DOI] [PubMed] [Google Scholar]
- 37.Kimani, G., C. N. Chunge, G. Gachihi, T. Kamau, B. Mungai, and G. M. Mkoji. 1992. Immune responses after treatment for Schistosoma mansoni infections. Scand. J. Immunol. Suppl. 11:29-33. [DOI] [PubMed] [Google Scholar]
- 38.King, C. H. 2001. Epidemiology of schistosomiasis: determinants of transmission of infection, p. 115-132. In A. A. F. Mahmoud (ed.), Schistosomiasis. Imperial College Press, London, United Kingdom.
- 39.Krouwels, F. H., L. C. Kerstens, H. W. van der Maarel, H. J. Degenhart, and H. J. Neijens. 1995. Density of eosinophils reflects activity of disease in allergic asthmatic children. Clin. Exp. Allergy 2 5:1171-1178. [DOI] [PubMed] [Google Scholar]
- 40.Krug, N., U. Napp, I. Enander, E. Eklund, C. H. Rieger, and U. Schauer. 1999. Intracellular expression and serum levels of eosinophil peroxidase (EPO) and eosinophil cationic protein in asthmatic children. Clin. Exp. Allergy 29:1507-1515. [DOI] [PubMed] [Google Scholar]
- 41.Limaye, A. P., J. S. Abrams, J. E. Silver, K. Awadzi, H. F. Francis, E. A. Ottesen, and T. B. Nutman. 1991. Interleukin-5 and the posttreatment eosinophilia in patients with onchocerciasis. J. Clin. Investig. 88:1418-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Limaye, A. P., E. A. Ottesen, V. Kumaraswami, J. S. Abrams, J. Regunathan, V. Vijayasekaran, K. Jayaraman, and T. B. Nutman. 1993. Kinetics of serum and cellular interleukin-5 in posttreatment eosinophilia of patients with lymphatic filariasis. J. Infect. Dis. 7:1396-1400. [DOI] [PubMed] [Google Scholar]
- 43.McLaren, D. J., C. G. Peterson, and P. Venge. 1984. Schistosoma mansoni: further studies of the interaction between schistosomula and granulocyte-derived cationic proteins in vitro. Parasitology 88:491-503. [DOI] [PubMed] [Google Scholar]
- 44.Meeusen, E. N. T., and A. Balic. 2000. Do eosinophils have a role in the killing of helminth parasites? Parasitol. Today 16:95-101. [DOI] [PubMed] [Google Scholar]
- 45.Mehlhorn, H., B. Becker, P. Andrews, H. Thomas, and J. K. Frenkel. 1981. In vivo and in vitro experiments on the effect of praziquantel on Schistosoma mansoni. A light and electron microscopy study. Arzneimittelforschung 31:544-554. [PubMed] [Google Scholar]
- 46.Mutapi, F., P. D. Ndhlovu, P. Hagan, J. T. Spicer, T. Mduluza, C. M. Turner, S. K. Chandiwana, and M. E. Woolhouse. 1998. Chemotherapy accelerates the development of acquired immune responses to Schistosoma haematobium infection. J. Infect. Dis. 178:289-293. [DOI] [PubMed] [Google Scholar]
- 47.Nakajima, H., G. J. Gleich, and H. Kita. 1996. Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J. Immunol. 156:4859-4866. [PubMed] [Google Scholar]
- 48.Ottesen, E. A., and P. F. Weller. 1979. Eosinophilia following treatment of patients with schistosomiasis mansoni and Bancroft's filariasis. J. Infect. Dis. 139:343-347. [DOI] [PubMed] [Google Scholar]
- 49.Owhashi, M., Y. Horii, and A. Ishii. 1983. Eosinophil chemotactic factor in schistosome eggs: a comparative study of eosinophil chemotactic factors in the eggs of Schistosoma japonicum and S. mansoni in vitro. Am. J. Trop. Med. Hyg. 32:359-366. [DOI] [PubMed] [Google Scholar]
- 50.Pearlman, E., L. Toe, B. A. Boatin, A. A. Gilles, A. W. Higgins, and T. R. Unnasch. 1999. Eotaxin expression in Onchocerca volvulus-induced dermatitis after topical application of diethylcarbamazine. J. Infect. Dis. 180:1394-1397. [DOI] [PubMed] [Google Scholar]
- 51.Petersen, B., R. Dahl, B. B. Larsen, and P. Venge. 1993. The effect of salmeterol on the early- and late-phase reaction to bronchial allergen and postchallange variation in bronchial reactivity, blood eosinophils, serum eosinophil cationic protein, and serum eosinophil protein X. Allergy 48:377-382. [DOI] [PubMed] [Google Scholar]
- 52.Peterson, C. G., R. C. Garcia, M. Carlson, and P. Venge. 1987. Eosinophil cationic protein (ECP), eosinophil protein-X (EPX) and eosinophil peroxidase (EPO): granule distribution degranulation and characterization of released proteins, p. 1-19. In C. G. Peterson (ed.), Eosinophil granule proteins. Biochemical and functional studies. Uppsala University, Uppsala, Sweden.
- 53.Prin, L., M. Capron, A. B. Tonnel, O. Bletry, and A. Capron. 1983. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int. Arch. Allergy Appl. Immunol. 72:336-346. [DOI] [PubMed] [Google Scholar]
- 54.Reimert, C. M., U. Minuva, A. Kharazmi, and K. Bendtzen. 1991. Eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Detection by enzyme-linked immunosorbent assay and purification from normal human urine. J. Immunol. Methods 141:97-104. [DOI] [PubMed] [Google Scholar]
- 55.Reimert, C. M., L. K. Poulsen, C. Bindslev-Jensen, A. Kharazmi, and K. Bendtzen. 1993. Measurement of eosinophil cationic protein (ECP) and eosinophil protein X/eosinophil derived neurotoxin (EPX/EDN). Time and temperature dependent spontaneous release in vitro demands standardized sample processing. J. Immunol. Methods 166:183-190. [DOI] [PubMed] [Google Scholar]
- 56.Reimert, C. M., P. Venge, A. Kharazmi, and K. Bendtzen. 1991. Detection of eosinophil cationic protein (ECP) by an enzyme-linked immunosorbent assay. J. Immunol. Methods 138:285-290. [DOI] [PubMed] [Google Scholar]
- 57.Reimert, C. M., P. S. Skov, and L. K. Poulsen. 1998. A microtiter assay for activation of eosinophils. Simultaneous monitoring of eosinophil adhesion and degranulation. Allergy 53:129-138. [DOI] [PubMed] [Google Scholar]
- 58.Royer, B., S. Varadjalou, P. Saas, J. J. Guillosson, J. P. Kantelip, and M. Arock. 2001. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin. Exp. Allergy 31:694-704. [DOI] [PubMed] [Google Scholar]
- 59.Sher, A., B. F. Hall, and M. A. Vadas. 1978. Acquisition of murine major histocompatibility complex gene products by schistosomula of Schistosoma mansoni. J. Exp. Med. 148:46-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spry, C. F. J. 1988. Eosinophils: a comprehensive review, and a guide to the scientific literature. Oxford University Press, Oxford, United Kingdom.
- 61.Supali, T., I. S. Ismid, P. Rückert, and P. Fischer. 2002. Treatment of Brugia timori and Wuchereria bancrofti infections in Indonesia using DEC or a combination of DEC and albendazole: adverse reactions and short-term effects on microfilariae. Trop. Med. Int. Health 7:894-901. [DOI] [PubMed] [Google Scholar]
- 62.Sur, S., D. G. Glitz, H. Kita, S. M. Kujawa, E. A. Peterson, D. A. Weiler, G. M. Kephart, J. M. Wagner, T. J. George, G. J. Gleich, and K. M. Leiferman. 1998. Localization of eosinophil-derived neurotoxin and eosinophil cationic protein in neutrophilic leukocytes. J. Leukoc. Biol. 63:715-722. [DOI] [PubMed] [Google Scholar]
- 63.Tarleton, R. L., and W. M. Kemp. 1981. Demonstration of IgG-Fc and C3 receptors on adult Schistosoma mansoni. J. Immunol. 126:379-384. [PubMed] [Google Scholar]
- 64.Vadas, M., J. David, A. E. Butterworth, V. Houba, L. David, and N. Pisani. 1979. Comparison of the ability of eosinophils and neutrophils, and of eosinophils from patients with S. mansoni infection and normal individuals, to mediate in vitro damage to schistosomula of S. mansoni. Adv. Exp. Med. Biol. 114:677-682. [DOI] [PubMed] [Google Scholar]
- 65.Vadas, M. A., J. R. David, A. E. Butterworth, V. Houba, R. F. Sturrock. L. David, R. Herson, T. A. Siongok, and R. Kimani. 1980. Functional studies on purified eosinophils and neutrophils from patients with Schistosoma mansoni infections. Clin. Exp. Immunol. 39:683-694. [PMC free article] [PubMed] [Google Scholar]
- 66.Vadas, M. A., J. R. David, A. E. Butterworth, N. T. Pisani, and T. A. Siongok. 1979. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J. Immunol. 122:1228-1236. [PubMed] [Google Scholar]
- 67.Van Dam, G. J., F. F. Stelma, B. Gryssels, S. T. Falcao Ferreira, I. Talla, M. Niang, J. P. Rotmans, and A. M. Deelder. 1996. Antibody response patterns against Schistosoma mansoni in a recently exposed community in Senegal. J. Infect. Dis. 173:1232-1241. [DOI] [PubMed] [Google Scholar]
- 68.Venge, P., and R. Dahl. 1989. Are blood eosinophil number and activity important for the development of the late asthmatic reaction after allergen challenge? Eur. Respir. J. Suppl. 6:430s-434s. [PubMed] [Google Scholar]
- 69.Warren, K. S. 1973. Regulation of the prevalence and intensity of schistosomiasis in man: immunology or ecology? J. Infect. Dis. 127:595-609. [DOI] [PubMed] [Google Scholar]
- 70.Webster, M., P. G. Fallon, A. J. Fulford, A. E. Butterworth, J. H. Ouma, G. Kimani, and D. W. Dunne. 1997. Effect of praziquantel and oxamniquine treatment on human isotype responses to Schistosoma mansoni: elevated IgE to adult worm. Parasite Immunol. 19:333-335. [DOI] [PubMed] [Google Scholar]
- 71.Webster, M., A. J. C. Fulford, G. Braun, J. H. Ouma, H. C. Kariuki, J. C. Havercroft, K. Gachuhi, R. F. Sturrock, A. E. Butterworth, and D. W. Dunne. 1996. Human immunoglobulin E responses to a recombinant 22.6-kilodalton antigen from Schistosoma mansoni adult worms are associated with low intensities of reinfection after treatment. Infect. Immun. 64:4042-4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woolhouse, M. E. J., and P. Hagan. 1999. Seeking the ghost of the worms past. Nat. Med. 5:1225-1227. [DOI] [PubMed] [Google Scholar]