Abstract
Evidence indicates that human T-cell lymphotropic virus type 1 (HTLV-1) infection leads to chronic immunosuppression and a greater susceptibility to infectious diseases. Spontaneous in vitro proliferation of peripheral blood mononuclear cells (PBMC) is an important immunological feature of HTLV-1-infected individuals. However, the association between spontaneous proliferation and immunosuppression is not clear. In this study, we evaluated the cellular immune responses of PBMC from 58 asymptomatic HTLV-1-infected individuals with PBMC showing or not showing spontaneous proliferation. Individuals with PBMC that spontaneously proliferated had increased proportions of CD4 T cells expressing CD45RO and dramatically reduced responses to recall antigens. In addition, frequencies of positive responses to recall antigens were also decreased in HTLV-infected individuals without spontaneous proliferation of PBMC. There was a polyclonal expansion of multiple T-cell receptor Vβ families of CD4+ T lymphocytes in patients with spontaneous proliferation. We observed that HTLV-1 induced an immunosuppression characterized by a decrease in the stimulation index to a recall antigen, even in individuals who did not present spontaneous proliferation. On the other hand, only patients with PBMC presenting spontaneous proliferation showed polyclonal activation and increased proportion of CD4 T cells expressing CD45RO.
Human T-cell lymphotropic virus type 1 (HTLV-1) is a human retrovirus that has infected 10 to 20 million people worldwide (3). There are areas in Japan, the Caribbean, Melanesia, central and western Africa, and South America that are large endemic foci (17, 35). In Brazil, the overall prevalence of HTLV-1 infection in blood donors is 0.45%, and the city of Salvador (located in the northeast) has the highest prevalence (1.35%) in the country (4). Recently, a population-based cross-sectional study performed in Salvador, Brazil, found an HTLV-1 seroprevalence of 1.76% (2).
Two diseases are clearly associated with HTLV-1 infection: adult T-cell leukemia/lymphoma (6, 26, 34) and HTLV-1-associated myelopathy/tropical spastic paraparesis (5, 24). Although 95% of infected people remain asymptomatic (7), there is evidence that HTLV-1 has a much broader spectrum of disease manifestations, such as uveitis (20), arthritis (8, 12), polymyositis (22), lymphocytic interstitial pneumonia (31), and infective dermatitis in children (15). Severe immunosuppression is well documented in patients with adult T-cell leukemia/lymphoma (10, 19). However, a growing body of literature suggests that many HTLV-1-infected individuals show chronic immunosuppression, even in the absence of malignant disease. HTLV-1-infected individuals are more susceptible to several infectious diseases, such as strongyloidiasis (1, 30), Hansen's disease (11), and tuberculosis (21, 25). In addition, they have reduced cutaneous delayed-type hypersensitivity responses to purified protein derivative (PPD) of Mycobacterium tuberculosis (14, 23, 33, 36).
The immunologic hallmark of HTLV-1-infected individuals is a spontaneous in vitro proliferation of their peripheral blood mononuclear cells (PBMC) (without any added antigen) observed in approximately fifty percent of patients (13, 27, 29). The memory CD4+ CD45RO+ subset is the principal T-cell population involved in this phenomenon (29). In this study, we evaluated the cellular immune responses of HTLV-1-infected individuals (with or without PBMC spontaneous proliferation) against candidin, cytomegalovirus (CMV), PPD, and tetanus toxoid (TT) recall antigens.
We observed that HTLV-1-infected individuals had reduced stimulation indexes to recall antigens, even when their PBMC did not spontaneously proliferate. On the other hand, only the individuals with spontaneous PBMC proliferation (SP+) had polyclonal T-cell activation (as shown by the study of T-cell receptor Vβ [TCR-Vβ] families) and an increase in the proportion of CD4 T cells expressing CD45RO.
MATERIALS AND METHODS
Patients.
Fifty-eight asymptomatic HTLV-1-infected individuals studied at the HTLV-1 Reference Development of Science Foundation (Salvador, Bahia, Brazil) were included in the study. The group consisted of 47 women (81%) and 11 men (19%) and had a mean age of 43 years. Blood samples from 12 healthy individuals from the Bahia State Blood Bank (HEMOBA) were used as controls. All samples were screened for HTLV-1/2 antibodies by an enzyme-linked immunosorbent assay (ELISA) (Ab-Capture ELISA test system; Ortho-Clinical Diagnostics, Inc., Raritan, New Jersey), and results were confirmed by Western blot assay (HTLV Blot 2.4; Genelabs Technologies, Singapore). Informed consent was obtained from all enrolled patients, and the Oswaldo Cruz Foundation (FIOCRUZ) ethics committee approved this study.
Media and reagents.
RPMI 1640 medium (Sigma Chemical Co., St. Louis, MO) was supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and pooled human AB serum (10%) (Sigma).
Cells.
PBMC from HTLV-infected individuals and healthy blood donors were obtained from heparinized venous blood samples by Ficoll-Hypaque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden). All experiments were performed with freshly isolated PBMC.
Flow cytometry.
For direct labeling, 50-μl portions of whole-blood samples from HTLV-infected individuals and healthy blood donors were incubated with antibodies for 30 min at room temperature. Erythrocytes were subsequently lysed with fluorescence-activated cell sorting (FACS) lysing solution (Becton-Dickinson Immunocytometry System, San Jose, CA). Cells were then washed three times in 2 ml of phosphate-buffered saline containing 1% bovine serum albumin. After a final wash, cells were fixed in phosphate-buffered saline containing 4% paraformaldehyde. In order to quantify CD4 and CD8 T cells, the following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-labeled anti-CD3 (Immunothec, a Beckman Coulter Company), phycoerythrin (PE)-labeled anti-CD4 (BD Pharmingen Technical), and phycoerythrin-cyanin-labeled anti-CD8 (Immunothec). To analyze CD4 T-cell memory and naïve subsets, cells were stained with phycoerythrin-cyanin CD4, PE-labeled CD45RO, and FITC-labeled CD45RA (Immunothec). We studied TCR-Vβ repertoires in five HTLV-1-infected individuals with PBMC with spontaneous proliferation and five HTLV-1-infected individuals with PBMC without spontaneous proliferation (SP−). To study TCR-Vβ family expression on T lymphocytes, cells were analyzed for surface expression of peridinin chlorophyll protein-labeled anti-CD4 (clone RPA-T4; Becton Dickinson), PE-labeled anti-CD8 (clone RPA-T8; Becton Dickinson), and FITC-labeled anti-TCR-Vβ (clones Vβ 2 [E22E7.2], Vβ 3.1 [LE-89], Vβ 5.1 [IMMU 157], Vβ 5.2 [36213], Vβ 6.1 [CRI 304.3], Vβ 8 [56CS.2], Vβ 11 [C21], Vβ 12 [VER2.32.1], Vβ 13.1 [IMMU 222], Vβ 13.6 [JU-74], Vβ 16 [TAMAYA 1.2], Vβ 17 [E17.5F3.15.13], Vβ 20 [ELL 1.4], Vβ 21.3 [IG 125], and Vβ 22 [IMMU 546]; Immunotech). Analyses were performed using a FACSort and Cell Quest software (Becton Dickinson, Mountain View, Calif.). At least 105 events were analyzed per sample.
Antigen-specific and nonspecific proliferation assays.
Antigen-specific and nonspecific proliferation assays were performed with unfractionated PBMC. The PBMC were cultured in RPMI 1640 culture medium with 10% AB serum, using 96-well U-bottom culture plates (Costar, Cambridge, MA) in triplicate at 37°C in a 5% CO2 humidified atmosphere for 5 days. Briefly, 105 cells/well were cultured in the presence of purified candidin (25 μg/ml; Sanofi Pasteur, France), PPD of Mycobacterium tuberculosis (2 μg/ml; Statens Serum Institute, Denmark), and CMV (10 μg/ml; Behring, Marburg, Germany), all kindly provided by Brigitte Autran, and TT (1 μg/ml) kindly provided by Daniel Scott, Institut Pasteur, Paris, France. Antigens were dialyzed and frozen prior to use. Controls consisted of supplemented medium. After 5 days of culture, cells were pulsed overnight with 1 μCi [3H]thymidine (specific activity, 2 Ci/mmol; ICN, Costa Mesa, CA). Incorporated [3H]thymidine was measured with a liquid scintillation beta counter (matrix 9600 direct beta counter; Packard). Results were expressed as mean counts per minute. The stimulation index represents the ratio of mean counts obtained in the presence of antigen to mean counts obtained without antigen. A stimulation index of ≥3 indicated a positive proliferative response. Therefore, in this study, a mean counts per minute of ≥500 for nonstimulated cells (i.e., three times the mean counts per minute for uninfected control nonstimulated cells, namely, 159 ± 138 cpm; range, 6 to 462) was considered spontaneous proliferation of PBMC.
Statistical analyses.
Data are expressed as means and standard deviations. We compared mean values of the percentages of CD4 and CD8 T-cell subsets and stimulation indexes of proliferative responses to recall antigen for the three groups (patients with spontaneous proliferation of PBMC, those without spontaneous proliferation, and controls) using the Kruskal-Wallis test. If a significant difference was found, a Mann-Whitney U test was performed. The statistical analysis of TCR-Vβ was performed by the Wilcoxon signed-rank test. A P value of less than 0.05 denoted a statistically significant difference. BioEstat 3.0 software (Sociedade Civil Mamirua/MCT-CNPq) was used for all statistical analyses.
RESULTS
PBMC spontaneous proliferation.
As shown in Table 1, spontaneous PBMC proliferation was observed in 39 out of 58 (67%) HTLV-1-infected individuals (1,656 cpm ± 956 cpm; range, 520 to 4,629 cpm).
TABLE 1.
Spontaneous proliferation of PBMC from HTLV-1-infected individualsa
| PBMC | No. of HTLV-1-infected individuals | Mean cpm ± SD (range) |
|---|---|---|
| SP+ | 39 | 1,656 ± 956 (520-4,629) |
| SP− | 19 | 217 ± 152 (18-476) |
| Total | 58 |
The mean counts per minute of nonstimulated PBMC from uninfected controls was 159 ± 138 cpm (range, 6 to 462 cpm). For HTLV-1-infected individuals, the mean cpm from nonstimulated cells was ≥500 cpm at day 6 for individuals with SP+ PBMC, while it was ≤500 cpm at day 6 for HTLV-1-infected individuals with SP− PBMC.
On the other hand, no spontaneous PBMC proliferation was observed in 19 out of 58 (33%) HTLV-1-infected individuals (217 ± 152 cpm; range, 18 to 476). In this case, the mean counts per minute for asymptomatic HTLV-1-infected individuals did not differ from the mean counts per minute for uninfected controls (159 ± 138 cpm; range, 6 to 462) (P = 0.32 by the Mann-Whitney U test).
HTLV-1-infected individuals have an expansion of the memory CD4+ CD45RO+ T-cell subset.
As shown in Table 2, the percentage of circulating CD4+ T lymphocytes in the HTLV-1-infected group with PBMC with spontaneous proliferation (41% ± 14%) was similar to those of HTLV-1-infected individuals without spontaneous PBMC proliferation (43% ± 9%) and of uninfected controls (42% ± 9%) (P = 0.84). However, the memory CD4+ CD45RO+ T-cell subset was expanded in HTLV-1-infected individuals with spontaneous PBMC proliferation (79% ± 10%) compared to that in HTLV-1-infected individuals without spontaneous proliferation (67% ± 18%) (P = 0.04) and uninfected controls (67% ± 11%) (P = 0.01). Additionally, the proportion of CD4 T cells expressing CD45RO was positively correlated to spontaneous proliferation of PBMC in culture (Spearman's r = 0 37; P = 0.04) (data not shown).
TABLE 2.
Percentages of T-cell subsets from HTLV-1-infected individuals and uninfected controls
| Group and parameter | % T cells
|
CD4/CD8 ratio | |||
|---|---|---|---|---|---|
| CD4+ | CD4+ CD45RA+a | CD4+ CD45RO+b | CD8+ | ||
| Group A (SP+ HTLV-infected subjects [n = 19]) | |||||
| Mean | 41 | 29 | 79 | 24 | 1.7 |
| SD | 14 | 12 | 10 | 6 | 0.7 |
| Median | 44 | 30 | 79 | 23 | 2.0 |
| Range | 10-61 | 10-53 | 55-92 | 12-37 | 1.0-3.0 |
| Group B (SP− HTLV-infected subjects [n = 10]) | |||||
| Mean | 43 | 32 | 67 | 21 | 2.5 |
| SD | 9 | 14 | 18 | 10 | 1.0 |
| Median | 45 | 31 | 68 | 19 | 2.5 |
| Range | 26-56 | 13-56 | 46-99 | 10-41 | 1.0-4.0 |
| Group C (uninfected controls [n = 10]) | |||||
| Mean | 42 | 37 | 67 | 21 | 2.2 |
| SD | 9 | 11 | 11 | 4 | 0.8 |
| Median | 40 | 37 | 64 | 22 | 2.0 |
| Range | 31-55 | 23-57 | 51-86 | 15-30 | 1.0-4.0 |
| P valuec | |||||
| Group A vs group B vs group C | 0.84 | 0.36 | 0.02 | 0.20 | 0.09 |
| Group A vs group B | 0.84 | 0.65 | 0.04 | 0.12 | 0.07 |
| Group A vs group C | 0.60 | 0.15 | 0.01 | 0.27 | 0.18 |
| Group B vs group C | 0.65 | 0.43 | 0.85 | 0.31 | 0.49 |
CD4+ cells expressing CD45RA.
CD4+ cells expressing CD45RO.
Differences between the values for SP+ and SP− HTLV-1-infected groups and uninfected controls were compared by the Kruskal-Wallis test (group A versus group B versus group C). Differences between two groups were analyzed by the Mann-Whitney U test (group A versus group B, group A versus group C, and group B versus group C). Differences between the values for SP+ and SP− HTLV-1-infected groups and uninfected controls that were statistically significant are shown in bold type.
There were no significant differences in the frequencies of naïve CD4+ CD45RA+ and CD8+ T-cell subsets in HTLV-infected individuals (with or without spontaneous PBMC proliferation) and in uninfected controls.
PBMC from HTLV-1-infected individuals have a lower capacity to proliferate in response to recall antigens.
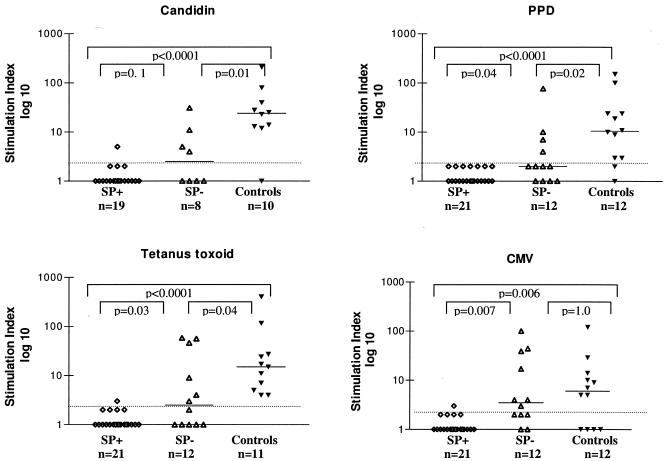
HTLV-1-infected individuals with spontaneous proliferation showed the lowest stimulation indexes to all antigens tested (candidin, PPD, tetanus toxoid, and cytomegalovirus antigens) compared to HTLV-1-infected individuals without spontaneous proliferation and uninfected controls. Only 5.3% (1 out of 19) responded to candidin, while 4.8% (1 out of 21) responded to CMV or TT (Fig. 1). Besides that, HTLV-1-infected SP− patients also showed decreases in stimulation indexes: 50% of them (4 out of 8) responded to candidin, 33% (4 out of 12) responded to PPD, 42% (5 out of 12) to cytomegalovirus, and 50% (6 out of 12) to tetanus toxoid antigen. Almost all uninfected controls had positive responses to recall antigens: 90% to candidin (9 out of 10), 83% to PPD (10 out of 12), 100% to tuberculin (11 out of 11), and 67% (8 out of 12) to CMV.
FIG. 1.
Proliferative responses to recall antigens from HTLV-1-infected asymptomatic individuals with PBMC with and without spontaneous proliferation, respectively, and uninfected controls. PBMC were cultured in the presence of candidin, PPD, tetanus toxoid, and cytomegalovirus. At day 5 of culture, cells were pulsed overnight with 1 μCi [3H]thymidine. Results are expressed as stimulation indexes (average counts per minute in the presence of antigens/average counts per minute obtained without antigen). Tests were carried out in triplicate. A stimulation index of ≥3 was considered positive for proliferative responses (above dashed lines). Bars depict median values. Differences were considered significant when P was <0.05 (Mann-Whitney U test).
In addition, PBMC from HTLV-1-infected individuals responded to fewer recall antigens than uninfected controls: while 83% of uninfected controls had specific PBMC proliferative responses to three or more different antigens, PBMC from HTLV-1-infected SP+ individuals were unable to recognize more than one antigen (P < 0.0001). Moreover, only 33% of HTLV-1-infected individuals without spontaneous PBMC proliferation responded to three or more antigens (P = 0.038).
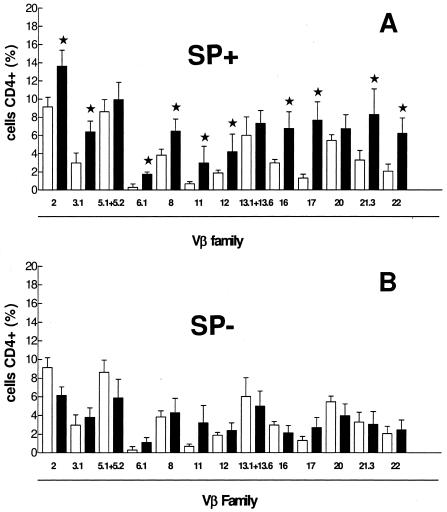
TCR-Vβ analysis disclosed polyclonal expansion only in HTLV-1-infected individuals with spontaneous proliferation of PBMC.
As shown in Fig. 2A, polyclonal expansion of CD4+ cells (TCR-Vβ families 2, 3.1, 6.1, 8, 11, 12, 16, 17, 21.3, and 22) was observed in patients with spontaneous PBMC proliferation. In contrast, HTLV-1-infected individuals without spontaneous PBMC proliferation had CD4 TCR-Vβ repertoires similar to those of uninfected individuals (Fig. 2B).
FIG. 2.
TCR-Vβ repertoire from five SP+ and five SP− HTLV-1-infected individuals. A total of 1 × 105 PBMC were analyzed for surface expression of CD4 TCR-Vβ family repertoire with specific monoclonal antibodies by flow cytometry. Analysis was performed on the FACScan using Cell Quest software. At least 10,000 events were analyzed per sample. Significant differences between uninfected controls and HTLV-1-infected individuals (P < 0.05 by the Wilcoxon signed-rank test) are indicated (★). (A) CD4+ T cells from 5 HTLV-1-infected patients with PBMC exhibiting spontaneous proliferation (black bars) and from 14 uninfected controls (white bars). (B) CD4+ T cells from 5 HTLV-1-infected patients with PBMC not exhibiting spontaneous proliferation (black bars) and from 14 uninfected controls (white bars).
DISCUSSION
Spontaneous proliferation is an immunological hallmark of PBMC from HTLV-1-infected individuals. This phenomenon is observed in up to 50% of cultures from PBMC from HTLV-1 carriers (28). In this study, we evaluated the cellular immune responses from HTLV-1-infected individuals with PBMC showing and not showing spontaneous proliferation. Compared to uninfected controls, HTLV-1-infected individuals with PBMC presenting spontaneous proliferation had an increased proportion of CD4 T cells expressing CD45RO. Moreover, TCR-Vβ analysis showed a polyclonal expansion of almost all Vβ families studied concerning CD4 T-cell subsets. In addition, HTLV-1-infected individuals with spontaneous PBMC proliferation had markedly reduced stimulation indexes for candidin, PPD, tetanus toxoid, and cytomegalovirus. Therefore, responses to these recall antigens could not be detected by cell proliferation.
The absence of detectable cellular immune responses to recall antigens in individuals with spontaneous PBMC proliferation was expected, since high levels of spontaneous proliferation would mask a specific response to the recall antigens evaluated by the stimulation index. However, previous findings support the idea that there could be different levels of T-cell anergy among HTLV-1-infected individuals.
One of the most important observations of this study was that cells from HTLV-1-infected individuals with PBMC without spontaneous proliferation showed significant decreases in the stimulation indexes to candidin, PPD, and tetanus toxoid. In addition, only 33% were able to recognize more than two antigens; in contrast, 83% of uninfected controls were able to recognize three or more antigens. In those individuals, detectable expansion of CD4+ T lymphocytes was not identified by quantification of memory or naïve CD4+ T-cell subsets, and analysis of TCR-Vβ by flow cytometry showed that their cells did not have polyclonal activation. Therefore, the mechanisms involved in the impairment of T lymphocytes from HTLV-1-infected individuals without spontaneous proliferation are unclear. These mechanisms could differ from those in HTLV-1-infected individuals with spontaneous PBMC proliferation. In addition, several other hypotheses are possible, such as the presence of regulatory T cells (9), impairment of antigen-presenting cells or inability of PBMC from HTLV-1-infected individuals to respond to interleukin-12 (32). Dendritic cells are the most potent antigen-presenting cells and produce interleukin-12, are targeted by HTLV-1, and stimulate the autologous proliferation of T lymphocytes in vitro (16, 18).
In conclusion, our results strongly suggest that HTLV-1-infected individuals show immunosuppression, as reflected by decreases in the stimulation indexes to recall antigens, even in individuals without spontaneous PBMC proliferation. The implication of these findings on the risk of HTLV-infected individuals developing other infectious diseases remains unknown. Further studies should be conducted in an attempt to clarify these questions.
Acknowledgments
Support for this study was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo a Pesquisa da Bahia (FAPESB).
We thank Lain Pontes de Carvalho for critical review of the manuscript. We also thank José Fernando O. Costa for technical assistance.
REFERENCES
- 1.Chieffi, P. P., C. S. Chiattone, E. N. Feltrim, R. C. Alves, and M. A. Paschoalotti. 2000. Coinfection by Strongyloides stercoralis in blood donors infected with human T-cell leukemia/lymphoma virus type 1 in Sao Paulo City, Brazil. Mem. Inst. Oswaldo Cruz 95:711-712. [DOI] [PubMed] [Google Scholar]
- 2.Dourado, I., L. C. Alcantara, M. L. Barreto, M. da Gloria Teixeira, and B. Galvao-Castro. 2003. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J. Acquir. Immune Defic. Syndr. 34:527-531. [DOI] [PubMed] [Google Scholar]
- 3.Edlich, R. F., J. A. Arnette, and F. M. Williams. 2000. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J. Emerg. Med. 18:109-119. [DOI] [PubMed] [Google Scholar]
- 4.Galvao-Castro, B., L. Loures, L. G. Rodriques, A. Sereno, O. C. Ferreira, Jr., L. G. Franco, M. Muller, D. A. Sampaio, A. Santana, L. M. Passos, and F. Proietti. 1997. Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. Transfusion 37:242-243. [DOI] [PubMed] [Google Scholar]
- 5.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 6.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollsberg, P., and D. A. Hafler. 1993. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N. Engl. J. Med. 328:1173-1182. [DOI] [PubMed] [Google Scholar]
- 8.Ijichi, S., T. Matsuda, I. Maruyama, T. Izumihara, K. Kojima, T. Niimura, Y. Maruyama, S. Sonoda, A. Yoshida, and M. Osame. 1990. Arthritis in a human T lymphotropic virus type I (HTLV-I) carrier. Ann. Rheum. Dis. 49:718-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwashiro, M., R. J. Messer, K. E. Peterson, I. M. Stromnes, T. Sugie, and K. J. Hasenkrug. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA 98:9226-9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan, M. H. 1993. Human retroviruses and neoplastic disease. Clin. Infect. Dis. 17(Suppl. 2):S400-S406. [DOI] [PubMed] [Google Scholar]
- 11.Kashala, O., R. Marlink, M. Ilunga, M. Diese, B. Gormus, K. Xu, P. Mukeba, K. Kasongo, and M. Essex. 1994. Infection with human immunodeficiency virus type 1 (HIV-1) and human T cell lymphotropic viruses among leprosy patients and contacts: correlation between HIV-1 cross-reactivity and antibodies to lipoarabinomannan. J. Infect. Dis. 169:296-304. [DOI] [PubMed] [Google Scholar]
- 12.Kitajima, I., I. Maruyama, Y. Maruyama, S. Ijichi, N. Eiraku, Y. Mimura, and M. Osame. 1989. Polyarthritis in human T lymphotropic virus type I-associated myelopathy. Arthritis Rheum. 32:1342-1344. [DOI] [PubMed] [Google Scholar]
- 13.Kramer, A., S. Jacobson, J. F. Reuben, E. L. Murphy, S. Z. Wiktor, B. Cranston, J. P. Figueroa, B. Hanchard, D. McFarlin, and W. A. Blattner. 1989. Spontaneous lymphocyte proliferation in symptom-free HTLV-I positive Jamaicans. Lancet ii:923-924. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda, Y., and H. Takashima. 1990. Impairment of cell-mediated immune responses in HTLV-I-associated myelopathy. J. Neurol. Sci. 100:211-216. [DOI] [PubMed] [Google Scholar]
- 15.LaGrenade, L., B. Hanchard, V. Fletcher, B. Cranston, and W. Blattner. 1990. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet 336:1345-1347. [DOI] [PubMed] [Google Scholar]
- 16.Macatonia, S. E., J. K. Cruickshank, P. Rudge, and S. C. Knight. 1992. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res. Hum. Retrovir. 8:1699-1706. [DOI] [PubMed] [Google Scholar]
- 17.Madeleine, M. M., S. Z. Wiktor, J. J. Goedert, A. Manns, P. H. Levine, R. J. Biggar, and W. A. Blattner. 1993. HTLV-I and HTLV-II world-wide distribution: reanalysis of 4,832 immunoblot results. Int. J. Cancer 54:255-260. [DOI] [PubMed] [Google Scholar]
- 18.Makino, M., S. Shimokubo, S. I. Wakamatsu, S. Izumo, and M. Baba. 1999. The role of human T-lymphotropic virus type 1 (HTLV-1)-infected dendritic cells in the development of HTLV-1-associated myelopathy/tropical spastic paraparesis. J. Virol. 73:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh, B. J. 1996. Infectious complications of human T cell leukemia/lymphoma virus type I infection. Clin. Infect. Dis. 23:138-145. [DOI] [PubMed] [Google Scholar]
- 20.Mochizuki, M., K. Yamaguchi, K. Takatsuki, T. Watanabe, S. Mori, and K. Tajima. 1992. HTLV-I and uveitis. Lancet 339:1110. [DOI] [PubMed] [Google Scholar]
- 21.Moreira, E. D., Jr., T. T. Ribeiro, P. Swanson, C. Sampaio Filho, A. Melo, C. Brites, R. Badaro, G. Toedter, H. Lee, and W. Harrington, Jr. 1993. Seroepidemiology of human T-cell lymphotropic virus type I/II in northeastern Brazil. J. Acquir. Immune Defic. Syndr. 6:959-963. [PubMed] [Google Scholar]
- 22.Morgan, O. S., P. Rodgers-Johnson, C. Mora, and G. Char. 1989. HTLV-1 and polymyositis in Jamaica. Lancet ii:1184-1187. [DOI] [PubMed] [Google Scholar]
- 23.Murai, K., N. Tachibana, S. Shioiri, E. Shishime, A. Okayama, J. Ishizaki, K. Tsuda, and N. Mueller. 1990. Suppression of delayed-type hypersensitivity to PPD and PHA in elderly HTLV-I carriers. J. Acquir. Immune Defic. Syndr. 3:1006-1009. [PubMed] [Google Scholar]
- 24.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 25.Pedral-Sampaio, D. B., E. Martins Neto, C. Pedrosa, C. Brites, M. Duarte, and W. Harrington, Jr. 1997. Co-infection of tuberculosis and HIV/HTLV retroviruses: frequency and prognosis among patients admitted in a Brazilian hospital. Braz. J. Infect. Dis. 1:31-35. [PubMed] [Google Scholar]
- 26.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popovic, M., N. Flomenberg, D. J. Volkman, D. Mann, A. S. Fauci, B. Dupont, and R. C. Gallo. 1984. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science 226:459-462. [DOI] [PubMed] [Google Scholar]
- 28.Prince, H. E., H. Lee, E. R. Jensen, P. Swanson, D. Weber, L. Fitzpatrick, M. Doyle, and S. Kleinman. 1991. Immunologic correlates of spontaneous lymphocyte proliferation in human T-lymphotropic virus infection. Blood 78:169-174. [PubMed] [Google Scholar]
- 29.Prince, H. E., J. York, S. M. Owen, and R. B. Lal. 1995. Spontaneous proliferation of memory (CD45RO+) and naive (CD45RO−) subsets of CD4 cells and CD8 cells in human T lymphotropic virus (HTLV) infection: distinctive patterns for HTLV-I versus HTLV-II. Clin. Exp. Immunol. 102:256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, R. D., J. F. Lindo, F. A. Neva, A. A. Gam, P. Vogel, S. I. Terry, and E. S. Cooper. 1994. Immunoepidemiologic studies of Strongyloides stercoralis and human T lymphotropic virus type I infections in Jamaica. J. Infect. Dis. 169:692-696. [DOI] [PubMed] [Google Scholar]
- 31.Setoguchi, Y., S. Takahashi, T. Nukiwa, and S. Kira. 1991. Detection of human T-cell lymphotropic virus type I-related antibodies in patients with lymphocytic interstitial pneumonia. Am. Rev. Respir. Dis. 144:1361-1365. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, M., C. S. Dezzutti, A. Okayama, N. Tachibana, H. Tsubouchi, N. Mueller, and R. B. Lal. 1999. Modulation of T-cell responses to a recall antigen in human T-cell leukemia virus type 1-infected individuals. Clin. Diagn. Lab. Immunol. 6:713-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tachibana, N., A. Okayama, J. Ishizaki, T. Yokota, E. Shishime, K. Murai, S. Shioiri, K. Tsuda, M. Essex, and N. Mueller. 1988. Suppression of tuberculin skin reaction in healthy HTLV-I carriers from Japan. Int. J. Cancer 42:829-831. [DOI] [PubMed] [Google Scholar]
- 34.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 35.Vrielink, H., and H. W. Reesink. 2004. HTLV-I/II prevalence in different geographic locations. Transfus. Med. Rev. 18:46-57. [DOI] [PubMed] [Google Scholar]
- 36.Welles, S. L., N. Tachibana, A. Okayama, S. Shioiri, S. Ishihara, K. Murai, and N. E. Mueller. 1994. Decreased reactivity to PPD among HTLV-I carriers in relation to virus and hematologic status. Int. J. Cancer 56:337-340. [DOI] [PubMed] [Google Scholar]