Abstract
Enzyme-linked immunosorbent assays (ELISAs) for the diagnosis of Johne's disease (JD), caused by Mycobacterium avium subsp. paratuberculosis, were developed using whole bacilli treated with formaldehyde (called WELISA) or surface antigens obtained by treatment of M. avium subsp. paratuberculosis bacilli with formaldehyde and then brief sonication (called SELISA). ELISA plates were coated with either whole bacilli or sonicated antigens and tested for reactivity against serum obtained from JD-positive and JD-negative cattle or from calves experimentally inoculated with M. avium subsp. paratuberculosis, Mycobacterium avium subsp. avium, or Mycobacterium bovis. Because the initial results obtained from the WELISA and SELISA were similar, most of the subsequent experiments reported herein were performed using the SELISA method. To optimize the SELISA test, various concentrations (3.7 to 37%) of formaldehyde and intervals of sonication (2 to 300 s) were tested. With an increase in formaldehyde concentration and a decreased interval of sonication, there was a concomitant decrease in nonspecific binding by the SELISA. SELISAs prepared by treating M. avium subsp. paratuberculosis with 37% formaldehyde and then a 2-s burst of sonication produced the greatest difference (7×) between M. avium subsp. paratuberculosis-negative and M. avium subsp. paratuberculosis-positive serum samples. The diagnostic sensitivity and specificity for JD by the SELISA were greater than 95%. The SELISA showed subspecies-specific detection of M. avium subsp. paratuberculosis infections in calves experimentally inoculated with M. avium subsp. paratuberculosis or other mycobacteria. Based on diagnostic sensitivity and specificity, the SELISA appears superior to the commercial ELISAs routinely used for the diagnosis of JD.
Mycobacterium avium subsp. paratuberculosis is the etiologic agent of Johne's disease (JD), which occurs worldwide, affecting many domestic and wild animals including cattle, sheep, and many other ruminants. Infections with M. avium subsp. paratuberculosis often lead to chronic granulomatous enteritis with clinical signs of diarrhea, weight loss, decreased milk production, and mortality. Recent studies have shown that more than 20% of the dairy herds in the United States have JD (2, 8), leading to an estimated annual economic loss of more than $200 million.
Control of JD has proven to be exceptionally difficult primarily due to the nature of M. avium subsp. paratuberculosis infections. Animals become infected at an early age by ingesting organisms shed in the feces or milk from older animals. Although infected, several years are required before animals become patent and shed mycobacteria in their feces. Generally, prepatent animals do not show symptoms of disease, so the majority of M. avium subsp. paratuberculosis infections go unnoticed and undiagnosed.
Most current diagnostic tests are based on testing for humoral immune responses or by detecting M. avium subsp. paratuberculosis in the feces by culture or by PCR. In general, these tests, especially the fecal culture and PCR tests, are incapable of detecting prepatent M. avium subsp. paratuberculosis infections and of distinguishing between pass-through M. avium subsp. paratuberculosis and colonizing M. avium subsp. paratuberculosis arising from infection. The fecal culture test requires 5 to 16 weeks for cultivation, and its sensitivity level is estimated to be only approximately 38% (11). Current enzyme-linked immunosorbent assays (ELISAs) were found to be less sensitive than the fecal culture test. For example, two independent studies reported that sera tested by ELISA detected only 45% and 57% of M. avium subsp. paratuberculosis-infected cattle that were fecal culture positive (7, 9). McKenna et al. (6) tested tissue, fecal, and serum samples from 994 dairy cattle for the presence of M. avium subsp. paratuberculosis or anti-M. avium subsp. paratuberculosis antibodies and found that the fecal culture test diagnosed only 22.5% of M. avium subsp. paratuberculosis infections. Sensitivity of commercial ELISAs for detecting M. avium subsp. paratuberculosis infections ranged from 13.9 to 27.8% when compared to the fecal culture test and 6.9 to 16.9% when compared to the tissue culture test.
A dogma exists that tests based on humoral immune responses are not feasible because antibodies to M. avium subsp. paratuberculosis are not produced during early infections. However, Koets et al. (5) and Waters et al. (10) found that M. avium subsp. paratuberculosis antibodies could be detected during early M. avium subsp. paratuberculosis infections. In a recent study involving several cattle, we discovered that a flow cytometric method (FCM) detected natural M. avium subsp. paratuberculosis infections 6 to 44 months earlier than the fecal culture test and 17 to 67 months earlier than a commercial ELISA (4). Also, the FCM was capable of subspecies-specific detection of M. avium subsp. paratuberculosis infections in calves experimentally inoculated with M. avium subsp. paratuberculosis or other mycobacteria (4). Furthermore, in contrast to most extracted antigen preparations, we used whole M. avium subsp. paratuberculosis bacilli to detect the presence of M. avium subsp. paratuberculosis-specific antibodies because they contain a complete repertoire of unaltered surface antigens.
Recognizing that the FCM might be too expensive and that a dedicated flow cytometer is not available in most diagnostic laboratories, we decided to develop a user-friendly diagnostic test that does not require a major equipment purchase. First, we observed that by using intact bacilli rather than lysate preparations, antibodies to M. avium subsp. paratuberculosis were detected in cattle sera by dot blots and whole-cell ELISA. Furthermore, a brief sonication of whole M. avium subsp. paratuberculosis bacilli rendered them nonreactive with serum from M. avium subsp. paratuberculosis-infected cattle, indicating that the M. avium subsp. paratuberculosis-specific immunoreactive antigens might be shed in the supernatant. We then designed a strategy for extracting these antigens without destroying their antigenic specificity and sensitivity. Here, we report the development of a novel ELISA as a candidate for JD diagnosis based on chemical and physical methods of extracting specific antigens from the surface of intact M. avium subsp. paratuberculosis bacilli.
MATERIALS AND METHODS
Mycobacterial cultures.
The Linda strain of Mycobacterium avium subsp. paratuberculosis was obtained from the Agricultural Research Service, USDA (Ames, Iowa). Stock cultures of the Linda strain of M. avium subsp. paratuberculosis were grown in Middlebrook medium (Becton Dickinson, Cockeysville, MD) containing 10% OADC (oleic acid, albumin, dextrose, and catalase; Franklin Labs, NJ) and 2 μg/ml mycobactin J (Allied Monitor, Fayette, MO) at 37°C. Mycobacteria were harvested from stationary-phase cultures that had reached an optical density of 0.68 based on absorbance set at 600 nm and then used immediately for experimental purposes.
Serum samples.
Serum samples were obtained from cattle that were noninfected, naturally infected, or experimentally infected with M. avium subsp. paratuberculosis, separated into aliquots of 20 to 1,000 μl and then stored at −20°C for short-term storage (<6 months) or at −80°C for long-term storage (>6 months).
M. avium subsp. paratuberculosis-negative and M. avium subsp. paratuberculosis-positive serum samples were prepared by pooling serum collected from five female Holstein-Friesian cattle (2 to 8 years old) that tested negative or positive for M. avium subsp. paratuberculosis by the FCM (4) and by ELISA (IDEXX, Westbrook, ME). M. avium subsp. paratuberculosis-negative serum samples were also obtained from 35 Holstein-Friesian cattle (5-month- to 9-year-old females) obtained from two dairy herds that had tested JD negative by ELISA (Kyoritsu Seiyaku Co., Japan) for 5 consecutive years and by ELISA, fecal culture, and PCR tests performed at the time of the last serum collection. Twenty-three M. avium subsp. paratuberculosis-positive serum samples were obtained from 2- to 8-year-old Holstein-Friesian cattle from Pennsylvania whose fecal samples produced two or more colonies of M. avium subsp. paratuberculosis during several fecal culture tests.
Serum samples were also collected from male Holstein-Friesian calves that had been experimentally inoculated with M. avium subsp. paratuberculosis, M. avium subsp. avium, or M. bovis at the National Animal Disease Center (Ames, Iowa) (10). At 2 to 5 weeks of age, the calves were each challenged by intratonsillar inoculation of 1.6 × 107 of M. avium subsp. paratuberculosis, M. avium subsp. avium, or M. bovis (two or three calves/organism) and serum samples were collected at 1- or 2-week intervals for up to 320 days.
WELISA plate preparation.
The WELISA method uses whole bacilli treated with formaldehyde. The WELISA plates were prepared as follows. Sixteen milliliters of culture medium containing M. avium subsp. paratuberculosis that had an optical density of 0.68 based on absorbance set at 600 nm were placed in a 50-ml conical centrifuge tube and centrifuged at 3,500 × g for 10 min. The pellet containing approximately 100 mg of mycobacteria was resuspended in 10 ml of phosphate-buffered saline (PBS) or in a 10-ml solution of 4.6, 9.3, or 18.5% formaldehyde (Fisher Scientific, Pittsburgh, PA) in distilled water, 37% formaldehyde, or distilled water. The stock formaldehyde solution (at 37%) also contained 10 to 15% methanol as a preservative. The solutions containing M. avium subsp. paratuberculosis were agitated by vortex for 1 min and then rocked on a Rocker II (model 260350) (Boekel Scientific, Feasterville, PA) at 15 rpm for 20 min at room temperature. After inoculation of 100 μl of the M. avium subsp. paratuberculosis suspension into each well of a 96-well plate (PolySorp Nunc-Immuno 96-microwell plate; Nalge Nunc International, Rochester, NY), the plates were stored in a fume hood at room temperature or in a refrigerator at 4°C until used.
SELISA plate preparation.
The SELISA method uses surface antigens obtained by treating the bacilli with formaldehyde followed by a brief sonication. The SELISA plates were prepared as follows. Mycobacteria were prepared as described for the WELISA plates, except that the bacterial suspensions were sonicated in 10-ml solutions containing 3.7, 18.5, and 27.8% formaldehyde in distilled water, 37% formaldehyde (undiluted stock formaldehyde; Fisher Scientific) or distilled water only. Sonication was performed by submerging the tip of the point source sonicator (Fisher Sonic Dismembrator model 100; Fisher Scientific, Feasterville, PA) in the solution containing the mycobacteria and sonicating at 22.5 kHz for 2 to 300 s. After centrifugation at 3,500 × g for 10 min, 100 μl of the supernatant was inoculated into each well of a 96-well PolySorp plate and incubated overnight as described above to allow the solutions to evaporate and the antigens to adhere to the wells. This method was used to prepare several batches of SELISA plates, which were used in various experiments. Each well contained 3.2 μg protein as determined by a Coomassie protein assay (Pierce Biotechnology, Rockford, IL) and 2.3 μg carbohydrate as determined by a phenol/sulfuric acid assay (3).
ELISAs and analyses.
Each well coated with whole M. avium subsp. paratuberculosis or with sonicated M. avium subsp. paratuberculosis antigens was treated with 200 μl of PBS (pH 7.0) containing 10% SuperBlock (Pierce Biotechnology, Rockford, IL), 0.05% Tween 80 (Acros Organics, Geel, Belgium), and 0.12% hydrogen peroxide for 1 h at 37°C, washed once with 200 μl/well buffer A (PBS, pH 7.0, containing 10% SuperBlock and 0.5% Tween 80), and then incubated with a solution containing 100 μl/well buffer A and 2 μl/well of each control or serum sample for 2 h at room temperature and agitated at 100 rpm on a lab rotator (Lab Line, Barnstead International, Dubuque, IA). After washing the wells four times with PBST (PBS, pH 7.0, containing 0.5% Tween 80), each well was inoculated with 100 μl buffer A containing biotinylated anti-bovine immunoglobulin G (IgG) polyclonal antibody (1:500 dilution; Jackson ImmunoResearch Laboratories, Westgroup, PA), incubated for 1 h at room temperature, rinsed four times with 200 μl PBST, inoculated with 100 μl/well horseradish peroxidase (HRP)-streptavidin (0.5 μg/ml; Pierce Biotechnology), incubated for 1 h, and then rinsed four times with 200 μl PBST. Controls consisted of treating whole M. avium subsp. paratuberculosis or M. avium subsp. paratuberculosis antigens with biotinylated anti-bovine IgG polyclonal antibody and HRP-streptavidin only. The ELISA reactions were developed according to the manufacturer's instructions using Pierce ImmunoPure ABTS tablets (2,2′-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt). After a 15-min incubation, optical density (absorbance) of each well was measured with a microplate reader (model 680; Bio-Rad, Hercules, CA) at 415 nm.
Optical densities obtained with M. avium subsp. paratuberculosis-negative and -positive serum samples were used to calculate S/P values of SELISA. The following formula was used to convert optical absorption data in nanometers to S/P ratios using the pool of five M. avium subsp. paratuberculosis-negative and -positive sera: S/P = (S − N)/(P − N), where S is the absorbance value of the sample, N is the absorbance value obtained using M. avium subsp. paratuberculosis-negative serum and P is the absorbance value obtained using M. avium subsp. paratuberculosis-positive serum. An S/P value of 0.23 was used as a cutoff value to determine positive samples. All SELISAs were repeated two to five times, and all samples were tested in blinded experiments.
RESULTS
Formaldehyde treatment.
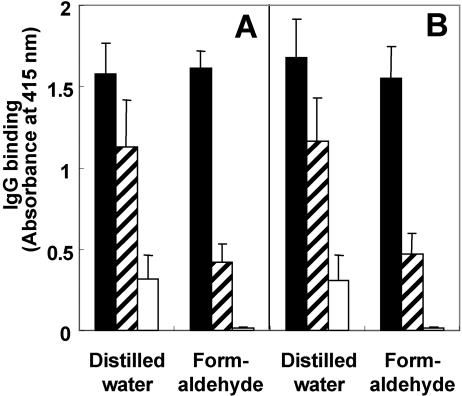
In the WELISA, when whole bacilli were treated with stock formaldehyde (37% formaldehyde plus 10 to 15% methanol), an absorbance value obtained with M. avium subsp. paratuberculosis-positive serum was approximately 3.8 times greater than that of M. avium subsp. paratuberculosis-negative serum and more than 110 times greater than that obtained with secondary antibody and HRP-streptavidin only (Fig. 1A). When formaldehyde treatment was omitted, the absorbance obtained with M. avium subsp. paratuberculosis-positive serum was only slightly higher than that obtained with M. avium subsp. paratuberculosis-negative serum and five times greater than those obtained with secondary antibody and HRP-streptavidin only. Similar results were obtained with the SELISA in which M. avium subsp. paratuberculosis bacilli were treated with or without stock formaldehyde and sonicated for 2 s, and the supernatant was tested for reactivity (Fig. 1B). These data demonstrate that treatment with formaldehyde minimized nonspecific antibody reactivity irrespective of the antigen preparation. Because the WELISA and SELISA showed similar levels of reactivity with M. avium subsp. paratuberculosis-positive and -negative serum and the SELISA utilized extracted antigens and not intact bacilli, all subsequent experiments were conducted with the SELISA only.
FIG. 1.
Effect of formaldehyde treatment on the ability of IgG in bovine serum to bind to whole organisms or to sonicated antigens of Mycobacterium avium subsp. paratuberculosis in an ELISA. The ELISA was conducted using whole cells (WELISA) (A) and sonicated antigens (SELISA) (B). M. avium subsp. paratuberculosis bacilli were treated with distilled water or 37% formaldehyde and not sonicated (A) or sonicated for 2 s and with the supernatant collected (B) immobilized via evaporation to the wells of 96-well plates. Immobilized M. avium subsp. paratuberculosis antigens were then treated with M. avium subsp. paratuberculosis-positive (solid bars) or -negative (cross-hatched bars) serum and then treated with biotinylated anti-bovine IgG polyclonal antibody. Controls (open bars) consisted of M. avium subsp. paratuberculosis treated with distilled water or formaldehyde and then secondary biotinylated antibody and HRP-streptavidin only. Each bar represents the mean + standard deviation of five replications. In both the WELISA and SELISA, treatment with formaldehyde minimized nonspecific antibody reactions. This experiment was repeated three times with similar results.
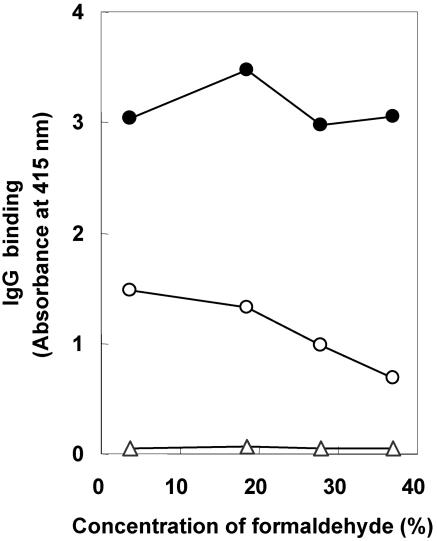
Various concentrations of formaldehyde were tested for their effects on the ability of the SELISA to differentiate between M. avium subsp. paratuberculosis-positive and -negative serum (Fig. 2). Similar absorbance levels were obtained with M. avium subsp. paratuberculosis-positive serum when M. avium subsp. paratuberculosis soluble antigens were prepared by treating M. avium subsp. paratuberculosis with 3.7, 18.5, 27.8, or 37% formaldehyde followed by sonication for 2 s. In contrast, decreasing levels of absorbance occurred with M. avium subsp. paratuberculosis-negative serum as the formaldehyde concentration increased. With 3.7% formaldehyde, there was only a twofold difference in absorbance between M. avium subsp. paratuberculosis-negative and -positive serum. At 37%, there was a 4.5-fold difference in absorbance between M. avium subsp. paratuberculosis-negative and -positive serum. Essentially no reactions occurred when serum treatment was omitted (Fig. 2). From these data, we conclude that undiluted (37%) formaldehyde yielded the highest specificity.
FIG. 2.
Effects of various formaldehyde concentrations on IgG binding to M. avium subsp. paratuberculosis antigens by SELISA. After formaldehyde treatment, whole M. avium subsp. paratuberculosis bacilli were sonicated for 2 s and the supernatant was used for the SELISA. Solid circles, pool of serum from five cattle that previously tested M. avium subsp. paratuberculosis positive by the FCM; open circles, a pool of serum from five cattle previously tested M. avium subsp. paratuberculosis negative by the FCM; open triangles, no serum. Each data point represents the mean of duplicate experiments. The greatest difference between M. avium subsp. paratuberculosis-positive and -negative serum occurred with the highest concentration of formaldehyde (37%) tested.
Sonication.
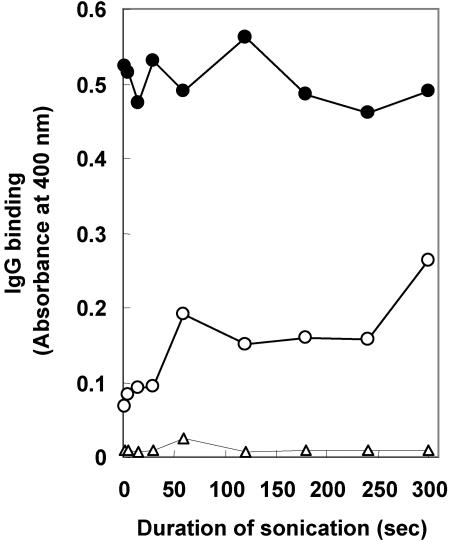
Various intervals of sonication ranging from 2 to 300 s were tested for effects on the ability of the SELISA to differentiate between M. avium subsp. paratuberculosis-positive and -negative serum. M. avium subsp. paratuberculosis bacilli were treated with 37% formaldehyde, sonicated, and centrifuged, and the supernatant was tested by SELISA. A 7.7-fold difference between M. avium subsp. paratuberculosis-positive and -negative serum was obtained with a short, 2-s burst of sonication, whereas a 2.6- to 3.8-fold difference was obtained with sonication intervals of 60 to 240 s; a 1.9-fold difference was obtained with a sonication interval of 300 s (Fig. 3). As the sonication intervals increased from a few seconds to 60 s, there was an increase in level of nonspecific reactivity of M. avium subsp. paratuberculosis antigen with M. avium subsp. paratuberculosis-negative serum. The level of nonspecific reactivity of M. avium subsp. paratuberculosis antigen with M. avium subsp. paratuberculosis-negative serum remained relatively constant up to 250 s and then increased substantially at 300 s.
FIG. 3.
Effects of duration of sonication on IgG binding to M. avium subsp. paratuberculosis antigens by SELISA. M. avium subsp. paratuberculosis bacilli were treated with 37% formaldehyde and sonicated for various time intervals, and the supernatant was used in the SELISA. Solid circles, pool of serum from five cattle previously tested M. avium subsp. paratuberculosis positive by the FCM; open circles, pool of serum from five cattle previously tested as M. avium subsp. paratuberculosis negative by the FCM; open triangles, no serum. Each data point represents the mean of duplicate experiments. A 2-s sonication gave the greatest difference between M. avium subsp. paratuberculosis-positive and -negative serum.
Diagnostic sensitivity and specificity.
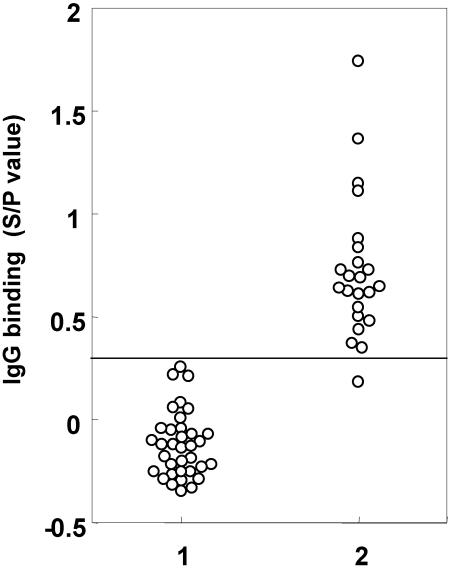
Once test variables were optimized, diagnostic specificity and sensitivity of the SELISA were determined by testing serum samples from 58 cattle that tested M. avium subsp. paratuberculosis positive or negative. Thirty-five M. avium subsp. paratuberculosis-negative serum samples were obtained from dairy farms that had tested JD negative for 5 consecutive years by ELISA and were negative by fecal culture, PCR, and ELISA during the fifth year (unpublished data). Of the 58 samples, 23 samples were obtained from cattle that had previously tested JD positive by fecal culture (11). Based on a cutoff S/P value of 0.23, the SELISA had a diagnostic sensitivity of 95.6% detecting 22 of 23 M. avium subsp. paratuberculosis-positive samples and had no false-positive reactions (Fig. 4). SELISA plates prepared at different times gave similar results, indicating that the method of antigen preparation was consistent from one batch to the next.
FIG. 4.
Diagnostic sensitivity of SELISA. (Column 1) Serum samples from cattle on dairy farms that had tested JD negative for 5 consecutive years by ELISA and by fecal culture, PCR, and ELISA performed during the fifth year. (Column 2) Serum samples from dairy cattle that tested JD positive by fecal culture. A cutoff value of 0.23 was used to distinguish M. avium subsp. paratuberculosis negatives and positives. Similar results were obtained in two separate experiments. The SELISA showed no false positives and only one negative with M. avium subsp. paratuberculosis-positive serum. (Note that this negative might have been a false positive [pass-through] by the fecal culture test.)
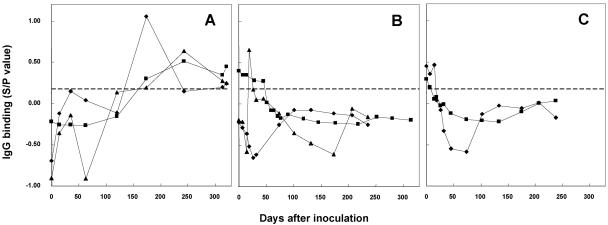
To determine if IgG binding to M. avium subsp. paratuberculosis by SELISA was specific, serum samples from three groups of experimentally infected calves (10) were tested by SELISA. These serum samples, collected from day 0 to day 321 after the inoculation, were obtained from animals exposed to M. avium subsp. avium and M. bovis, as well as M. avium subsp. paratuberculosis. Two calves inoculated with M. avium subsp. paratuberculosis showed positive IgG binding (i.e., S/P value of >0.23) at 174 days after inoculation and the other calf became positive at 243 days after inoculation (Fig. 5A). In one of the calves inoculated with M. avium subsp. paratuberculosis, the level of IgG binding dropped below the cutoff value of 0.23 at 240 days, but became positive again at 321 days. Beginning at 45 days after inoculation of calves with M. avium subsp. avium or M. bovis, the level of IgG binding to M. avium subsp. paratuberculosis stayed lower than the cutoff value (Fig. 5B and C). Initial IgG levels in these animals were attributed to maternal or colostral antibodies.
FIG. 5.
Specific detection of M. avium subsp. paratuberculosis infections by SELISA in cattle experimentally inoculated with M. avium subsp. paratuberculosis, Mycobacterium avium subsp. avium, and M. bovis. Serum was obtained from calves at time of intratonsillar inoculation of mycobacteria (M. avium subsp. paratuberculosis, M. avium subsp. avium, or M. bovis [panels A to C, respectively]) and then at 1- or 2-week intervals thereafter for up to 320 days. The SELISA was used to test for reactivity against M. avium subsp. paratuberculosis. Each symbol indicates an individual animal. (A) SELISA of IgG binding to M. avium subsp. paratuberculosis antigens in serum from calves inoculated with M. avium subsp. paratuberculosis (n = 3; solid squares, 5903; solid diamonds, 5902; and solid triangles, 5904). (B) SELISA of IgG binding to M. avium subsp. paratuberculosis antigens by serum from calves inoculated with M. avium subsp. avium (n = 3; solid diamonds, 6137; solid triangles, 193; and solid squares, 2016). (C) SELISA of IgG binding to M. avium subsp. paratuberculosis in serum from calves inoculated with M. bovis (n = 2; squares, 202; and triangles, 2354). Similar results were obtained in two separate experiments. The positive fluorescence levels from day 0 to approximately day 100 in panels B and C are probably due to the presence of maternal antibodies against M. avium subsp. paratuberculosis. The SELISA detected M. avium subsp. paratuberculosis-specific antibodies at 174 days after inoculation (A) but did not cross-react with serum from M. avium subsp. avium- or M. bovis-inoculated calves (B and C).
DISCUSSION
A unique aspect of this antigen-based JD test is that it incorporates antigens extracted directly from the surface of M. avium subsp. paratuberculosis bacilli. We found previously that M. avium subsp. paratuberculosis bacilli treated briefly with sonication did not react by the FCM (unpublished data), indicating sonication removes the antigens from the surface. Even so, it is possible that some internal M. avium subsp. paratuberculosis antigens might occur in the SELISA antigen preparations. In addition, we found that with an increase in duration of sonication there was an increase in nonspecific antigen-antibody binding. This indicates that longer sonication intervals may result in the release of nonspecific internal antigens or in structural alteration of the dislodged surface antigens.
All other current antigen-based tests use either proteins such as the purified protein derivative preparation or a whole-cell lysate or homogenate of M. avium subsp. paratuberculosis. These complex antigen preparations may be too heterogeneous for high sensitivity and certainly contain proteins that cross-react with other mycobacteria, thus lowering specificity. We hypothesize that the gentle agitation from the brief sonication used in the SELISA method dislodged M. avium subsp. paratuberculosis-specific cell surface antigens. This novel technique has shown much higher sensitivity and specificity in a single-test format. The surface components that contribute to this diagnostic sensitivity and specificity are the subject of ongoing studies in our laboratories.
In an earlier report, we developed a flow cytometric assay for the diagnosis of early JD in cattle based on using whole bacilli to detect subspecies-specific M. avium subsp. paratuberculosis IgG antibodies in the serum of JD-positive cattle (4). In the FCM, M. avium subsp. paratuberculosis-positive serum did not cross-react with other closely related mycobacteria, including M. avium subsp. avium and M. scrofulaceum (4), as well as M. bovis, Mycobacterium phlei, Mycobacterium smegmatis, Mycobacterium gordonae, Mycobacterium szugai, and Escherichia coli (unpublished data). The diagnostic sensitivity and specificity of the FCM were 95.2% and 96.7%, respectively (4). In a study involving several cattle, the FCM detected M. avium subsp. paratuberculosis infections 6 to 44 months earlier than the fecal culture test and 17 to 67 months earlier than a commercial ELISA (4). However, a potential disadvantage is that flow cytometry can be costly and may require initial training to achieve consistent and dependable results.
Nonetheless, the FCM provided evidence that M. avium subsp. paratuberculosis-specific antigens occur on the surface of M. avium subsp. paratuberculosis. While investigating other test formats that are more user friendly, preliminary experiments showed whole immobilized M. avium subsp. paratuberculosis bacilli could be used in a dot blot assay and in a 96-well ELISA to detect M. avium subsp. paratuberculosis antibodies in JD-positive cattle (unpublished data). But there was a significant increase in nonspecific reactivity (i.e., binding of antibodies in M. avium subsp. paratuberculosis-negative serum to M. avium subsp. paratuberculosis) indicating that drying to immobilize M. avium subsp. paratuberculosis bacilli on the nitrocellulose paper or the 96-well plates causes cross-reactive epitopes to be exposed for antibody binding. Preliminary experiments also showed that sonicated M. avium subsp. paratuberculosis bacilli did not react with M. avium subsp. paratuberculosis-positive serum, indicating that sonication dislodged immunologically reactive components from the surface of M. avium subsp. paratuberculosis. In the present study, we used formaldehyde to chemically fix M. avium subsp. paratuberculosis bacilli because in some cases it is known to maintain the antigenic integrity and reactivity with specific antibodies (1). After formaldehyde treatment, we used a brief burst of sonication to dislodge the surface antigens. When whole M. avium subsp. paratuberculosis bacilli (as in the WELISA) or surface antigens (as in the SELISA) were dried on 96-well plates without pretreatment in formaldehyde, only small differences in absorbance occurred between M. avium subsp. paratuberculosis-positive and -negative sera. In contrast, when whole bacilli and surface antigens were treated with formaldehyde and then dried on the 96-well plates, the reactivity of M. avium subsp. paratuberculosis-positive serum was much greater than that of M. avium subsp. paratuberculosis-negative serum. Also, bacilli and surface antigens treated with formaldehyde did not show nonspecific binding of secondary antibody or of streptavidin. These findings suggest that drying bacilli or antigens without formaldehyde treatment evidently causes exposure of nonspecific binding sites that react with irrelevant antibodies. Although the WELISA and SELISA were equally capable of differentiating M. avium subsp. paratuberculosis-positive from M. avium subsp. paratuberculosis-negative serum samples, we concentrated our efforts on the SELISA since it uses extracted antigens and not intact bacilli and the sonicated antigen might adhere better to the wells than whole bacilli and be more resistant to rinsing.
The ability of the SELISA to detect M. avium subsp. paratuberculosis infections in cattle was evaluated further by testing serum obtained from 35 JD-negative and 23 JD-positive cattle. The SELISA clearly differentiated between M. avium subsp. paratuberculosis-positive and -negative samples with a diagnostic sensitivity of 95.6% and specificity of 100%. By using the same serum samples, a commercial ELISA detected only 6 of 23 (26.1%) JD-positive cattle. According to the criteria used by Whitlock et al. (11), 13 M. avium subsp. paratuberculosis-positive cattle were categorized as low shedders. Ninety-two percent of the low shedders tested positive by the SELISA, whereas none tested M. avium subsp. paratuberculosis positive by a commercial ELISA (Biocor Animal Health, Omaha, NE). Thus, these data indicate that the diagnostic sensitivity of the SELISA, especially for low shedders, is much greater than that reported for commercial ELISAs. For example, in a comparative study, Whitlock et al. (11) found that the estimated sensitivity of fecal culture was 33% and an absorbed ELISA detected approximately 35% of known fecal-culture positives. In addition, Whitlock et al. (11) suggested that the absorbed ELISA would likely have a sensitivity of less than 10% for animals in stage 1 JD (i.e., prepatent and preclinical). In contrast, the SELISA showed diagnostic sensitivity levels similar to those of the FCM (4). In further studies, we suspect that the SELISA or a similar ELISA based on surface antigen preparations will prove to be superior to the fecal culture test and commercial ELISAs.
The SELISA was also capable of detecting M. avium subsp. paratuberculosis antibodies in experimentally infected calves. No positive levels of cross-reacting antibodies were detected in the serum of calves inoculated with M. avium subsp. avium or M. bovis. Similar results were obtained when serum samples from the same calves were tested by FCM for reactivity against M. avium subsp. avium, M. bovis, and M. avium subsp. paratuberculosis (4).
In conclusion, we report a novel method of using formaldehyde and sonication to prepare M. avium subsp. paratuberculosis antigens for use in an ELISA, which we call SELISA. As with the FCM (4), the SELISA is subspecies specific for M. avium subsp. paratuberculosis and does not cross-react with other closely related mycobacteria, and its sensitivity, especially for low shedders, was much higher than those of current ELISAs.
Acknowledgments
This work was supported by the following grants to C.A.S.: a Veterinary Services grant from the Animal and Plant Health Inspection Service, U.S. Department of Agriculture, and grants from the Food Safety Center of Excellence and Veterinary Medicine at the University of Tennessee, the B. Ray Thompson Fund, and the Tennessee Agricultural Experiment Station. We also wish to acknowledge funding by USDA CSREES-NRI grants to J.P.B. and by the USDA Agricultural Research Service.
REFERENCES
- 1.Brown, W. J., and M. V. G. Farquhar. 1989. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 31:553-569. [DOI] [PubMed] [Google Scholar]
- 2.Chi, J., J. A. VanLeeuwen, A. Weersink, and G. P. Keefe. 2002. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subsp. paratuberculosis and Neospora caninum. Prev. Vet. Med. 55:137-153. [DOI] [PubMed] [Google Scholar]
- 3.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric methods for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 4.Eda, S., B. Elliott, M. C. Scott, W. R. Waters, J. P. Bannantine, R. H. Whitlock, and C. A. Speer. 2005. New method of serological testing for Mycobacterium avium subsp. paratuberculosis (Johne's disease) by flow cytometry. Foodborne Pathog. Dis. 2:250-262. [DOI] [PubMed] [Google Scholar]
- 5.Koets, A. P., V. P. M. G. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKenna, S. L., G. P. Keefe, H. W. Barkema, and D. C. Sockett. 2005. Evaluation of three ELISAs for Mycobacterium avium subsp. paratuberculosis using tissue and fecal culture as comparison standards. Vet. Microbiol. 110:105-111. [DOI] [PubMed] [Google Scholar]
- 7.Milner, A. R., W. N. Mack, K. J. Coates, J. Hill, I. Hill, and P. Sheldrick. 1990. The sensitivity and specificity of a modified ELISA for the diagnosis of Johne's disease from a field trial in cattle. Vet. Microbiol. 25:193-198. [DOI] [PubMed] [Google Scholar]
- 8.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179-192. [DOI] [PubMed] [Google Scholar]
- 9.Sweeney, R. W., R. H. Whitlock, C. L. Buckley, and P. A. Spencer. 1995. Evaluation of a commercial enzyme-linked immunosorbent assay for the diagnosis of paratuberculosis in dairy cattle. J. Vet. Diagn. Investig. 7:488-493. [DOI] [PubMed] [Google Scholar]
- 10.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock, R. H., S. J. Wells, R. W. Sweeney, and J. Van Tiem. 2000. ELISA and fecal culture for paratuberculosis (Johne's disease): sensitivity and specificity of each method. Vet. Microbiol. 77:387-398. [DOI] [PubMed] [Google Scholar]