Abstract
Phosphoinositide lipids regulate complex events via the recruitment of proteins to a specialized region of the membrane at a specific time. Precise control of both the synthesis and turnover of phosphoinositide lipids is integral to membrane trafficking, signal transduction, and cytoskeletal rearrangements. Little is known about the acute regulation of the levels of these signaling lipids. When Saccharomyces cerevisiae cells are treated with hyperosmotic medium the levels of phosphatidylinositol 3,5-bisphosphate (PI3,5P2) increase 20-fold. Here we show that this 20-fold increase is rapid and occurs within 5 min. Surprisingly, these elevated levels are transient. Fifteen minutes following hyperosmotic shock they decrease at a rapid rate, even though the cells remain in hyperosmotic medium. In parallel with the rapid increase in the levels of PI3,5P2, vacuole volume decreases rapidly. Furthermore, concomitant with a return to basal levels of PI3,5P2 vacuole volume is restored. We show that Fig4p, consistent with its proposed role as a PI3,5P2 5-phosphatase, is required in vivo for this rapid return to basal levels of PI3,5P2. Surprisingly, we find that Fig4p is also required for the hyperosmotic shock-induced increase in PI3,5P2 levels. These findings demonstrate that following hyperosmotic shock, large, transient changes occur in the levels of PI3,5P2 and further suggest that Fig4p is important in regulating both the acute rise and subsequent fall in PI3,5P2 levels.
Regulation of the levels of phosphoinositide lipids is central to understanding the spatial and temporal control of diverse membrane-related events. In the yeast Saccharomyces cerevisiae, four phosphoinositides have been identified, phosphatidylinositol 3-phosphate (PI3P), PI4P, phosphatidylinositol 3,5-biphosphate (PI3,5P2), and PI4,5P2. The most recently identified phosphoinositide is PI3,5P2 (7, 22, 34) and this phospholipid is also found in plants and mammals.
In S. cerevisiae, the sole means of generating PI3,5P2 is through phosphorylation of PI3P at the D-5 position by the kinase Fab1p (35). The levels of PI3,5P2 are approximately 20-fold lower than those of the other phosphoinositides (3). Despite this low abundance, PI3,5P2 is important for several cellular processes. For example, PI3,5P2 levels modulate vacuole volume. Cells defective in PI3,5P2 production have grossly enlarged vacuoles (12, 19). Conversely, when PI3,5P2 levels increase in response to hyperosmotic shock, vacuole volume decreases (3). This decrease in volume is likely regulated by PI3,5P2. Further support for this postulate comes from the observation that the volume of grossly enlarged fab1Δ vacuoles (no PI3,5P2) does not decrease after hyperosmotic shock (3). PI3,5P2 is also required for proper acidification of the vacuole. However, the absence of PI3,5P2 does not affect the localization of the vacuolar ATPase (2, 12). PI3,5P2 is also required for vacuole fission and retrograde traffic from the vacuole to the late endosome (3, 4, 9). In addition, PI3,5P2 is required for proper sorting of specific proteins into the lumen of the vacuole (8, 19, 26).
One of the most striking characteristics of PI3,5P2 is the dramatic increase in its levels that occurs following acute hyperosmotic stress (7). Cells exposed to high-osmolarity medium for 10 min show a 20-fold increase in PI3,5P2 levels (3). This change is at least 10 times greater than the changes observed for the other phosphoinositides (3). This suggests that PI3,5P2 plays an important role in cellular adaptation to changes in extracellular osmolarity.
Two proteins, Vac7p and Vac14p, have been identified as potential activators of the PI3P 5-kinase Fab1p. vac7Δ and vac14Δ strains have no detectable PI3,5P2 under basal conditions and are grossly defective in PI3,5P2 elevation following hyperosmotic shock (3, 8).
In addition to synthesis, turnover of phosphoinositides plays an important role in the maintenance of steady-state levels of these phospholipids. A strong candidate for PI3,5P2 turnover is Fig4p. This protein contains a Sac1 domain which has homology to known phosphoinositide phosphatases. Importantly, recombinant glutathione S-transferase (GST)-Fig4p functions as a PI3,5P2-specific 5-phosphatase in vitro (24) and Fig4p interacts with the putative Fab1p activator Vac14p (8, 11, 24). Both proteins localize to the yeast vacuole membrane, as does Fab1p and the other putative Fab1p activator, Vac7p (2, 3, 8, 12, 24). Thus, Fig4p is in close proximity to the site of PI3,5P2 synthesis. Despite the fact that Fig4p has activity as a PI3,5P2 5-phosphatase in vitro, under basal conditions, fig4Δ and wild-type cells have similar levels of PI3,5P2 (11, 24). Therefore, it has been unclear whether Fig4p plays a major role in the regulation of PI3,5P2 levels in vivo.
Previous studies measured PI3,5P2 levels following a 10-min exposure to hyperosmotic medium (3, 7, 12). The time it takes to reach this level and the maximum level of PI3,5P2 that is achieved had not been determined. Similarly, while vac7Δ and vac14Δ mutants show little to no detectable PI3,5P2 at 10 min following hyperosmotic shock, it is not known whether this represents a defect in maximum elevation of PI3,5P2 or simply a delay in the production of the phospholipid.
Here we show that the 20-fold rise in the level of PI3,5P2 following hyperosmotic shock is very rapid and transient. By 5 min the level of PI3,5P2 had risen 20-fold and by 30 min it had returned to basal levels, even though the cells remained in hyperosmotic medium. Analysis of the decrease in the level of PI3,5P2 reveals that, in vivo, Fig4p is the major PI3,5P2 phosphatase required for this rapid turnover. In addition, we present the surprising discovery that Fig4p, like Vac7p and Vac14p, is necessary for the 20-fold elevation of PI3,5P2 levels following hyperosmotic shock.
MATERIALS AND METHODS
Strains, media, and expression vectors.
The strains used in this study are listed in Table 1. Strains were grown at 24°C in either yeast extract-peptone-dextrose or synthetic complete minimal medium. All plasmid expression was done with the pRS400 series of vectors (5, 28).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype | Reference |
|---|---|---|
| LWY7217 | MATaleu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | 2 |
| LWY7235 | MATaleu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | 2 |
| LWY7240 | MATα leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | This study |
| LWY6725 | LWY7235 × LWY7240 | This study |
| LWY2054 | LWY7217 vac7Δ::HIS3 | 3 |
| LWY2055 | LWY7217 fab1Δ::LEU2 | 3 |
| LWY5177 | LWY7235 vac14Δ::TRP1 | 3 |
| LWY6474 | LWY7235 fig4Δ::TRP1 | This study |
| LWY6830 | LWY7235 HA-FAB1 | This study |
Genetic manipulations.
PCRs were performed using PfuUltra HF (Stratagene, La Jolla, CA) according to the manufacturer's instructions. Restriction enzyme digests were performed using enzymes and buffers from New England Biolobs (Beverly, MA) obtained through the University of Iowa Tissue Culture/Hybridoma Facility.
The FIG4 open reading frame was PCR amplified from LWY7235 using a GCGAGGATCCCCCACGTATCTGATCTTCGC primer, which adds a BamHI site 1,200 bp upstream of FIG4, and a GCGCAAGCTTATGACAGCGATCGGGCTTCC primer, which adds a HindIII site about 740 bp downstream of FIG4. The PCR product was ligated into BamHI- and HindIII-gapped pRS415 to generate pRS415_FIG4. The PCR product was also ligated into BamHI- and HindIII-gapped pUC18 as a first step towards generating a FIG4 knockout strain (see below).
Chromosomal deletion of FIG4 was generated by PCR amplifying the TRP1 marker from pRS424 and adding 5′ SpeI and 3′ SwaI sites. The PCR product was ligated into SpeI- and SwaI-gapped pUC18-FIG4. This pUC18-fig4::TRP1 construct was cut with BamHI and PvuI and the resulting 2.5-kb fragment was transformed into the LWY6725 wild-type diploid. TRP1+ transformants were sporulated and tetrads were analyzed by PCR to verify correct integration of the fig4::TRP1 cassette.
The FIG4-HA (hemagglutinin tagged) construct was generated by PCR amplifying FIG4 plus the promoter sequence using the primers TACGATGCCCAAGCTTGGGTAGTACATATCTTCTGTG and GTAACCTTTTGCGGCCGCCAAGTTGTATATCTTTAG. This resulted in a product with a HindIII site at the 5′ end and a NotI site at the 3′ end. This product was then ligated into a HindIII- and NotI-gapped pRS415-3xHA plasmid, resulting in a FIG4-HA fusion construct.
Chromosomal HA-FAB1 was generated by transposon insertion of a FAB1-specific construct obtained through the TRIPLES database (http://ygac.med.yale.edu/triples/default.htm) (16, 23). Briefly, the transposon clone V24B2 was linearized with NotI and the ∼8-kb linearized transposon was transformed into the LWY6725 wild-type diploid and transformants were selected on synthetic complete-Ura plates. The transposon was then popped out through galactose-induced cre recombinase activity, removing the URA3 marker and leaving behind a 3× HA tag at amino acid position 112 of Fab1p. Heterozygous diploid candidates were sporulated and spores were tested for correct insertion of the 3× HA construct, wild-type growth rate, normal vacuole morphology, and expression of HA-Fab1p.
FM4-64 labeling of yeast vacuoles.
Approximately 0.3 units of cells at an optical density at 600 nm (OD600) were collected and resuspended in 600 μl fresh medium. To this solution was added 2 μl of FM4-64 (2 μg/μl dissolved in dimethyl sulfoxide) (Molecular Probes, Eugene, OR) and 50 μl 1 M piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 6.8. The mixture was incubated at 24°C for 15 min. Cells were washed twice with fresh medium and allowed to chase for 2.5 h. Following this chase cells were untreated or subjected to hyperosmotic shock by resuspension in medium containing 0.45 M NaCl. This concentration of NaCl elicits the same changes in vacuole volume as 0.9 M NaCl (data not shown) but causes less perturbation of the refractive index of the medium, which leads to better imaging. Cells were viewed on a Zeiss Axioplan II microscope, and images were captured with an RT-Spot camera (Diagnostic Instruments) and subsequently analyzed with Metamorph software (Universal Imaging Corporation).
Inositol extraction.
Cells were labeled with [3H]inositol, and total cellular phosphatidylinositol was extracted, deacylated and measured as described previously (3) with the following changes. Cells were lysed in the presence of 0.5-mm zirconia beads (Biospec, Bartlesville, OK) on a Beadbeater (Biospec) for 2 min at room temperature followed by 2 min on ice. This was repeated two more times. The value of each phosphatidylinositol (PI3P, PI4P, PI3,5P2, and PI4,5P2) reported here is its percent of total phosphatidylinositol (PI, PI3P, PI4P, PI3,5P2, and PI4,5P2) extracted for each sample. Thus, each sample is normalized for number of cells and incorporation of [3H]inositol.
Preparation of antibodies to Vac7p.
To obtain antigen for generation of anti-Vac7p sera we constructed a pGEX-KG-GST-VAC7 vector. The full-length VAC7 open reading frame was PCR amplified using the primers GGGGGTGATGACAGAAGAAGATAGA and ACGCGTCGACTCACTTCTTACCAGGATGGA. This product was ligated into SmaI-cut pGEX-KG and transformed into Escherichia coli DH5α. Since full-length GST-Vac7p was insoluble, the pGEX-KG-VAC7 construct was cut with SacI and religated. The result was a vector expressing GST fused to the first 152 amino acids of Vac7p. Soluble GST-Vac7p was isolated on glutathione-Sepharose beads and this antigen was used to generate Vac7p antibodies in rabbits.
Immunofluorescence.
Immunofluorescence was carried out as described previously (2). Briefly, 37% formaldehyde was added to 5 OD600 units of log-phase cells for 50 min at 24°C. Cells were treated with Oxalyticase (Enzogenetics, Eugene, OR) for 15 min at 30°C. Spheroplasts were then fixed on slides and blocked with 1% bovine serum albumin-phosphate-buffered saline for 15 min. Primary antibodies were added for 2 h at 24°C, diluted 1:25 in 1% bovine serum albumin-phosphate-buffered saline. The primary antibodies used were goat anti-Vac14p (3) and mouse anti-Vph1p (Molecular Probes) for wild-type cells or rabbit anti-Vac8p (32) and mouse anti-HA (Covance, Princeton, NJ) for FIG4-HA cells. Secondary antibodies were added for 1 h at 24°C, diluted 1:100 in 1% bovine serum albumin-phosphate-buffered saline. These included donkey anti-goat-Alexa Fluor 488 (Molecular Probes) and donkey anti-mouse-rhodamine red (Jackson ImmunoResearch Laboratories, West Grove, PA) for wild-type cells or goat anti-rabbit-Alexa Fluor 488 (Molecular Probes) and donkey anti-mouse-rhodamine red (Jackson ImmunoResearch Laboratories) for FIG4-HA cells. Images were collected using a Bio-Rad 1024 confocal microscope and analyzed using Metamorph software (Universal Imaging Corporation).
Subcellular fractionation.
Cells were grown at 24°C to log phase and 12.5 OD600 units of cells were harvested. All subsequent steps were carried out on ice. Pelleted cells were resuspended in 250 μl cold cytosol buffer (20 mM HEPES [pH 6.8], 0.15 M potassium acetate, 10 mM MgCl2, 0.25 M sorbitol) containing 2.5 μl protease inhibitor cocktail (Sigma catalog no. P8215) and 1 μl chymostatin (Sigma catalog no. C7268). To this slurry was added 0.1 g acid-washed glass beads. Cells were lysed using a microtube mixer (Tomy Tech, Fremont, CA) at maximum speed for 2 min followed by 2 min of rest. This was repeated four times. Lysates were spun at 300 × g for 1 min to clear extracts and the supernatant was spun again at 13,000 × g for 10 min. The resulting supernatant (S13) was harvested and the pellet (P13) was resuspended in an equal volume of fresh cytosol cocktail. A unique recipe of 5× Laemmli sample buffer was used when working with high-molecular-weight proteins, containing 62.5 mM Tris (pH 6.8), 15% glycerol, 5% sodium dodecyl sulfate (SDS), 5% 2-mercaptoethanol, and bromophenol blue. This sample buffer was added to the fractions and heated at 80°C for 5 min before subjecting the samples to SDS-polyacrylamide gel electrophoresis. Gels were wet transferred to nitrocellulose for >800 Vh to ensure transfer of the higher-molecular-weight proteins analyzed in this study.
Western blot analysis of proteins was performed using goat anti-Vac14p, mouse anti-HA (Covance), or rabbit anti-Vac7p sera as primary antibodies. Horseradish peroxidase-conjugated donkey anti-goat, rabbit anti-mouse, or goat anti-rabbit antibodies (Molecular Probes) were used as secondary antibodies along with SuperSignal chemiluminescence (Pierce, Rockford, IL) to visualize immunoreactive proteins.
RESULTS
PI3,5P2 levels increase transiently in response to hyperosmotic shock.
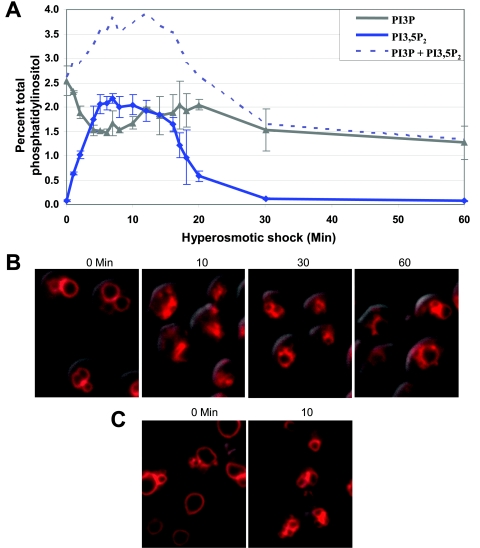
A detailed analysis of changes in the level of PI3,5P2 following hyperosmotic shock revealed that initiation of the change is rapid. At the earliest time point measurable, 1 min after introduction into hyperosmotic medium, the cellular level of PI3,5P2 rose fivefold (Fig. 1A). Surprisingly, the maximum increase (20-fold) in PI3,5P2 level occurred within 5 min of exposure to hyperosmotic medium. This maximum level persisted for 10 min. Strikingly, the PI3,5P2 level then decreased at a rate similar to its increase. By 30 min following hyperosmotic shock, PI3,5P2 levels returned to near the basal level. The rapid decrease in PI3,5P2 level occurred even though the cells remained in hyperosmotic medium. This suggests that PI3,5P2 is part of a rapid response to acute changes in the osmolarity of the environment.
FIG. 1.
Both PI3,5P2 levels and vacuole volume transiently change after hyperosmotic shock. (A) Wild-type (LWY7235) cells were labeled with [3H]inositol for 12 h and then treated with NaCl for the indicated times. Total cellular phosphatidylinositol was extracted, deacylated, and analyzed by high-pressure liquid chromatography. To normalize each data point for number of cells and [3H]inositol incorporation, each value is shown as the percent of total [3H]phosphatidylinositol extracted. Each data point is an average of at least two independent experiments. (B) Wild-type cells were labeled with FM4-64 dye to visualize vacuole volume and number of vacuole lobes. Cells were then treated with NaCl for the indicated times and viewed by fluorescence microscopy. Representative fields are shown. (C) Vacuole membranes undergo scission as vacuole volume is reduced following hyperosmotic shock. Mutant cells (fig4Δ) which have basal and hyperosmotic shock-induced PI3,5P2 levels that are lower than normal and thus larger vacuoles were labeled with FM4-64 dye to visualize vacuole volume and number of vacuole lobes. Cells were then treated with NaCl for 10 min and viewed by fluorescence microscopy. Representative fields are shown.
Vacuole volume parallels PI3,5P2 levels.
Hypoosmotic shock induces vacuole fusion and a subsequent increase in vacuole volume (33). Therefore, we tested the effect of hyperosmotic shock on vacuole volume. Ten minutes following hyperosmotic shock, vacuole volume decreased greatly (Fig. 1B). Thirty minutes following hyperosmotic shock, when the level of PI3,5P2 had returned close to the original level, vacuoles returned close to their original volume. In addition, mutants that lack PI3,5P2 have unusually large vacuoles, whereas strains with elevated levels of PI3,5P2 have vacuoles that are smaller than normal (3). These studies suggest that one of the downstream consequences of the acute regulation of PI3,5P2 levels is the regulation of vacuole volume.
The hyperosmotic shock-induced decrease in vacuole volume is likely due to transport of water and osmolytes out of the vacuole to help minimize changes in cytoplasmic osmolarity. PI3,5P2 may play a role in this process by activating water channels (see Discussion). Similarly, PI3,5P2 may help accommodate the water loss by increasing vacuole fission. This would result in a decrease in vacuole volume without a decrease in membrane surface area. Due to the small size of wild-type vacuoles, it is difficult to determine if membrane fission increases following hyperosmotic shock (Fig. 1B). Therefore, we used a mutant (fig4Δ) that has larger vacuole lobes under basal conditions but has a fourfold increase in PI3,5P2 level in response to hyperosmotic shock. We observed that the number of vacuole lobes increased upon salt treatment (Fig. 1C). This is consistent with an increase in vacuole membrane fission. These results demonstrate a strong correlation between PI3,5P2 level and the degree of vacuole fission.
PI3P levels decrease as the levels of PI3,5P2 increase following hyperosmotic shock.
Following hyperosmotic shock, the level of PI3P decreased at a rate similar to the rate of PI3,5P2 increase (Fig. 1A). This decrease in the levels of PI3P could be due to increased conversion to PI3,5P2, supporting the hypothesis that Fab1p activity is increased. Alternatively, the decrease in the level of PI3P may indicate that the level of PI3,5P2 increases because PI3,5P2 turnover is inhibited, suppressing generation of PI3P from PI3,5P2. This second hypothesis is improbable because it is highly unlikely that the cell uses turnover of PI3,5P2 as a significant means of generating PI3P. Specifically, when PI3,5P2 is absent from the cell, as in the fab1Δ mutant, PI3P levels under basal conditions are normal and then increase following hyperosmotic shock (3). Furthermore, PI3,5P2 levels are normally 20-fold lower than those of PI3P, making turnover of PI3,5P2 a poor source for PI3P production. These observations taken together strongly support the hypothesis that the depletion of PI3P following hyperosmotic shock is due to the activation of Fab1p and subsequent synthesis of PI3,5P2.
Note that the decrease in the level of PI3P following hyperosmotic shock is half of what would be expected from the increase in PI3,5P2. This is likely due to a stimulation in the synthesis of both PI3P and PI3,5P2. This hypothesis is supported by the observation that total PI3P levels, assessed as the sum of PI3P and PI3,5P2, increased in a wild-type strain (Fig. 1A). These observations demonstrate that hyperosmotic shock increases the rate of synthesis of PI3P via activation of Vps34p and increases the rate of synthesis of PI3,5P2 via activation of Fab1p.
Rates of synthesis and turnover of macromolecules are often measured directly via pulse-chase experiments. However, this is not technically feasible for studies of metabolites of PI. Nonphosphorylated PI constitutes roughly 95% of the phosphoinositides in yeast. Moreover, the PI pool changes rapidly due to interconversion between phosphoinositide species. Thus, rates of synthesis and turnover of PI3,5P2 must be inferred indirectly from changes in the steady-state level of the lipid.
PI4P and PI4,5P2 levels also change in response to hyperosmotic shock.
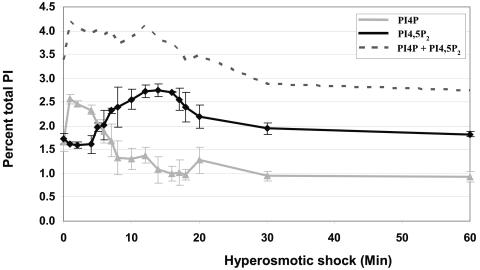
Following hyperosmotic shock, the levels of PI4P and PI4,5P2 also change. Within 1 min there was a very rapid increase in PI4P level (Fig. 2). At 5 min there was a subsequent increase in PI4,5P2 level. Note that as PI4,5P2 levels increased, levels of its precursor PI4P decreased. This suggests that the increase in PI4,5P2 likely depends on the initial increase in PI4P. This analysis also suggests that PI4P and PI4,5P2 are part of a response to hyperosmotic shock. Since PI4P and PI4,5P2 are reported to localize almost exclusively to the Golgi apparatus and plasma membrane (1, 29), they are unlikely to be involved in the vacuole response to hyperosmotic shock.
FIG. 2.
PI4P and PI4,5P2 levels change transiently in response to hyperosmotic shock. Total phosphatidylinositol was extracted from wild-type (LWY7235) cells labeled with [3H]inositol as described in the legend for Fig. 1. The percentages of PI4P and PI4,5P2 in the cells after hyperosmotic stimulation are shown here. Each data point is an average of at least two independent experiments.
In strains lacking Fab1p or any of its putative activators, hyperosmotic shock-induced changes in PI4P and PI4,5P2 levels are similar to those observed in a wild-type strain (data not shown). The regulation of PI4P and PI4,5P2 levels following hyperosmotic shock appears to be independent of the regulation of PI3,5P2 levels.
Membrane localization of Fab1p, Vac7p, Vac14p, and Fig4p does not change upon hyperosmotic shock.
The requirement for PI3,5P2 in several vacuole-related functions suggests that the vacuole is the site of PI3,5P2 synthesis and turnover. In addition, Fab1p, along with its putative activators Vac7p and Vac14p, is found on the vacuole membrane (2, 3, 8, 12). Furthermore, the PI3,5P2 5-phosphatase Fig4p is also found on the vacuole membrane (24). We tested whether, concomitant with a hyperosmotic shock-induced change in vacuole volume (Fig. 1B and C), there is a change in the localization of any of the proteins that regulate PI3,5P2 levels.
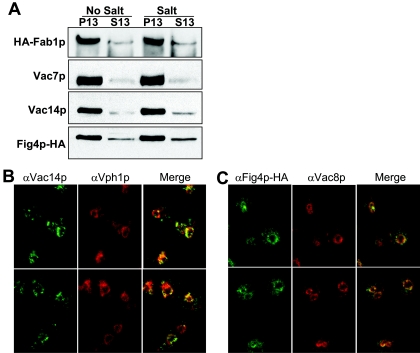
Protein distribution was measured using cellular fractionation because fluorescence microscopy cannot accurately measure the distribution of proteins between the membrane and cytoplasm; the membrane pool is more concentrated, leading to a higher fluorescence signal. In fractionation experiments, large organelles, such as the vacuole, Golgi apparatus, endoplasmic reticulum, and nucleus pellet after brief centrifugation at 13,000 × g (P13). We observed that, under basal conditions, Fab1p, Vac7p, Vac14p, and Fig4p associated with the vacuole-rich fraction (Fig. 3A). In addition, a small amount of Fig4p also localized to a soluble fraction. Hyperosmotic shock did not affect the distribution of Fab1p, Vac7p, Vac14p, or Fig4p (Fig. 3A). Thus, the hyperosmotic shock-induced changes in the level of PI3,5P2 are likely due to regulation of the enzymatic activity of Fab1p and/or Fig4p.
FIG. 3.
Fab1p and its putative activators localize on the vacuole membrane. (A) Wild-type (LWY7235), chromosomal HA-FAB1 (LWY6830), and fig4Δ cells expressing FIG4-HA were lysed in the absence of detergent and extracts were separated into membrane (P) and soluble (S) fractions by centrifugation at 13,000 × g. The P13 fraction contains large organelles such as the vacuole, Golgi apparatus, endoplasmic reticulum, and nucleus. Western blot analysis revealed the levels of the proteins in each fraction. The same strains were also treated with NaCl for 10 min prior to lysis. Data are representative of three independent experiments.(B) Vac14p colocalizes with the vacuole membrane. Wild-type (LWY7235) cells were fixed with formaldehyde and probed with anti-Vac14p and anti-Vph1p sera. (C) Fig4p-HA colocalizes with the vacuole membrane. fig4Δ cells expressing FIG4-HA were treated as described above and probed with anti-Vac8p serum and anti-HA monoclonal antibody.
The localization of Vac14p and Fig4p as assessed by immunofluorescence microscopy was consistent with the fractionation studies and showed that Vac14p and its binding partner Fig4p localize on the vacuole membrane (Fig. 3B and C). Some Fig4p was present in the cytoplasm (Fig. 3C).
Vac7p and Vac14p are required for the hyperosmotic shock-induced increase in the level of PI3,5P2 but not PI3P.
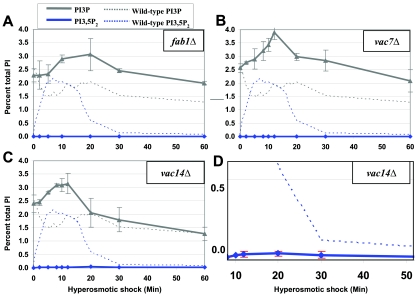
The changes in the levels of PI3P and PI3,5P2 following hyperosmotic shock were assessed for strains lacking Fab1p, Vac7p, or Vac14p. In both a fab1Δ and a vac7Δ strain there was no detectable PI3,5P2 (Fig. 4A and B). The vac14Δ strain produced no detectable PI3,5P2 under basal conditions (Fig. 4C). However, following hyperosmotic shock, a low level of PI3,5P2 was observed (Fig. 4D). Consistent with this ability to produce a low level of PI3,5P2, the phenotypes observed in the vac14Δ strain are less severe than those found in vac7Δ or fab1Δ strains (3). Note that the low level of PI3,5P2 observed in the vac14Δ strain was only 2% of the level observed in wild-type cells.
FIG. 4.
Vac7p and Vac14p play a role in PI3,5P2 synthesis and possibly turnover. (A to C) fab1Δ (LWY2055), vac7Δ (LWY2054), and vac14Δ (LWY5177) cells were labeled with [3H]inositol for 12 h and total phosphatidylinositol was extracted. PI3P and PI3,5P2 levels are shown for each strain after exposure to 0.9 M NaCl for the indicated times. These levels are superimposed over wild-type PI3P and PI3,5P2 levels (dotted lines). Each data point is an average of at least two independent experiments. (D) Data from panel C rescaled to show that the low level of PI3,5P2 does not decrease after 30 min.
The mutants defective in PI3,5P2 synthesis showed an increase in PI3P level following hyperosmotic shock (Fig. 4A to C). This again demonstrates that PI3P synthesis is also stimulated by hyperosmotic shock. Note that neither Fab1p nor its putative activators Vac7p and Vac14p play a role in the hyperosmotic shock-induced increase in PI3P synthesis.
Fig4p and Vac14p are required for rapid turnover of PI3,5P2 following hyperosmotic shock.
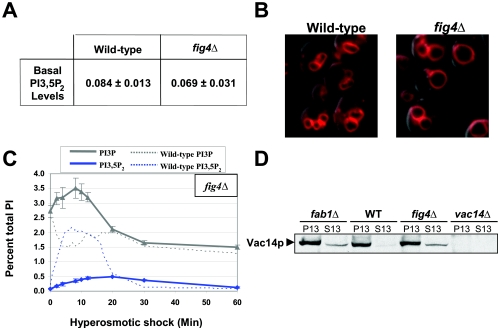
Recombinant Fig4p catalyzes the conversion of PI3,5P2 to PI3P in vitro. Yet, in a fig4Δ mutant, the basal levels of PI3,5P2 are the same as in a wild-type strain (Fig. 5A) (see also reference 24). Likewise, vacuole volume in a fig4Δ strain is similar to that of the wild type (Fig. 5B). These findings suggest that Fig4p may not catalyze the conversion of PI3,5P2 to PI3P in vivo. However, following hyperosmotic shock, turnover of PI3,5P2 is defective. At 30 min there was very little decrease in the hyperosmotic shock-induced level of PI3,5P2 (Fig. 5C). This provides the first evidence that Fig4p functions in vivo as a PI3,5P2 5-phosphatase. This also strongly suggests that Fig4p is the major enzyme that functions in the rapid disappearance of PI3,5P2 following hyperosmotic shock. Since PI3,5P2 levels in a fig4Δ strain return to normal by 60 min, other pathways must exist that are capable of rapidly degrading PI3,5P2 (see Discussion). This redundancy is likely to be the reason why fig4Δ cells have a nearly normal level of PI3,5P2 under basal conditions.
FIG. 5.
Fig4p is required for both PI3,5P2 synthesis and turnover. (A) PI3,5P2 levels were determined for wild-type (LWY7235) and fig4Δ (LWY6474) cells. The data are averages of three independent experiments. (B) Wild-type and fig4Δ cells were labeled with FM4-64 to visualize vacuole membranes. Images were captured by fluorescence microscopy. Representative fields are shown. (C) Time course of PI3P and PI3,5P2 level changes in fig4Δ cells after exposure to NaCl. These levels are superimposed over the PI3P and PI3,5P2 levels in the wild type (dotted lines). Each data point is an average of at least two independent experiments. (D) Vac14p is partially mislocalized in fig4Δ and fab1Δ cells. Cells were lysed under nondetergent conditions and extracts were spun at 13,000 × g to determine the extent of membrane distribution. Protein levels were determined using Western blot analysis. The data are representative of three independent experiments.
Fig4p is not required for the rapid turnover of PI3P following hyperosmotic shock (Fig. 5C). This is consistent with the observation that, in vitro, Fig4p removes the 5′ phosphate from PI3,5P2 but not the 3′ phosphate from PI3P (24). PI3P levels are quite similar in the absence of Fig4p (fig4Δ) (Fig. 5C) and in the presence of Fig4p (fab1Δ, vac7Δ, and vac14Δ) (Fig. 4), before and after hyperosmotic shock.
Since Fig4p physically associates with Vac14p it is possible that Vac14p also plays a role in the turnover of PI3,5P2. Indeed, the low level of PI3,5P2 produced in the vac14Δ strain following hyperosmotic shock was subsequently turned over more slowly than in the wild-type strain (Fig. 4D). This delay in the turnover of PI3,5P2 may be due to the absence of Vac14p-dependent regulation of Fig4p and/or to the mislocalization of Fig4p in the absence of Vac14p. In fact, the level of Fig4p protein in a vac14Δ strain is greatly reduced (see Fig. 5C in reference 10). Thus, Vac14p appears to be important for the stability of Fig4p and at a minimum plays an important role in the regulation of PI3,5P2 turnover via the localization of Fig4p.
Fig4p plays a role in the hyperosmotic shock-induced increase in PI3,5P2 levels.
Surprisingly, fig4Δ cells synthesize only 25% of the wild-type PI3,5P2 level following hyperosmotic shock (Fig. 5C). This suggests Fig4p also plays a role in the hyperosmotic shock-induced elevation of PI3,5P2 levels. This role may be direct or indirect. For example, all of the proteins required for PI3,5P2 metabolism may form a complex and, in the absence of one protein, the other proteins may not localize properly. Indeed, Vac14p localization to the vacuole is partially defective in fab1Δ cells (Fig. 5D) (3). In addition, about 25% of Vac14p is mislocalized in fig4Δ cells compared to wild-type cells (Fig. 5D). Thus, Vac14p associates with both Fab1p and Fig4p on the vacuole membrane.
While 25% of Vac14p is mislocalized in fig4Δ cells, this small degree of mislocalization is unlikely to be the sole cause of the large defect in hyperosmotic shock-induced PI3,5P2 level elevation. This raises the possibility that Fig4p has a more direct role in increasing Fab1p activity.
We have recently found that a previously identified Fig4p point mutation (fig4G519R) (24) and a second mutation (fig4D469N) do not affect the localization of Vac14p (10). These point mutants are defective in PI3,5P2 5-phosphatase activity (10, 24). Notably, these mutants also have a defect in the hyperosmotic shock-induced elevation in PI3,5P2 (10). These findings support the hypothesis that Fig4p likely has dual roles and functions in both the synthesis and turnover of PI3,5P2.
DISCUSSION
Regulation of PI3,5P2 levels plays an important role in an array of organisms and cell types. In plants, as in yeast, the level of PI3,5P2 changes in response to an acute hyperosmotic challenge (7, 18, 37). In mammalian cells, PI3,5P2 functions in both endomembrane maintenance and intracellular trafficking. Reduced levels of this phospholipid result in abnormally large endosomal structures (14, 15, 31). In addition, tight control of the levels of PI3,5P2 and other phosphoinositides is important for mammalian cell resistance to selected bacterial pathogens. Some pathogens have evolved mechanisms to subvert this response. For example, Salmonella species secrete SopB (13), a phosphoinositide phosphatase which uses PI3,5P2 as one of its substrates (17). Secretion of such phosphoinositide phosphatases likely impedes trafficking of the phagocytosed pathogen to the lysosome, thereby promoting survival of the pathogen in the host cell (reviewed in reference 21).
The rapid, large, and transient changes observed in the level of PI3,5P2 in yeast following hyperosmotic shock make it tempting to speculate that in higher eukaryotes there may be many signaling pathways that use PI3,5P2 where response to a specific stimulus occurs within minutes. Indeed, epidermal growth factor-induced degradation of the epidermal growth factor receptor is significantly decreased in mammalian cells which overexpress the PI3,5P2 binding domain of mVps24 (36). Also, a defect in production of PI3,5P2 in mammalian cells causes a defect in insulin-induced GLUT4 trafficking from endosomal stores to the plasma membrane (14, 25).
In yeast cells, within minutes following hyperosmotic shock, PI3,5P2 is used to decrease vacuole volume. This control of vacuole volume may be achieved in part by Atg18p (Svp1p). Atg18p binds PI3,5P2 and is required for retrograde traffic from the vacuole (9). Likewise, an atg18Δ mutant has grossly enlarged vacuoles. For both retrograde traffic and vacuole volume control, Atg18p may function in vacuole membrane fission and be directly regulated by the level of PI3,5P2.
Vacuole volume may also be controlled in part by the movement of ions, osmolytes, and water to and from the vacuole interior. In support of this hypothesis, maximum release of vacuolar Ca2+ through Yvc1p occurs at 1 min following hyperosmotic shock (6), a time at which the PI3,5P2 level has already risen fivefold. Similarly, within 1 min following hyperosmotic shock, the glycerol export channel at the plasma membrane (Fps1p) becomes inactivated (30), allowing glycerol to accumulate as the cell responds to the dramatic change in intracellular osmolarity. Both Fps1p inactivation and PI3,5P2 synthesis are independent of the well-characterized osmotic stress HOG1 mitogen-activated protein kinase pathway (7, 30).
Detailed analysis of PI3,5P2 levels following hyperosmotic shock in the fig4Δ mutant demonstrates that Fig4p functions in vivo as the primary PI3,5P2 5-phosphatase. In wild-type cells, the hyperosmotic shock-induced 20-fold increase in PI3,5P2 is degraded by 30 min. In contrast, in cells lacking Fig4p, the 30-min time point shows very little decrease in the hyperosmotic shock-induced PI3,5P2 level. In addition, the absence of Vac14p, a Fig4p binding partner, also leads to a dramatic defect in PI3,5P2 turnover.
While Fig4p is the primary means of PI3,5P2 turnover, there also appears to be a secondary mechanism. In S. cerevisiae, this secondary means of degradation is likely carried out by phosphoinositide 5-phosphatases and/or polyphosphatases. PI3,5P2 is unlikely to be degraded by a phospholipase because no PI3,5P2-specific phospholipase has been found nor has soluble inositol 1,3,5-trisphosphate been discovered (27). Similarly, PI3,5P2 is unlikely to be turned over by a phosphoinositide 3-phosphatase because no PI5P has been detected in S. cerevisiae. Like Fig4p, the proteins Sac1p, Sjl1p, Sjl2p, Sjl3p, and Inp54p have a Sac1 phosphoinositide polyphosphatase and/or a synaptojanin-like phosphoinositide 5-phosphatase domain. Moreover, PI3,5P2 levels are elevated under basal conditions when multiple combinations of these genes are knocked out (11, 20, 29). However, none of these other phosphatases localizes to the vacuole membrane. Thus, they likely provide only a secondary means to break down PI3,5P2.
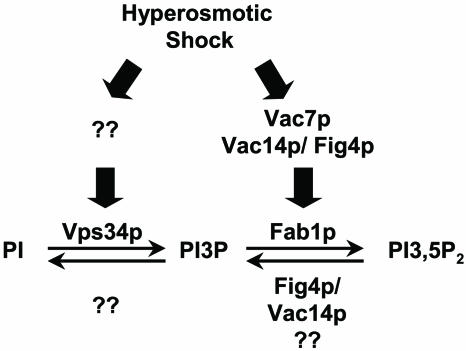
Surprisingly, our studies also revealed that Fig4p is necessary for proper elevation of PI3,5P2 levels in response to hyperosmotic shock (Fig. 6). There are several possible explanations. Since Fig4p and Vac14p form a complex, the absence of Fig4p may result in defects in Vac14p-dependent Fab1p activation. In this scenario Fig4p does not play a role in Fab1p activation other than to help localize Vac14p. However, because 75% of Vac14p is localized to the vacuole membrane even in the absence of Fig4p, it is likely that Fig4p plays a more direct role.
FIG. 6.
Model for the roles of the Vac14p/Fig4p complex in both synthesis and turnover of PI3,5P2. Hyperosmotic shock increases the levels of PI3P and PI3,5P2. The PI3,5P2 increase is dramatically compromised in the absence of Fig4p, Vac7p, or Vac14p, demonstrating that all three proteins are important for Fab1p activation. Turnover of PI3,5P2 following hyperosmotic stimulation is compromised in the absence of Fig4p and Vac14p. Turnover of PI3P in the absence of any Fab1p activator or Fab1p is normal.
Fig4p may have a direct role in Fab1p activation and function as a protein phosphatase that activates Vac14p and/or Fab1p. Recent studies from our laboratory (10) have analyzed the in vivo roles of Vac7p, Vac14p, and Fig4p in hyperosmotic shock-induced elevation of the PI3,5P2 level. These studies confirm that Vac7p, Vac14p, and Fig4p are all required for the normal elevation of PI3,5P2 induced by hyperosmotic shock and support the model that Fig4p has a direct role in the activation of Fab1p. In vitro assays will provide a direct approach to test these possibilities.
Acknowledgments
We thank Robert Piper and Mark Stamnes for helpful discussions. We also thank Cecilia Bonangelino for generating antibodies to Vac7p and Vac14p.
This work was supported by National Institutes of Health grant GM50403 to L.S.W. J.E.D. was supported by American Heart Predoctoral Fellowships 0215265Z and 0315239Z and by an NRSA Fellowship from the University of Iowa Center on Aging.
REFERENCES
- 1.Audhya, A., and S. D. Emr. 2002. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Dev. Cell 2:593-605. [DOI] [PubMed] [Google Scholar]
- 2.Bonangelino, C. J., N. L. Catlett, and L. S. Weisman. 1997. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol. Cell. Biol. 17:6847-6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonangelino, C. J., J. J. Nau, J. E. Duex, M. Brinkman, A. E. Wurmser, J. D. Gary, S. D. Emr, and L. S. Weisman. 2002. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14p, an activator of the lipid kinase Fab1p. J. Cell Biol. 156:1015-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant, N. J., R. C. Piper, L. S. Weisman, and T. H. Stevens. 1998. Retrograde traffic out of the yeast vacuole to the TGN occurs via the prevacuolar/endosomal compartment. J. Cell Biol. 142:651-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110:119-122. [DOI] [PubMed] [Google Scholar]
- 6.Denis, V., and M. S. Cyert. 2002. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 156:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dove, S. K., F. T. Cooke, M. R. Douglas, L. G. Sayers, P. J. Parker, and R. H. Michell. 1997. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390:187-192. [DOI] [PubMed] [Google Scholar]
- 8.Dove, S. K., R. K. McEwen, A. Mayes, D. C. Hughes, J. D. Beggs, and R. H. Michell. 2002. Vac14 controls PtdIns(3, 5)P2 synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr. Biol. 12:885-893. [DOI] [PubMed] [Google Scholar]
- 9.Dove, S. K., R. C. Piper, R. K. McEwen, J. W. Yu, M. C. King, D. C. Hughes, J. Thuring, A. B. Holmes, F. T. Cooke, R. H. Michell, P. J. Parker, and M. A. Lemmon. 2004. Svp1p defines a family of phosphatidylinositol 3,5-bisphosphate effectors. EMBO J. 23:1922-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duex, J. E., F. Tang, and L. S. Weisman. 2006. The Vac14p/Fig4p complex and Vac7p independently increase PI3,5P2 levels in response to hyperosmotic shock. J. Cell Biol. 172:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gary, J. D., T. K. Sato, C. J. Stefan, C. J. Bonangelino, L. S. Weisman, and S. D. Emr. 2002. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol. Biol. Cell 13:1238-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gary, J. D., A. E. Wurmser, C. J. Bonangelino, L. S. Weisman, and S. D. Emr. 1998. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J. Cell Biol. 143:65-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez, L. D., K. Hueffer, M. R. Wenk, and J. E. Galan. 2004. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304:1805-1807. [DOI] [PubMed] [Google Scholar]
- 14.Ikonomov, O. C., D. Sbrissa, K. Mlak, M. Kanzaki, J. Pessin, and A. Shisheva. 2002. Functional dissection of lipid and protein kinase signals of PIKfyve reveals the role of PtdIns 3,5-P2 production for endomembrane integrity. J. Biol. Chem. 277:9206-9211. [DOI] [PubMed] [Google Scholar]
- 15.Ikonomov, O. C., D. Sbrissa, and A. Shisheva. 2001. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J. Biol. Chem. 276:26141-26147. [DOI] [PubMed] [Google Scholar]
- 16.Kumar, A., K. H. Cheung, P. Ross-Macdonald, P. S. Coelho, P. Miller, and M. Snyder. 2000. TRIPLES: a database of gene function in Saccharomyces cerevisiae. Nucleic Acids Res. 28:81-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus, S. L., M. R. Wenk, O. Steele-Mortimer, and B. B. Finlay. 2001. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 494:201-207. [DOI] [PubMed] [Google Scholar]
- 18.Meijer, H. J., D. Nullin, H. Ende, A. Musgrave, and T. Munnik. 1999. Hyperosmotic stress induces rapid synthesis of phosphatidyl-d-inositol 3,5-bisphosphate in plant cells. Planta 208:294-298. [Google Scholar]
- 19.Odorizzi, G., M. Babst, and S. D. Emr. 1998. Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95:847-858. [DOI] [PubMed] [Google Scholar]
- 20.Parrish, W. R., C. J. Stefan, and S. D. Emr. 2004. Essential role for the myotubularin-related phosphatase Ymr1p and the synaptojanin-like phosphatases Sjl2p and Sjl3p in regulation of phosphatidylinositol 3-phosphate in yeast. Mol. Biol. Cell 15:3567-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizarro-Cerda, J., and P. Cossart. 2004. Subversion of phosphoinositide metabolism by intracellular bacterial pathogens. Nat. Cell Biol. 6:1026-1033. [DOI] [PubMed] [Google Scholar]
- 22.Rameh, L. E., K. F. Tolias, B. C. Duckworth, and L. C. Cantley. 1997. A new pathway for synthesis of phosphatidylinositol-4,5-bisphosphate. Nature 390:192-196. [DOI] [PubMed] [Google Scholar]
- 23.Ross-Macdonald, P., P. S. Coelho, T. Roemer, S. Agarwal, A. Kumar, R. Jansen, K. H. Cheung, A. Sheehan, D. Symoniatis, L. Umansky, M. Heidtman, F. K. Nelson, H. Iwasaki, K. Hager, M. Gerstein, P. Miller, G. S. Roeder, and M. Snyder. 1999. Large-scale analysis of the yeast genome by transposon tagging and gene disruption. Nature 402:413-418. [DOI] [PubMed] [Google Scholar]
- 24.Rudge, S. A., D. M. Anderson, and S. D. Emr. 2004. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol. Biol. Cell 15:24-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sbrissa, D., O. C. Ikonomov, J. Strakova, and A. Shisheva. 2004. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology 145:4853-4865. [DOI] [PubMed] [Google Scholar]
- 26.Shaw, J. D., H. Hama, F. Sohrabi, D. B. DeWald, and B. Wendland. 2003. PtdIns(3,5)P2 is required for delivery of endocytic cargo into the multivesicular body. Traffic 4:479-490. [DOI] [PubMed] [Google Scholar]
- 27.Shears, S. B. 2004. How versatile are inositol phosphate kinases? Biochem. J. 377:265-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stefan, C. J., A. Audhya, and S. D. Emr. 2002. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Mol. Biol. Cell 13:542-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamas, M. J., K. Luyten, F. C. Sutherland, A. Hernandez, J. Albertyn, H. Valadi, H. Li, B. A. Prior, S. G. Kilian, J. Ramos, L. Gustafsson, J. M. Thevelein, and S. Hohmann. 1999. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 31:1087-1104. [DOI] [PubMed] [Google Scholar]
- 31.Tsujita, K., T. Itoh, T. Ijuin, A. Yamamoto, A. Shisheva, J. Laporte, and T. Takenawa. 2004. Myotubularin regulates the function of the late endosome through the gram domain-phosphatidylinositol 3,5-bisphosphate interaction. J. Biol. Chem. 279:13817-13824. [DOI] [PubMed] [Google Scholar]
- 32.Wang, Y. X., N. L. Catlett, and L. S. Weisman. 1998. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol. 140:1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, Y. X., E. J. Kauffman, J. E. Duex, and L. S. Weisman. 2001. Fusion of docked membranes requires the armadillo repeat protein Vac8p. J. Biol. Chem. 276:35133-35140. [DOI] [PubMed] [Google Scholar]
- 34.Whiteford, C. C., C. A. Brearley, and E. T. Ulug. 1997. Phosphatidylinositol 3,5-bisphosphate defines a novel PI 3-kinase pathway in resting mouse fibroblasts. Biochem. J. 323:597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto, A., D. B. DeWald, I. V. Boronenkov, R. A. Anderson, S. D. Emr, and D. Koshland. 1995. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol. Biol. Cell 6:525-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan, Q., P. R. Hunt, L. Frelin, T. A. Vida, J. Pevsner, and A. J. Bean. 2005. mVps24p functions in epidermal growth factor receptor sorting/trafficking from the early endosome. Exp. Cell Res. 304:265-273. [DOI] [PubMed] [Google Scholar]
- 37.Zonia, L., and T. Munnik. 2004. Osmotically induced cell swelling versus cell shrinking elicits specific changes in phospholipid signals in tobacco pollen tubes. Plant Physiol. 134:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]