Abstract
Giardia lamblia cell populations show 90% detachment from glass under normal forces of 2.43 ± 0.33 nN applied by centrifugation. Detachment forces were not significantly different for cells attached to positively charged, hydrophobic, or inert surfaces than for cells attached to plain glass. The insensitivity of attachment force to surface treatment is consistent with a suction-based mechanism of attachment.
For many parasitic organisms, the ability to bind tightly to host tissue is necessary for pathogenicity and survival. One such organism is Giardia lamblia (synonymous with G. intestinalis and G. duodenalis), a protistan parasite of the mammalian small intestine, which attaches securely but reversibly to the microvillus brush border of the host intestinal epithelium. Giardia is the most frequently identified cause of human intestinal infection in the United States and worldwide (5, 15, 23).
The ventral disk, a structure unique to Giardia, is a 9-μm-diameter concave spiral of cross-linked microtubules and associated proteins across the anterior underside of the cell. Attachment mechanisms proposed usually indicate the ventral disk as the principal source of attachment force (3). Proposed mechanisms of attachment to hard surfaces can be grouped into two categories: (i) binding and (ii) suction mechanisms. Binding models require close contact between a portion of the cell (such as the ventral disk or the cell periphery) and the substrate in order to generate attachment force via molecular-level interactions. Possible binding-based mechanisms include nonspecific interactions (electrostatic and van der Waals), hydrophobic interactions, or ligand-mediated specific binding. In contrast, suction-based mechanisms are those requiring a pressure differential under the ventral disk for generation of attachment force.
Previous theoretical and experimental studies of Giardia attachment (reviewed in reference 3) have revealed many aspects of cell behavior at surfaces; however, inconsistencies in methods and a lack of quantitative data on attachment forces have allowed only limited conclusions to be drawn about the mechanism of attachment. It is known that Giardia cells attach easily and reversibly in vitro to a variety of surfaces, including glass and polystyrene, allowing us to ask two important questions that provide insight into the mechanism of attachment. How strongly do Giardia trophozoites attach to a model surface in vitro? How does attachment change with surface treatments?
We developed a centrifugation technique to measure the force of attachment of Giardia cell populations on plain glass and chemically modified substrates. The use of this simplified system allows separation of physical attachment from molecular interactions with host cells, enabling greater control of substrate surface properties, ease of modeling, and comparison to other eukaryotic cell-glass adhesion results. Additionally, centrifugation offers the advantage of being able to apply uniform normal forces to large populations of cells. The method is similar to that of other centrifugation studies (2, 7, 12, 16, 20, 21), except that our instrument was constructed specifically to be used in medium with Giardia, which can be exposed to only relatively low amounts of oxygen.
Giardia lamblia trophozoites of the WBC6 strain were cultured for 48 h at 37°C in supplemented growth media (11), detached from culture tubes by being chilled for 20 min at 4°C, transferred into custom-made sample holders capped with KOH-ethanol-cleaned circular glass slides, and incubated for 60 min at 37°C to allow cells to attach to the glass. Sample holders fit into a hanging bucket centrifuge rotor in which cells were spun for 5 min over a range of spin speeds from 500 to 13,000 rpm (4.0 pN to 2.7 nN normal force) at 37°C. Controls were prepared in the same manner as spun samples except that they were inverted to subject cells to gravity (1 × g) for 10 min in lieu of centrifugation.
Immediately after centrifugation, the glass slide was removed from the sample holder, and images of 8 to 12 areas of the slide were taken (n = 3,000 to 4,000 cells per image). Means of cell counts were calculated for each image and compared to noncentrifuged samples to determine the fraction of cells detached over a range of forces, where force is given by F = (ρc − ρm) · Vc · RCF, ρc is the density of the cell, ρm is the density of the medium (taken to be 1.000 g/cm3), Vc is the cell volume, and the RCF, or relative centrifugal force in units of g, depends on the spin speed and centrifuge radius, r. Following the method of Nieminski et al. (17), cell mass density was measured to be 1.064 g/cm3. To obtain cell volume, we multiplied the average cell area as determined by phase-contrast microscopy (133.2 μm2 ± 17.9 μm2) by an overall cell thickness of 1.5 μm, estimated from published transmission electron micrographs (4).
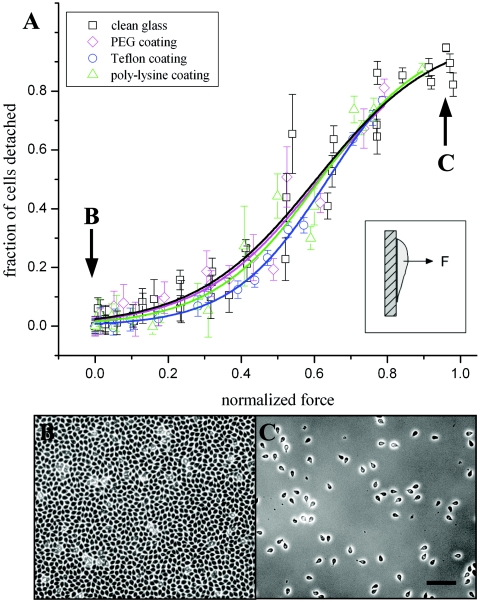
We measured 50% detachment of cells from plain glass at a force of 1.51 ± 0.20 nN and 90% detachment at 2.43 ± 0.33 nN (Fig. 1A). Typical numbers of attached cells per field of view dropped from more than 3,900 (Fig. 1B) to fewer than 500 (Fig. 1C) over the entire force range. A sigmoidal curve, which has been observed in other cell attachment studies (6, 19), was found to provide a good fit to the attachment data (R2 = 0.96, reduced χ2 = 0.005).
FIG. 1.
(A) Attachment of cells to chemically modified glass in comparison to plain glass. Data points are calculated as the fraction of detached cells after centrifugation at a given speed (up to 12,800 × g, or 2.7 nN normal force) compared to the fraction for unspun samples (1 × g). Data from coated surfaces are normalized by plain-glass-surface data from the same day. Error bars indicate standard deviations of mean cell counts over multiple images. No significant difference in cell detachment was observed with positively charged poly-l-lysine-coated (triangles), hydrophobic Teflon-like silane-coated (circles), or inert polyethylene glycol (PEG)-coated (diamonds) glass surfaces in comparison to plain glass (squares). Cell populations showed 50% detachment at forces of 1.81 nN for poly-l-lysine, 1.92 nN for PEG, and 1.92 nN for Teflon-like surfaces. All data fit were fit well by sigmoidal curves, with R2 values of 0.96 for plain glass (black line), 0.96 for poly-l-lysine (green line), 0.99 for Teflon (blue line), and 0.94 for PEG (magenta line). (B) Phase-contrast image showing typical cell density of unspun samples. (C) Phase-contrast image showing typical cell density after centrifugation at 12,000 × g. Bar, 100 μm.
To test the dependence of detachment force on surface properties, we chemically modified the glass surfaces in three ways and repeated the detachment force measurements. First, we measured detachment from positively charged poly-l-lysine-coated glass. If Giardia cells, which are thought to carry a negative surface charge (9), relied on electrostatic interactions with charged surfaces to produce some or all of the measured attachment, we would expect to see an attachment difference in these two cases. However, we found that detachment forces of Giardia cells on poly-l-lysine-coated glass did not vary significantly from plain-glass detachment forces (Fig. 1A). Cell confluence levels were similar on both poly-l-lysine surfaces and plain glass. This result suggests that nonspecific electrostatic binding is unlikely to be the primary mechanism of attachment.
We next tested the influence of hydrophobicity and van der Waals dispersion forces on cell attachment, using a Teflon-like coating of (tridecafluoro-1,1,2,2-tetrahydrooctyl)trichlorosilane (Gelest, Inc.) on glass. This surface is highly hydrophobic, with a static water droplet contact angle of 95.2° ± 1.9° (n = 12) compared to contact angles of <3° (smallest measurable angle) for plain glass, 33.9° for poly-l-lysine-coated glass, and 37.7° for pegylated glass. If cells attached primarily due to van der Waals forces, we would expect force to change in proportion to (ɛ − 1)/(ɛ + 1), where ɛ is the dielectric constant of the substrate (10). In our case, we would expect the magnitude of the attachment force to borosilicate glass (ɛ = 4.75) to be approximately twice as great as that of the attachment force to Teflon (ɛ = 2.0). Although previous studies of eukaryotic (22) and prokaryotic (18) cells have demonstrated markedly different adhesion levels between glass and Teflon surfaces, we again found no significant difference between Giardia detachment from plain glass and that from coated glass (Fig. 1A). This result indicates that van der Waals force is unlikely to be a chief component of attachment force, although it is undoubtedly present to a small extent in the overall interaction (13).
Finally, we tested cell detachment from glass surfaces coated with polyethylene glycol (M-SPA-5000; Nektar). Pegylation is known to inhibit adsorption of proteins and to screen surface charges from the glass (8). Proteins from the growth media adsorbed to the pegylated glass at extremely low levels compared to adsorption to plain glass and Teflon-coated glass when measured by fluorescence of fluorescein isothiocyanate-tagged bovine serum albumin or Coomassie staining. Despite the low adsorption rate, we found that Giardia cells attached well to pegylated glass (Fig. 1A), with normal confluence levels and negligible differences in detachment forces compared to those with plain glass. While it is likely that specific binding is important for parasite-host recognition or interactions in vivo (14), specific binding cannot explain Giardia's robust attachment to treated glass surfaces.
We have presented detachment force measurements based on centrifugation indicating that Giardia can resist detachment forces of 1.5 nN on plain glass. Three significantly different surface coatings were applied to glass, and the measured cell attachment forces were unaffected. This method allows consistent measurement of effects on Giardia attachment with higher sensitivity than previous assays and may be adapted to study drug treatments and attachment to epithelial cells.
Our results challenge the idea that specific or nonspecific binding is essential for generating attachment force and are consistent with suction-based models, in which the cell would generate attachment force by lowering the pressure underneath its ventral disk. A suction-based attachment mechanism may explain how Giardia cells are able to attach to a wide variety of substrates in vitro and infect a diverse population of vertebrate hosts, including in some cases transmission between different host species (1). Additionally, use of suction by a single-celled organism would represent a unique mechanism of cellular attachment to surfaces.
REFERENCES
- 1.Adam, R. D. 2001. Biology of Giardia lamblia. Clin. Microbiol. Rev. 14:447-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derhami, K., J. F. Wolfaardt, A. Wennerberg, and P. G. Scott. 2000. Quantifying the adherence of fibroblasts to titanium and its enhancement by substrate-attached material. J. Biomed. Mater. Res. 52:315-322. [DOI] [PubMed] [Google Scholar]
- 3.Elmendorf, H., S. C. Dawson, and J. M. McCaffery. 2003. The cytoskeleton of Giardia lamblia. Int. J. Parasitol. 33:3-28. [DOI] [PubMed] [Google Scholar]
- 4.Feely, D. E., S. L. Erlandsen, and D. C. Chase. 1984. Structure of the trophozoite and cyst, p. 3-31. In S. L. Erlandsen and E. A. Meyer (ed.), Giardia and giardiasis. Plenum Press, New York, N.Y.
- 5.Furness, B. W., M. J. Beach, and J. M. Roberts. 2000. Giardiasis surveillance—United States, 1992-1997. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 49:1-13. [PubMed] [Google Scholar]
- 6.Garcia, A. J., and N. D. Gallant. 2003. Stick and grip: measurement systems and quantitative analyses of integrin-mediated cell adhesion strength. Cell Biochem. Biophys. 39:61-73. [DOI] [PubMed] [Google Scholar]
- 7.Giacomello, E., J. Neumayer, A. Colombatti, and R. Perris. 1999. Centrifugal assay for fluorescence-based cell adhesion adapted to the analysis of ex vivo cells and capable of determining relative binding strengths. BioTechniques 26:758-766. [DOI] [PubMed] [Google Scholar]
- 8.Gölander, C.-G. H., J. N. Herron, K. Lim, P. Claesson, P. Stenius, and J. D. Andrade. 1992. Properties of immobilized PEG films and the interaction with proteins, p. 221-245. In J. M. Harris (ed.), Poly(ethylene glycol) chemistry: biotechnical and biomedical applications. Plenum Press, New York, N.Y.
- 9.González-Robles, A., C. Argüello, B. Chávez, R. Cedillo-Rivera, G. Ortega-Pierres, and A. Martinez-Palomo. 1989. Giardia lamblia: surface charge of human isolates in culture. Trans. R. Soc. Trop. Med. Hyg. 83:642-643. [DOI] [PubMed] [Google Scholar]
- 10.Israelachvili, J. 1992. Intermolecular and surface forces. Academic Press, New York, N.Y.
- 11.Keister, D. B. 1983. Axenic culture of Giardia lamblia in Tyi-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 12.Koo, L. Y., D. J. Irvine, A. M. Mayes, D. A. Lauffenburger, and L. G. Griffith. 2002. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J. Cell Sci. 115:1423-1433. [DOI] [PubMed] [Google Scholar]
- 13.Leckband, D., and J. Israelachvili. 2001. Intermolecular forces in biology. Q. Rev. Biophys. 34:105-267. [DOI] [PubMed] [Google Scholar]
- 14.Magne, D., L. Favennec, C. Chochillon, A. Gorenflot, D. Meillet, N. Kapel, D. Raichvarg, J. Savel, and J. G. Gobert. 1991. Role of cytoskeleton and surface lectins in Giardia duodenalis attachment to Caco2 cells. Parasitol. Res. 77:659-662. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, M. M., D. Naumovitz, Y. Ortega, and C. R. Sterling. 1997. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 10:67-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClay, D. R., G. M. Wessel, and R. B. Marchase. 1981. Intercellular recognition: quantitation of initial binding events. Proc. Natl. Acad. Sci. USA 78:4975-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieminski, E. C., F. W. Schaefer III, and J. E. Ongerth. 1995. Comparison of two methods for detection of Giardia cysts and Cryptosporidium oocysts in water. Appl. Environ. Microbiol. 61:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong, Y.-L., A. Razatos, G. Georgiou, and M. M. Sharma. 1999. Adhesion forces between E. coli bacteria and biomaterial surfaces. Langmuir 15:2719-2725. [Google Scholar]
- 19.Piper, J. W., R. A. Swerlick, and C. Zhu. 1998. Determining force dependence of two-dimensional receptor-ligand binding affinity by centrifugation. Biophys. J. 74:492-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thoumine, O., A. Ott, and D. Louvard. 1996. Critical centrifugal forces induce adhesion rupture or structural reorganization in cultured cells. Cell Motil. Cytoskelet. 33:276-287. [DOI] [PubMed] [Google Scholar]
- 21.Trommler, A., D. Gingell, and H. Wolf. 1985. Red blood cells experience electrostatic repulsion but make molecular adhesions with glass. Biophys. J. 48:835-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kooten, T. G., J. M. Schakenraad, H. C. van der Mei, and H. J. Busscher. 1992. Influence of substratum wettability on the strength of adhesion on human fibroblasts. Biomaterials 13:897-904. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe, M. S. 1992. Giardiasis. Clin. Microbiol. Rev. 5:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]