Abstract
Endoplasmic reticulum-associated degradation (ERAD) mediates the turnover of short-lived and misfolded proteins in the ER membrane or lumen. In spite of its important role, only subtle growth phenotypes have been associated with defects in ERAD. We have discovered that the ERAD proteins Ubc7 (Qri8), Cue1, and Doa10 (Ssm4) are required for growth of yeast that express high levels of the sterol biosynthetic enzyme, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR). Interestingly, the observed growth defect was exacerbated at low temperatures, producing an HMGR-dependent cold sensitivity. Yeast strains lacking UBC7, CUE1, or DOA10 also assembled aberrant karmellae (ordered arrays of membranes surrounding the nucleus that assemble when HMGR is expressed at high levels). However, rather than reflecting the accumulation of abnormal karmellae, the cold sensitivity of these ERAD mutants was due to increased HMGR catalytic activity. Mutations that compromise proteasomal function also resulted in cold-sensitive growth of yeast with elevated HMGR, suggesting that improper degradation of ERAD targets might be responsible for the observed cold-sensitive phenotype. However, the essential ERAD targets were not the yeast HMGR enzymes themselves. The sterol metabolite profile of ubc7Δ cells was altered relative to that of wild-type cells. Since sterol levels are known to regulate membrane fluidity, the viability of ERAD mutants expressing normal levels of HMGR was examined at low temperatures. Cells lacking UBC7, CUE1, or DOA10 were cold sensitive, suggesting that these ERAD proteins have a role in cold adaptation, perhaps through effects on sterol biosynthesis.
Cellular membranes are a highly adaptable and their biochemical composition is tightly regulated (47). Unicellular organisms are able to survive changing environmental conditions in large part due to their ability to modify the composition of their membranes in response to environmental stresses such as low and high temperatures (48). In particular, sterol composition plays a major role in modulating membrane characteristics, and regulation of the sterol biosynthetic pathway has been well studied (10, 31, 35).
Wright and others have explored the role of sterol biosynthetic enzymes in membrane formation using a yeast model of membrane biogenesis. Elevated levels of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (HMGR) induce dramatic endoplasmic reticulum (ER) proliferations (1, 16, 50). In yeast, the membranes induced by the HMGR isozyme, Hmg1, form a nucleus-associated array of stacked ER membranes called karmellae and represent a well-studied example of how changes in physiological conditions can result in altered membrane structures. For example, studies of HMGR-induced karmellae formation in yeast have provided insights into the processes of ER proliferation, including the importance of continued flux though the secretory pathway (17, 26, 36, 38). However, these studies have not yet provided a comprehensive understanding of how ER regulatory networks and metabolic pathways contribute to the modulation of membrane composition and biogenesis. Nor have they examined how such regulatory pathways interface with environmental conditions, including temperature changes, to affect overall cell physiology and viability.
At least two ER quality control mechanisms coordinate cellular physiology with ER-associated protein synthesis and degradation. The unfolded protein response (UPR) induces the synthesis of ER-localized chaperones such as Kar2 (BiP) in response to accumulation of abnormal or excess proteins (24, 42). Another ER quality control mechanism, ER-associated degradation (ERAD), eliminates short-lived or misfolded proteins from the ER (8, 15, 32). ERAD requires the activity of the ER-associated E2 ubiquitin-conjugating enzymes Ubc6 and Ubc7. Ubc6 is directly anchored to the ER membrane by a carboxyl-terminal transmembrane domain; Ubc7 is a peripheral membrane protein that associates with the ER via its binding to the ER membrane protein, Cue1 (5, 11, 19). Other ER proteins that are required for ubiquitination and degradation of the ERAD substrates include the E3 ubiquitin ligases Hrd1 (Der3) and Doa10 (4, 6, 45), Hrd3 (14), and Der1 (25).
A synthetic lethality screen of a deletion mutant population suggests that ERAD has a role in cellular adaptation to chemical and physical changes that affect membranes. These screens revealed that ubc7Δ and cue1Δ mutants have impaired growth when cells are forced to express high levels of Hmg1 at low temperatures (51). Here we report results of our investigation of the relationships among the ubiquitin-proteasome pathway, ER membrane biogenesis, and low temperature. We analyzed the potential of more than 50 proteins in ubiquitin-dependent protein catabolism and related pathways to play a role in cell viability in the presence of elevated HMGR across a range of temperatures. This analysis identified Ubc7, Cue1, and Doa10 as being uniquely required for a cell's normal response to increased Hmg1at low temperatures. Subsequent experiments showed that mutants lacking any one of these ERAD genes are unable to grow at 10°C even when normal levels of HMGR are expressed. Based on the data reported here, we propose a role for a specific subset of ERAD enzymes, Ubc7, Cue1, and Doa10, in allowing cells to adapt membrane characteristics for survival at low temperatures.
MATERIALS AND METHODS
Yeast strains, media, and plasmids.
Most deletion mutant strains examined were homozygous diploid deletion mutants purchased from Research Genetics (Invitrogen Corp., Carlsbad, CA). These deletion strains are in the S288C background, BY4743 (MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/+ lys2Δ0/+). A full list of mutants screened is given in Table 1. The ubc7Δ, cue1Δ, doa10Δ, ubc6Δ, hrd1Δ, and hrd3Δ deletions were verified by PCR with primers that hybridized to sequences flanking the deleted gene and within the KanMX6 gene that was used to select for the recombination (sequence of oligonucleotides available upon request). Haploid ubc7Δ, cue1Δ, and doa10Δ strains were generated from another S288C strain, JRY527 (MATa ade2-101 his3Δ200 lys2-801 met2- ura3-52) for use in morphological studies (3). pre1-1 pre2-1 proteasome mutants were generated in the WCG4 strain (MATa/α his3-11/his3-11 leu2-3/leu2-3 112ura3/112ura3) (18).
TABLE 1.
Hmg1-dependent growth inhibition of ubiquitin-proteasome and related mutants
| Gene function | Mutation resulting ina:
|
||
|---|---|---|---|
| Severe growth inhibition | Growth inhibition at one or more of the temperatures tested | Normal growth at all temperatures tested | |
| ERAD | cue1Δ,* doa10Δ,* ubc7Δ,* | der1Δ, hrd1Δ, hrd3Δ | ubc6Δ |
| Non-ERAD ubiquitination-dependent protein catabolism | doa1Δ** | ate1Δ, cdh1Δ, doa4Δ, dsk2Δ, hul5Δ, nas6Δ, rad6Δ, rad23Δ, rpn10Δ, rub1Δ, tom1Δ, ubp2Δ, ubp3Δ, ubp6Δ, ubp14Δ, ubp15Δ, ubr1Δ, ubr2Δ, ufd4Δ, ump1Δ, yuh1Δ | atg10Δ, cdc26Δ, hul4Δ, npl4Δ, pex4Δ, rpn4Δ, rpn13Δ, ubc5Δ, ubc8Δ, ubc11Δ, ubc13Δ, ubp1Δ, ubp5Δ, ubp7Δ, ubp9Δ, ubp11Δ, ubp12Δ, ubp13Δ, ubp16Δ |
| Other (putative ERAD substrates or ubiquitin-like functions) | mga2Δ, spt23Δ | asi2Δ, atg7Δ, uba3Δ | |
The temperatures tested were 16, 26, and 37°C. *, Growth inhibition was observed at 16 and 26°C but not at 37°C; **, growth inhibition was observed at 37°C but not at 16 or 26°C.
Plasmid-expressing yeast strains were grown in rich minimal medium (0.17% yeast nitrogen base, 0.5% ammonium sulfate, 2% Casamino Acids, and 2% agar, with 30 μg of adenine/ml, 20 μg of histidine/ml, 40 μg of lysine/ml, 40 μg of leucine/ml, 20 μg of methionine/ml, 30 μg of tryptophan/ml, and 20 μg of tyrosine/ml) lacking uracil to select for retention of plasmids. The carbon source was 2% glucose or 2% galactose. Yeast strains used in 10°C studies were gown on YPD (2% yeast extract, 2% proteose peptone, and 2% glucose.) Solid medium contained 2% agar. Agar used in rich minimal medium was washed with distilled water prior to use to remove contaminating chemicals.
Deletion of UBC7, CUE1, and DOA10 in JRY527 was accomplished by using appropriate PCR products that carried portions of the 5′ and 3′ regions of the gene to be deleted and the Geneticin resistance marker, KanMX6 (49). Disruption was confirmed via PCR, using primers that hybridized to sequences flanking the deleted gene and within the KanMX6 sequence.
All plasmids used except p716 have been previously described. The vector control used in all cases was pBM150 (CEN4, ARS1, URA3, GAL1/10 promoter) (23). The galactose-inducible HMG1 plasmids, pAK266 (wild-type HMG1 under GAL1 promoter control in pRS316; CEN6 ARS4 URA3), pDP304 (hmg1, His1020Gln, catalytically inactive Hmg1, under GAL1 promoter control in pBM150; CEN4 ARS1 URA3), p558 (hmg1-K442M, L487; the mutant Hmg1 protein is catalytically active but unable to assemble karmellae; see reference 17), and p260 (encodes galactose-inducible mutant hmg1 protein with a 29-amino-acid deletion in the catalytic domain; the deletion results in loss of both catalytic activity and karmellae-inducing ability are described in references 37 and 38). pRH134-2 was received from Randy Hampton (University of California, San Diego) and was as described previously (11). To make p716, the GAL1 promoter was removed from pAK266 with BamHI. The GPDH promoter was PCR amplified from plasmid pCR436 [pBluescript KS(+) containing a HindIII-SalI fragment with the GPDH promoter sequence]. Yeast cells were then transformed with the gapped p716 plasmid and the PCR fragment. The resulting, stably transformed yeast strains constitutively assembled karmellae and were lovastatin resistant, confirming that the plasmid encoded functional Hmg1. In addition, the plasmid was rescued from the yeast and displayed the predicted restriction enzyme fragment sizes. In all cases, yeast were transformed with the Frozen-EZ Yeast Transformation II kit (Zymo Research, Orange, CA).
Growth assays by serial dilutions.
Serial dilutions from an overnight culture diluted to 0.5 Α600 units per ml were made into sterile distilled water at the ratios of 1:36, 1:5, 1:5, 1:5, and 1:5. Cell suspensions were transferred from the microtiter plate to appropriate solid medium using an ethanol-sterilized, 48-pin metal stamper. Plates were incubated at 10, 16, 26, or 37°C and photographed after an appropriate period of time (see figure legends). At least three independent replicate experiments were performed for each set of strains; a representative experiment is shown in the figures.
Microscopy.
For electron microscopy, ubc7Δ and cue1Δ cells transformed with pAK266 were grown in liquid medium containing galactose for 24 h at 16°C. Cells were then prefixed in glutaraldehyde, postfixed in potassium permanganate, and embedded in Spurr's resin as previously described (17, 38). Sections were stained with Reynold's lead citrate and examined on a Philips CM100 transmission electron microscope operating at 60 kV. Electron microscopic samples were coded so that observation was made without knowledge of sample identity.
The vital dye, 3,3′-dihexyloxacarbocyanine iodide (DiOC6), was used to visualize karmellae in living cells (27) using a fluorescein filter set (excitation, 480 ± 20 nm; barrier, 535 ± 40 nm) on a Nikon Microphot-FXA fluorescence microscope (Nikon USA, Melville, NY). Cells depicted were grown for 14 h on galactose at 16°C.
Sterol analysis.
Sterols were isolated as previously described (33) and analyzed by gas chromatography (GC). An HP5890 series II equipped with the Hewlett-Packard CHEMSTATION software package was used to analyze sterol content. The capillary column (DB-1) was 15 m by 0.25 mm (film thickness; J&W Scientific, Folsom, CA) and was programmed from 195 to 280°C (1 min at 195°C and then an increase at 20°C/min to 240°C, followed by 2°C/min until the final temperature of 280°C was reached). The linear velocity was 30 cm/s using nitrogen as the carrier gas, and all injections were run in the splitless mode.
GC/mass spectrometry analyses of sterols were done by using Thermoquest Trace 2000 gas chromatograph interfaced to a Thermoquest Voyager mass spectrometer. The GC separations were done on a fused silica column, DB-5MS (20 m by 0.18 mm by 0.18 μm [film thickness; J&W Scientific, Folsom, CA). The injector temperature was 190°C. The oven temperature was programmed to remain at 100°C for 1 min, followed by a temperature ramp of 6.0°C/min to a final temperature of 300°C. The final temperature was held for 25 min. Helium was the carrier gas with a linear velocity of 50 cm/s in the splitless mode. The mass spectrometer was in the electron impact ionization mode at an electron energy of 70 eV, with an ion source temperature of 150°C and scanning from 40 to 850 atomic mass units at 0.6-s intervals.
RESULTS
Elevated expression of Hmg1 in the ubc7Δ, cue1Δ, and doa10Δ ERAD mutants resulted in cold-sensitive growth.
A competitive growth experiment designed to identify genes involved in karmellae biogenesis revealed that ubc7Δ and cue1Δ mutants divided more slowly under karmellae-inducing conditions than other mutants within the population (51). To confirm these results, ubc7Δ and cue1Δ strains were individually transformed with a plasmid carrying a galactose-inducible HMG1 gene (plasmid AK266) and examined for a galactose-dependent growth phenotype. Both mutants displayed cold-sensitive growth on galactose (Fig. 1). Growth was particularly inhibited at 16°C, with moderate inhibition at 26°C. Thus, these ERAD genes appeared to be required for low-temperature viability of yeast cells expressing high levels of HMGR. This result was consistent with the previously observed slow-growth phenotype in the population of deletion mutants, since one of the competitive growth experiments was conducted at 26°C to enable the growth of temperature-sensitive mutants (51). To determine whether this cold sensitivity was a phenotype limited to the BY4743 strain background, we generated ubc7Δ and cue1Δ mutants in another strain background, JRY527. These new ERAD mutants were similarly cold sensitive in the presence of high levels of Hmg1 (data not shown). Thus, the cold sensitivity was unlikely to result from cryptic mutations or other characteristics unique to the BY4743 background.
FIG. 1.
The ERAD proteins Ubc7, Cue1, and Doa10 are required for normal growth in the presence of increased Hmg1. Wild-type, ubc7Δ, cue1Δ, and doa10Δ cells of strain BY4743 were transformed with a plasmid either encoding a galactose-inducible HMG1 gene (pAK266) or the vector control. The leftmost spot is inoculated from a culture at 1.4 × 106 cells per ml, followed by 1:5 serial dilutions. Images were taken after 2 days of growth on glucose or galactose at 37 and 26°C and after 5 days of growth at 16°C.
Only a subset of genes involved in ERAD or the ubiquitin-proteasome pathway was required for normal growth of cells with elevated levels of Hmg1.
In addition to Ubc7 and Cue1, other ER-associated proteins have a role in ERAD, including Doa10, Ubc6, Hrd1, Hrd3, and Der1 (6, 14, 25, 45). Based on analysis of growth on solid medium of these mutants, only doa10Δ mutants exhibited significant cold-sensitive growth in the presence of increased Hmg1 (Fig. 1 and Table 1). These results indicated that the ERAD proteins Ubc7, Cue1, and Doa10 were specifically required for normal growth in response to high levels of Hmg1 at low temperatures.
To determine whether the cellular response to increased Hmg1 requires other components of the ubiquitin-proteasome pathway, a panel of deletion mutants were transformed with pAK266 and screened for a growth phenotype at 16, 26, and 37°C (Table 1). Many of the mutants showed slight growth inhibition in response to increased Hmg1 at one or more of the temperatures tested. In addition, growth of the doa1Δ mutant expressing elevated HMGR was found to be strongly temperature sensitive. However, none of these mutants exhibited the pronounced cold sensitivity that was observed in ubc7Δ, cue1Δ, or doa10Δ strains.
ubc7Δ, cue1Δ, and doa10Δ cells displayed defective spatial and temporal regulation of karmellae assembly.
Since the Hmg1-dependent growth defects of ubc7Δ and cue1Δ cells were originally recognized as part of a screen to identify genes required for ER membrane biogenesis, we examined whether these mutants were able to assemble normal karmellae. After 24 h of karmellae induction at the nonpermissive temperature of 16°C, ubc7Δ and cue1Δ mutants contained unusual ER membrane proliferations that were not observed in wild-type controls or in previous experiments (50). In contrast to the highly organized stacks of karmellae membranes that surround the nucleus, forming a horseshoe pattern in sections (Fig. 2A), the ER membrane proliferations observed in ubc7Δ and cue1Δ were disorganized and were not always closely associated with the nucleus (Fig. 2B and C). Thus, although the mutants were able to proliferate their ER in response to elevated Hmg1, they were unable to properly modulate the organization and location of the resulting proliferations.
FIG. 2.
ubc7Δ, cue1Δ, and doa10Δ cells make aberrant karmellae. Wild-type, ubc7Δ, and cue1Δ cells of strain BY4743 transformed with pAK266 (carrying a galactose-inducible HMG1 gene) were fixed for electron microscopy after 24 h of growth on galactose at 16°C. (A) A representative wild-type cell exhibits a normal karmellae structure, which is labeled K. (B and C) Abnormal membrane proliferations observed in mutants, including disorganized membrane strips that are not closely associated with the nucleus, are indicated with arrows. (D, E, F and G) The lipophilic dye DiOC6 was used to stain wild-type and deletion cells of strain JRY527 after 14 h of growth on galactose at 16°C. A representative wild-type cell exhibits a normal karmellae structure (D). Mutants show a diversity of abnormal membrane proliferations including loops, whorls, and membrane strips (E, F, and G).
As previously reported, karmellae can be visualized in living cells with fluorescence microscopy using the vital dye, 3,3′-dihexyloxacarbocyanine iodide (DiOC6 [27]). Subsequent examination of homozygous diploid ubc7Δ, cue1Δ, and doa10Δ mutants using this method yielded unexpected new information about variation in karmellae morphology in wild-type strains of different backgrounds. All studies of karmellae morphology conducted in our laboratory prior to those presented here have been in yeast strain JRY527. Because ubc7Δ and cue1Δ mutants were identified from a pool of mutants created by the Deletion Consortium, initial investigation of ubc7Δ and cue1Δ mutants and other ubiquitin-proteasome mutants was conducted with mutants derived from the BY4743 parent strain. The wild-type BY4743 cells were difficult to stain with DiOC6; the cells were very densely stained and appeared to contain some disorganized membrane strips and whorls in addition to normal karmellae. The unusual staining characteristics in this wild-type strain made it difficult to use in vivo observations to determine the extent of karmellae abnormalities in the mutants. Therefore, we examined karmellae structure in ubc7Δ, cue1Δ, and doa10Δ mutants that were generated in the more easily stained JRY527 background.
As expected from past observations, staining of wild-type JRY527 cells with DiOC6 revealed normal karmellae, without unusual staining observed in the BY4743 background (Fig. 2D). Consistent with our observations of the ubc7Δ, cue1Δ, and doa10Δ mutants in the BY4743 background, the analogous mutant strains generated in the JRY527 background assembled both karmellae and abnormal membrane structures (Fig. 2E, F, and G). Thus, the abnormalities in karmellae assembly in ubc7Δ, cue1Δ, and doa10Δ mutants were unlikely to result from differences in strain background. In the absence of high levels of Hmg1, no unusual membrane structures were observed in any cells examined, although the BY4743 strain continued to be difficult to stain optimally with DiOC6.
Increased HMG-CoA reductase catalytic activity, not karmellae, was responsible for Hmg1 sensitivity in ERAD mutants.
Previous studies have shown that Hmg1-dependent induction of karmellae requires a region in the last ER lumenal region of the membrane domain (“loop G”) and is independent of HMGR catalytic activity (36, 37). These observations allowed us to test the hypothesis that the cold-sensitive phenotype and abnormal karmellae assembly in ubc7Δ, cue1Δ, and doa10Δ mutants were functionally related. To determine whether the observed Hmg1-dependent cold sensitivity was due to abnormal karmellae biogenesis, mutants were transformed with plasmids containing mutated or truncated forms of Hmg1 (Fig. 3A). Wild-type cells transformed with pDP304, which encodes a catalytically inactive form of Hmg1, make normal karmellae when grown on galactose-containing medium but do not express elevated HMGR activity. The ubc7Δ mutants expressing the catalytically inactive, karmellae-inducing form of Hmg1 (pDP304) grew as well as cells expressing the vector control plasmid. Thus, the catalytic activity was essential for the cold-sensitive phenotype. cue1Δ and doa10Δ mutants transformed with pDP304 also grew similarly to wild-type (data not shown.)
FIG. 3.
HMG-CoA reductase catalytic activity, not the presence of karmellae, is the cause of decreased growth in response to increased levels of Hmg1. (A) Wild-type and ubc7Δ cells were transformed with vector control or plasmids expressing galactose-inducible HMG1 (pAK266), galactose-inducible mutant hmg1 with no catalytic activity (pDP304 and p260), galactose-inducible hmg1 mutant that has catalytic activity but is unable to make karmellae (p558), a constitutively expressed HMG1 (p716), or galactose-inducible HMG2 (pRH134-2). The left-most spot is inoculated from a culture at 1.4 × 106 cells per ml, followed by 1:5 serial dilutions. Images were taken after 5 days of growth at 16°C. The asterisk indicates that the expression of high levels of Hmg2 results in the formation of membrane structures that are distinct from karmellae. (B) Wild-type, ubc7Δ, cue1Δ, and doa10Δ cells were transformed with either galactose-inducible HMG1 (pAK266) or vector control plasmids. Transformants were grown on glucose, galactose, or galactose plus 400 μg of lovastatin/ml (lova). The left-most spot is inoculated from a culture at 1.4 × 106 cells per ml, followed by 1:5 serial dilutions. Images were taken after 5 days of growth at 16°C.
ubc7Δ cells expressing a galactose-inducible, catalytically inactive form of Hmg1 that is unable to induce karmellae (p260) grew as well on galactose-containing media as cells transformed with pDP304. Conversely, ubc7Δ cells expressing a galactose-inducible mutant form of hmg1 that was unable to induce the formation of karmellae but retained catalytic activity (p558) was cold sensitive. ubc7Δ mutants constitutively expressing HMG1 under the control of the GPDH promoter (p716) showed a slight cold-sensitive phenotype on both glucose and galactose at 16°C, indicating that the observed phenotype is not carbon source dependent (Fig. 3A). Finally, ubc7Δ cells expressing galactose-inducible Hmg2 (pRH134-2) grew more poorly on galactose than vector control transformants. The Hmg2 protein has identical catalytic activity as Hmg1 but induces proliferation of short stacks of smooth membranes that can be associated with the nucleus or plasma membrane or present in the cytoplasm (28). Collectively, these observations indicate that increased HMGR activity, not abnormal karmellae assembly, was responsible for the cold-sensitive phenotype.
To confirm that the primary cause of Hmg1-dependent cold sensitivity in ubc7Δ, cue1Δ, and doa10Δ cells was HMGR catalytic activity, mutant cells expressing pAK266 were grown at 16°C in the presence of lovastatin, a competitive inhibitor of HMGR. As previously observed, the mutants expressing high levels of Hmg1 displayed cold-sensitive growth when they expressed elevated HMGR. However, when HMGR activity was reduced by the presence of lovastatin, nearly normal growth was restored at 16°C (Fig. 3B.) This result demonstrated that the increased catalytic activity of Hmg1 is toxic at cold temperatures to ubc7Δ, cue1Δ, and doa10Δ mutant cells.
The response of proteasome mutants to increased Hmg1 was similar to that of ubc7Δ, cue1Δ, and doa10Δ cells.
Because Ubc7, Cue1, and Doa10 are part of the molecular machinery that covalently attaches ubiquitin to target proteins, we hypothesized that the Hmg1-dependent cold sensitivity in these mutants was due to the failure of these mutants to ubiquitinate a specific target protein or proteins. Ubiquitination of this target might result in either activation of a proteasome-independent event (41) or degradation of the protein by the proteasome (29). To distinguish between these two possibilities, we examined the growth characteristics of proteasome mutants that expressed increased levels of Hmg1. If an inability to degrade the target protein were the basis for the Hmg1 sensitivity, then cells with defects in proteasome function should show similar cold-sensitive phenotypes as those observed in ubc7Δ, cue1Δ, and doa10Δ mutants.
Although genes encoding proteins that compromise the proteasome are essential, partial-loss-of-function mutants are viable. Strain WCG4/11-12 (pre1-1 pre1-2) exhibits partial loss of function of two essential 20s core particle components, Pre1 and Pre2, and has been shown to have 5% of normal proteasome activity (18). The growth of this mutant strain and a congenic wild-type expressing elevated Hmg1 was examined.
Under all conditions tested, pre1-1 pre2-1 mutants grew less well than the wild type. In addition, mutant transformants displayed variability in growth that was not observed in the congenic wild-type transformants. Consequently, to ensure that conclusions about the effects of increased levels of Hmg1 were not confounded by variability in growth of independent transformants, 29 randomly selected pre1-1 pre2-1 vector control transformants and 29 randomly selected pAK266 transformants were examined. Two representative transformants for each plasmid are shown in Fig. 4. As expected, all 29 of the pre1-1 pre2-1 vector control transformants that were examined grew as well with normal levels of Hmg1 (i.e., on glucose) as with high levels of Hmg1 (i.e., on galactose). Of the 29 pre1-1 pre2-1 mutants transformed with AK266, 25 exhibited Hmg1-induced cold sensitivity, a finding consistent with the hypothesis that normal growth of cells with elevated levels of HMGR requires ubiquitin-mediated protein degradation. Given the poor growth of pre1-1 pre2-1 mutants, the four transformants with normal growth may have gained reversion or suppressor mutations that elevate proteasomal function. Interestingly, the Hmg1-induced growth inhibition observed in proteasome mutants was not as extreme as that observed in ERAD mutants. Therefore, the cellular response to increased Hmg1 may require additional ERAD-dependent events that are proteasome independent.
FIG. 4.
Proteasome function mutants are sensitive to increased levels of Hmg1. A pre1-1, pre2-1 mutant strain and a congenic wild-type strain were transformed with either pAK266 or vector control plasmids. The left-most spot is inoculated from a culture at 1.4 × 106 cells per ml, followed by 1:5 serial dilutions. Images were taken after 12 days of growth at 16°C. Two independent pre1-1 pre2-1 transformants with the vector and pAK266 are shown for comparison.
Deletion of HMG2 did not suppress cold sensitivity in ubc7Δ and cue1Δ cells.
The cold sensitivity of the pre1-1 pre2-1 strain described above suggests that one component of the normal cellular response to increased levels of Hmg1 is the UBC7-, CUE1-, and DOA10-dependent degradation of a protein target. Hmg2 is a known ER-resident target of Ubc7 and the E3 ubiquitin ligase, Hrd1 (11, 16). Although Doa10 and Hrd1 have been shown to have distinct substrate specificity, experimental evidence suggests that Hrd1 and Doa10 have some overlapping function (45). Therefore, we hypothesized that under our unique experimental conditions, the inability of the ubc7Δ, cue1Δ, and doa10Δ mutants to degrade Hmg2 might be the underlying molecular cause of their sensitivity to elevated Hmg1 expression. If so, deletion of HMG2 in any of the mutant strains should suppress the cold-sensitive phenotype. Double-deletion mutants lacking both UBC7 and HMG2 were generated in the JRY527 background and transformed with either pAK266 or a vector control plasmid. As seen in Fig. 5, deletion of HMG2 did not restore the growth observed in vector controls to ubc7Δ cells and, therefore, did not suppress the Hmg1-induced cold sensitivity. Therefore, Hmg2 is unlikely to be the essential target of UBC7-, CUE1-, and DOA10-dependent ubiquitination.
FIG. 5.
Deletion of HMG2 does not suppress cold sensitivity in ubc7Δ cells. A ubc7Δ hmg2Δ double deletion mutant strain in the JRY527 background was transformed with either pAK266 or vector control plasmids. The left-most spot was inoculated from a culture at 1.4 × 106 cells per ml, followed by 1:5 serial dilutions. Images were taken after 5 days of growth at 16°C.
Although Hmg1 is not a substrate for ERAD under standard growth conditions (11), we hypothesized that it might become subject to degradation under certain conditions, such as growth at low temperatures. To test this hypothesis, total protein was isolated from wild-type, ubc7Δ, cue1Δ, and doa10Δ cells transformed with pAK266 after 24 h of karmellae induction at permissive and nonpermissive temperatures. Immunoblot analysis with an antibody specific to the Hmg1 isozyme revealed that total levels of Hmg1 in mutant cells were the same as or lower than that of wild-type cells (data not shown). Thus, the loss of these ERAD proteins did not lead to the elevation of Hmg1 levels, making it unlikely that Hmg1 itself is the essential target of Ubc7, Cue1, and Doa10.
Sterol metabolite profiles were altered in ubc7Δ cells.
HMGR catalyzes the formation of mevalonate, the rate-limiting step in the biosynthesis of sterols and other isoprenoids in animals and fungi (12). A reasonable molecular mechanism for the Hmg1 sensitivity observed in mutant cells is that increased flux through the sterol biosynthetic pathway results in accumulation of a toxic metabolite whose levels are normally held in check via the action of Ubc7, Cue1, and Doa10. For example, Donald et al. showed that cells overexpressing the Hmg1 catalytic domain accumulate increased squalene levels and show decreased growth rates (11).
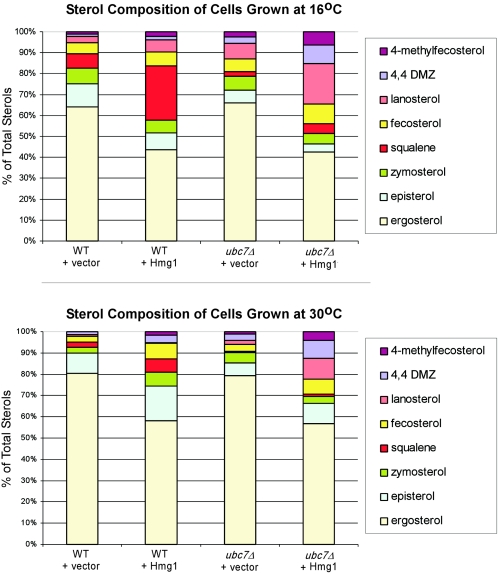
GC was used to analyze sterol metabolite composition of ubc7Δ and wild-type cells in the BY4743 background in the presence of normal and increased levels of Hmg1 (Fig. 6). Although this method did not measure absolute sterol levels, it provided quantitative data concerning the relative amounts of particular sterols within a sample. Interestingly, increased levels of Hmg1 resulted in a decrease in the percentage of ergosterol in both wild-type and ubc7Δ cells at both permissive and restrictive temperatures. This result suggested that one or more sterol biosynthetic enzymes that catalyze reactions downstream of squalene synthase were downregulated in response to elevated flux through the sterol biosynthetic pathway. In addition, this regulation appeared to be intact in ubc7Δ mutants.
FIG. 6.
The sterol metabolite profile of ubc7Δ cells in the presence of normal and increased levels of Hmg1 differs from the isogenic wild-type control. GC was used to measure the relative amounts of sterol metabolites as a percentage of total cellular sterol. Wild-type or ubc7Δ cells of strain JRY527 were transformed with galactose-inducible HMG1 (pAK266) or a vector control plasmid. Expression of Hmg1 was induced in strains containing pAK266 by growth on galactose for either 24 h at 16°C (A) or 14 h at 30°C (B). The data shown are representative of results observed in three similar experiments at each temperature range.
The proportion of squalene in wild-type cells expressing elevated levels of Hmg1 was higher than in the vector control. However, the proportion of squalene in ubc7Δ mutants was actually lower than that of wild-type cells. The observation that ubc7Δ cells exhibited lower squalene levels ruled out the possibility that accumulation of excess squalene in ubc7Δ cells was responsible for their Hmg1 sensitivity.
Although the proportion of squalene was lower in ubc7Δ cells than in the wild type, the proportion of several other sterol metabolites was elevated in ubc7Δ cells grown at 16°C with high levels of Hmg1. If the Ubc7/Cue1/Doa10 complex regulates flux through the sterol biosynthetic pathway by targeting ergosterol biosynthetic enzymes for degradation, then the loss of UBC7 function should lead to elevated levels of these enzymes, in turn producing inappropriately elevated amounts of their products. Thus, the elevated proportions of lanosterol (synthesized by Erg7), 4,4-dimethylzymosterol (synthesized by Erg24), fecosterol (synthesized by Erg6), and 4-methylfecosterol (resulting from incomplete C-4 demethylation by the Erg25, Erg26, and Erg27 complex) in ubc7Δ mutants suggest that the essential substrate(s) of the Ubc7/Cue1/Doa10 complex may be Erg6, Erg7, Erg24, Erg25, Erg26, and/or Erg27. Of these potential target proteins, only Erg6 and Erg24 are nonessential. Erg6 is a soluble protein that is associated with lipid particles and the ER (2). Erg24 is an ER transmembrane protein (30). Thus, the localization of both proteins is consistent with the possibility that they are ERAD targets. We constructed ubc7Δ erg6Δ and ubc7Δ erg24Δ double mutants to test directly whether loss of these erg genes suppressed the cold-sensitive phenotype of ubc7Δ mutants. In both cases, the double mutants were as cold sensitive as the ubc7Δ mutants alone (data not shown.) Thus, neither the loss of Erg6 nor the loss of Erg24 individually suppressed the cold sensitivity of ubc7Δ mutants.
ubc7Δ, cue1Δ, and doa10Δ mutants are cold sensitive in the absence of elevated levels of HMGR.
The results presented thus far suggested that the Ubc7-Doa10 ERAD pathway is important for regulating sterol biosynthesis. In addition, it appears that this regulation is particularly important at low temperatures. Interestingly, Fig. 6 shows that expression of increased levels of HMGR in wild-type cells growing at 30°C results in sterol profile changes that are similar to those seen in wild-type cells growing with normal levels of HMGR at 16°C. In addition, the sterol profiles of ubc7Δ mutants were abnormal under all conditions tested. Given these results, we hypothesized that increased levels of HMGR produce cellular responses similar to low temperature growth. According to this scenario, cells growing at 16°C with high HMGR levels would respond physiologically as if they were growing at a lower temperature. To begin exploring this hypothesis, we examined the ability of ubc7Δ, cue1Δ, and doa10Δ mutants expressing normal levels of HMGR to grow at 10°C. As predicted, these ERAD mutants, but not others, are cold sensitive, although ubc7Δ and cue1Δ mutants are more cold sensitive than the doa10Δ mutant (Fig. 7.)
FIG. 7.
The ERAD proteins Ubc7, Cue1, and Doa10 are required for normal growth at low temperature in the absence of high levels of Hmg1. Wild-type yeast strains and cue1Δ, doa10Δ, and ubc7Δ mutants were serially diluted (1:5) and plated onto YPD medium. After growth at 10°C for 12 days, significant slow growth was observed in the cue1Δ and ubc7Δ mutants. The doa10Δ mutant is slightly cold sensitive at 10°C.
DISCUSSION
ERAD as a regulator of the sterol biosynthetic pathway at low temperature.
Our results demonstrate that the ability of yeast cells to thrive at low temperature requires three specific ERAD enzymes: Ubc7, Cue1, and Doa10. The growth rate of cells lacking any one of these enzymes is decreased at 10°C. Impaired growth is also observed at 16°C when HMGR activity is expressed at high levels. In addition, ubc7Δ mutants have altered sterol composition profiles compared to the wild type in the presence of both normal and elevated levels of Hmg1. These results suggest that proper regulation of ergosterol biosynthesis in response to cold is an essential physiological adaptation that enables yeast to survive at low temperatures. Therefore, we propose that ERAD is needed for cold adaptation because it regulates ergosterol biosynthesis.
Unicellular organisms possess a remarkable ability to adjust to environmental changes including temperature extremes. Studies in yeast and bacteria have shown that these organisms adapt to low temperature in part through altering the lipid and sterol content of their membranes (see references 13 and 46). Interestingly, S. cerevisiae has a single desaturase that can introduce one double bond into the fatty acid chain (7, 43, 44). Thus, the fatty acids present in the phospholipids of budding yeast are either unsaturated or monounsaturated (10). Consequently, modifications of sterols may play a much more important role in the cold adaptation responses of yeast than in other organisms that produce a wider variety of phosopholipids.
Published reports by Rodriguez-Vargas et al. and Schade et al. provide evidence that alterations in membrane composition at 10°C may be due to changes in global transcriptional patterns (39, 40). Although changes in gene expression certainly play a role in altering membrane composition in response to cold, evidence presented in this report suggests that posttranslational regulation is also involved in cold adaptation.
The first indication that ubc7Δ, cue1Δ, and doa10Δ mutants might have defects in sterol metabolism was our observation that these mutants are hypersensitive to miconazole (unpublished results). Miconazole belongs to the azole class of antifungals that inhibit ergosterol synthesis by interfering with the function of Erg11, lanosterol demethylase (for a review, see reference 31). Mutations that result in altered sterol profiles can cause either hypersensitivity or resistance to azoles. Interestingly, the hypersensitivity of ubc7Δ, cue1Δ, and doa10Δ mutants to miconazole was exacerbated by increased HMGR levels; in contrast, increased expression of HMGR in the wild type resulted in decreased sensitivity to miconazole (unpublished results). These observations led us to examine the whether defects in ERAD affected sterol metabolism.
As suggested initially by their miconazole sensitivity, ubc7Δ mutants have altered sterol metabolite profiles, leading to the hypothesis that altered flux through the sterol biosynthetic pathway is the basis of the cold-sensitive phenotype. Based on the inability of erg6Δ or erg24Δ to suppress the cold sensitivity of ubc7Δ mutants, Erg6 and Erg24 are unlikely to be the essential targets of Ubc7-dependent ubiquitination. However, other ergosterol biosynthetic enzymes remain candidates. Hitchcock et al. identified 211 membrane-associated proteins that are ubiquitinated, 83 of which are potential targets of Ubc7-dependent ubiquitination (20). This analysis showed that several ergosterol biosynthetic proteins were ubiquitinated (Erg1, Erg2, Erg5, Erg9, Erg11, Erg25, and Erg27), and three were potential ERAD substrates (Erg1, squalene monooxygenase; Erg9, squalene synthetase; and Erg27, 3-keto sterol reductase). Because all of these genes are essential for viability, we were unable to test whether their loss suppressed the cold-sensitive phenotype of ubc7Δ mutants. Nevertheless, the data of Hitchcock et al. confirm that ergosterol biosynthetic enzymes are targets of ERAD, a finding consistent with our hypothesis.
Previous work by other labs has also suggested a relationship among cold adaptation, membrane composition, and ERAD. For example, transcription of OLE1, an essential gene that encodes the only Δ9 fatty acid desaturase found in yeast, is regulated by the transcription factors Mga2 and Spt23 (52). The activity of these transcription factors is, in turn, regulated by proteasomal processing initiated by an ERAD complex containing Npl4, Ufd1, and Cdc48 (21, 22). Importantly, Nakagawa et al. found that activation of Mga2 requires proteasomal processing that it is activated by cold temperatures, leading them to conclude that Mga2 is a low temperature sensor in yeast (34). In addition to regulating transcriptional activators of the OLE1 gene, ERAD components, including Ubc7, Ubc6, and Cue1, but not Doa10, directly regulate the stability of the Ole1 protein (7).
Taking all of these data into account, the essential nature of Ubc7, Cue1, and Doa10 at low temperatures and in the presence of elevated levels of HMGR could be explained in several ways. First, it is possible that improper regulation of Ole1 levels in the ERAD mutants could be the direct cause of cold sensitivity. However, given that mga2Δ mutants are not cold sensitive and are only slightly sensitive to elevated HMGR (Table 1) and that Braun et al. (7) have shown that Doa10 is not involved in modulating Ole1 protein stability, this simple explanation seems unlikely. A more likely explanation is that the ultimate cause of cold sensitivity in ubc7Δ, cue1Δ, and doa10Δ cells results from a combination of altered sterol levels and failure to regulate Ole1 levels.
ERAD as a process that integrates levels of proteins, lipids, and membranes.
The question of how cells sense the need to alter existing or synthesize additional membranes and how they subsequently coordinate production of the proteins and lipids required for these changes has long puzzled biologists. Cox et al. proposed that the unfolded protein response (UPR) is the nexus for coordinating the functional demand for membrane biogenesis with increased protein and lipid production (9). Part of this hypothesis is based on their observation that karmellae biogenesis requires the UPR. However, subsequent studies conducted in our laboratory could not confirm this observation and, instead, showed that the UPR is neither required for nor induced upon karmellae biogenesis (26). These observations, together with the results described here, suggest that the crucial coordination of membrane biogenesis, enzyme activity, and lipid production in yeast may be a specialized function of ERAD instead of the UPR. Specifically, deletion of UBC7, CUE1, or DOA10 resulted in a variety of defects, including altered sterol composition, inability to regulate the amount and morphology of the ER, and cold sensitivity. Thus, the ERAD functions carried out by Ubc7, Cue1, and Doa10 appear to be required for integration of protein stability, membrane lipid composition, and three-dimensional membrane structure.
The possibility of a broader role of ubiquitination in sterol metabolism and cold adaptation.
In addition to the strong Hmg1-induced cold sensitivity observed in ubc7Δ, cue1Δ, and doa10Δ mutants, we also observed less severe growth defects in 22 other mutants with defects in genes involved in ubiquitination or proteasome function. Few patterns arose among mutants found to be slightly sensitive versus insensitive to Hmg1. Both groups contained several ubiquitin-like proteins and several ubiquitin-specific proteases. Although both groups contained E2 and E3 proteins, the slightly sensitive group had five E3 and one E2 protein, while the nonsensitive group had one E3 and seven E2 proteins. Since many E3 proteins were only recently identified or remain unknown, the observed distribution could simply be due to selection criteria for mutants to include in this screen. Alternatively, this observation may suggest that the function of E3 enzymes may be less redundant than that of E2 enzymes. Regardless of the particular characteristics of each group, the fact that so many ubiquitination mutants were at least slightly sensitive to increased Hmg1 confirms the importance of the ubiquitin/proteasome pathway in allowing cells to properly respond to changes in sterol biosynthetic capacity. The impaired growth of ubc7Δ, cue1Δ, and doa10Δ mutants at 16°C expressing abnormally high levels of Hmg1 resembles the observed cold sensitivity of these mutants at 10°C expressing normal levels of Hmg1. Based on results presented here, we hypothesize that engineered overexpression of Hmg1 mimics the physiological consequences of low temperature. Thus, we predict that ubiquitination genes required for normal growth in the presence of elevated HMGR will also be required for survival at 10°C.
Acknowledgments
We acknowledge the assistance of Emily Cadera, Dangelei Fox, Brian Rezvani, and Jeff Merkel, talented undergraduates and good friends who not only provided experimental assistance but also were fearless in asking questions and who helped us laugh at both appropriate and inappropriate times. We also thank Jeff Simonson for providing the data for the ubc7Δ hmg2Δ double mutant experiment and Peter Jauert for assistance with strain construction.
This study was supported by National Science Foundation grant MBC-0078287 (R.W.) and National Institutes of Health grants GM62104 (M.B.) and GM67368-01 (J.L.).
REFERENCES
- 1.Anderson, R. G., L. Orci, M. S. Brown, L. M. Garcia-Segura, and J. L. Goldstein. 1983. Ultrastructural analysis of crystalloid endoplasmic reticulum in UT-1 cells and its disappearance in response to cholesterol. J. Cell Sci. 63:1-20. [DOI] [PubMed] [Google Scholar]
- 2.Athenstaedt, K., D. Zweytick, A. Jandrositz, S. D. Kohlwein, and G. Daum. 1999. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basson, M. E., M. Thorsness, and J. Rine. 1986. Saccharomyces cerevisiae contains two functional genes encoding 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc. Natl. Acad. Sci. USA 83:5563-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bays, N. W., R. G. Gardner, L. P. Seelig, C. A. Joazeiro, and R. Y. Hampton. 2001. Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat. Cell Biol. 3:24-29. [DOI] [PubMed] [Google Scholar]
- 5.Biederer, T., C. Volkwein, and T. Sommer. 1997. Role of Cue1 in ubiquitination and degradation at the ER surface. Science 278:1806-1809. [DOI] [PubMed] [Google Scholar]
- 6.Bordallo, J., R. K. Plemper, A. Finger, and D. H. Wolf. 1998. Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol. Biol. Cell 9:209-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun, S., K. Matuschewski, M. Rape, S. Thoms, and S. Jentsch. 2002. Role of the ubiquitin-selective CDC48(UFD1/NPL4)chaperone (segregase) in ERAD of OLE1 and other substrates. EMBO J. 21:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodsky, J. L., and A. A. McCracken. 1999. ER protein quality control and proteasome-mediated protein degradation. Semin. Cell Dev. Biol. 10:507-513. [DOI] [PubMed] [Google Scholar]
- 9.Cox, J. S., R. E. Chapman, and P. Walter. 1997. The unfolded protein response coordinates the production of endoplasmic reticulum protein and endoplasmic reticulum membrane. Mol. Biol. Cell 8:1805-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daum, G., N. D. Lees, M. Bard, and R. Dickson. 1998. Biochemistry, cell biology, and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 14:1471-1510. [DOI] [PubMed] [Google Scholar]
- 11.Donald, K. A., R. Y. Hampton, and I. B. Fritz. 1997. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 63:3341-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards, P. A., and J. Ericsson. 1999. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 68:157-185. [DOI] [PubMed] [Google Scholar]
- 13.Finegold, L. 1986. Molecular aspects of adaptation to extreme cold environments. Adv. Space Res. 6:257-264. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, R. G., G. M. Swarbrick, N. W. Bays, S. R. Cronin, S. Wilhovsky, L. Seelig, C. Kim, and R. Y. Hampton. 2000. Endoplasmic reticulum degradation requires lumen to cytosol signaling: transmembrane control of Hrd1p by Hrd3p. J. Cell Biol. 151:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampton, R. Y. 2002. ER-associated degradation in protein quality control and cellular regulation. Curr. Opin. Cell Biol. 14:476-482. [DOI] [PubMed] [Google Scholar]
- 16.Hampton, R. Y., R. G. Gardner, and J. Rine. 1996. Role of 26S proteasome and HRD genes in the degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, an integral endoplasmic reticulum membrane protein. Mol. Biol. Cell 7:2029-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hampton, R. Y., A. Koning, R. Wright, and J. Rine. 1996. In vivo examination of membrane protein localization and degradation with green fluorescent protein. Proc. Natl. Acad. Sci. USA 93:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinemeyer, W., A. Gruhler, V. Mohrle, Y. Mahe, and D. H. Wolf. 1993. PRE2, highly homologous to the human major histocompatibility complex-linked RING10 gene, codes for a yeast proteasome subunit necessary for chrymotryptic activity and degradation of ubiquitinated proteins. J. Biol. Chem. 268:5115-5120. [PubMed] [Google Scholar]
- 19.Hiller, M. M., A. Finger, M. Schweiger, and D. H. Wolf. 1996. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science 273:1725-1728. [DOI] [PubMed] [Google Scholar]
- 20.Hitchcock, A. L., K. Auld, S. P. Gygi, and P. A. Silver. 2003. A subset of membrane-associated proteins is ubiquitinated in response to mutations in the endoplasmic reticulum degradation machinery. Proc. Natl. Acad. Sci. USA 100:12735-12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchcock, A. L., H. Krebber, S. Frietze, A. Lin, M. Latterich, and P. A. Silver. 2001. The conserved npl4 protein complex mediates proteasome-dependent membrane-bound transcription factor activation. Mol. Biol. Cell 12:3226-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoppe, T., K. Matuschewski, M. Rape, S. Schlenker, H. D. Ulrich, and S. Jentsch. 2000. Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell 102:577-586. [DOI] [PubMed] [Google Scholar]
- 23.Johnston, M., and R. W. Davis. 1984. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol. Cell. Biol. 4:1440-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman, R. J. 2002. Orchestrating the unfolded protein response in health and disease. J. Clin. Investig. 110:1389-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knop, M., A. Finger, T. Braun, K. Hellmuth, and D. H. Wolf. 1996. Der1, a novel protein specifically required for endoplasmic reticulum degradation in yeast. EMBO J. 15:753-763. [PMC free article] [PubMed] [Google Scholar]
- 26.Koning, A. J., L. L. Larson, E. J. Cadera, M. L. Parrish, and R. L. Wright. 2002. Mutations that affect vacuole biogenesis inhibit proliferation of the endoplasmic reticulum in Saccharomyces cerevisiae. Genetics 160:1335-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koning, A. J., P. Y. Lum, J. M. Williams, and R. Wright. 1993. DiOC6 staining reveals organelle structure and dynamics in living yeast cells. Cell Motil. Cytoskel. 25:111-128. [DOI] [PubMed] [Google Scholar]
- 28.Koning, A. J., C. J. Roberts, and R. L. Wright. 1996. Different subcellular localization of Saccharomyces cerevisiae HMG-CoA reductase isozymes at elevated levels corresponds to distinct endoplasmic reticulum membrane proliferations. Mol. Biol. Cell 7:769-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostova, Z., and D. H. Wolf. 2003. For whom the bell tolls: protein quality control of the endoplasmic reticulum and the ubiquitin-proteasome connection. EMBO J. 22:2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai, M. H., M. Bard, C. A. Pierson, J. F. Alexander, M. Goebl, G. T. Carter, and D. R. Kirsch. 1994. The identification of a gene family in the Saccharomyces cerevisiae ergosterol biosynthesis pathway. Gene 140:41-49. [DOI] [PubMed] [Google Scholar]
- 31.Lees, N. D., M. Bard, and D. R. Kirsch. 1999. Biochemistry and molecular biology of sterol synthesis in Saccharomyces cerevisiae. Crit. Rev. Biochem. Mol. Biol. 34:33-47. [PubMed] [Google Scholar]
- 32.McCracken, A. A., and J. L. Brodsky. 2003. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD). Bioessays 2 5:868-877. [DOI] [PubMed] [Google Scholar]
- 33.Molzhan, S. W., and R. A. Woods. 1972. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J. Gen. Microbiol. 72:339-348. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa, Y., N. Sakumoto, Y. Kaneko, and S. Harashima. 2002. Mga2p is a putative sensor for low temperature and oxygen to induce OLE1 transcription in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 291:707-713. [DOI] [PubMed] [Google Scholar]
- 35.Parks, L. W., and W. M. Casey. 1995. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49:95-116. [DOI] [PubMed] [Google Scholar]
- 36.Parrish, M. L., C. Sengstag, J. D. Rine, and R. L. Wright. 1995. Identification of the sequences in HMG-CoA reductase required for karmellae assembly. Mol. Biol. Cell 6:1535-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Profant, D. A., C. J. Roberts, A. J. Koning, and R. L. Wright. 1999. The role of the 3-hydroxy 3-methylglutaryl coenzyme A reductase cytosolic domain in karmellae biogenesis. Mol. Biol. Cell 10:3409-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Profant, D. A., C. J. Roberts, and R. L. Wright. 2000. Mutational analysis of the karmellae-inducing signal in Hmg1, a yeast HMG-CoA reductase isozyme. Yeast 16:811-827. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Vargas, S., F. Estruch, and F. Randez-Gil. 2002. Gene expression analysis of cold and freeze stress in Baker's yeast. Appl. Environ. Microbiol. 68:3024-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schade, B., G. Jansen, M. Whiteway, K. D. Entian, and D. Y. Thomas. 2004. Cold adaptation in budding yeast. Mol. Biol. Cell 15:5492-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnell, J. D., and L. Hicke. 2003. Non-traditional functions of ubiquitin and ubiquitin-binding proteins. J. Biol. Chem. 278:35857-35860. [DOI] [PubMed] [Google Scholar]
- 42.Schroder, M., and R. J. Kaufman. 2005. ER stress and the unfolded protein response. Mutat. Res. 569:29-63. [DOI] [PubMed] [Google Scholar]
- 43.Stukey, J. E., V. M. McDonough, and C. E. Martin. 1989. Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. J. Biol. Chem. 264:16537-16544. [PubMed] [Google Scholar]
- 44.Stukey, J. E., V. M. McDonough, and C. E. Martin. 1990. The OLE1 gene of Saccharomyces cerevisiae encodes the delta 9 fatty acid desaturase and can be functionally replaced by the rat stearoyl-CoA desaturase gene. J. Biol. Chem. 265:20144-20149. [PubMed] [Google Scholar]
- 45.Swanson, R., M. Locher, and M. Hochstrasser. 2001. A conserved ubiquitin ligase of the nuclear envelope/endoplasmic reticulum that functions in both ER-associated and Matalpha2 repressor degradation. Genes Dev. 15:2660-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thieringer, H. A., P. G. Jones, and M. Inouye. 1998. Cold shock and adaptation. Bioessays 2 0:49-57. [DOI] [PubMed] [Google Scholar]
- 47.Veen, M., and C. Lang. 2004. Production of lipid compounds in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 63:635-646. [DOI] [PubMed] [Google Scholar]
- 48.Vigh, L., B. Maresca, and J. L. Harwood. 1998. Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem. Sci. 23:369-374. [DOI] [PubMed] [Google Scholar]
- 49.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 50.Wright, R., M. Basson, L. D'Ari, and J. Rine. 1988. Increased amounts of HMG-CoA reductase induce “karmellae”: a proliferation of stacked membrane pairs surrounding the yeast nucleus. J. Cell Biol. 107:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright, R., M. L. Parrish, E. Cadera, L. Larson, C. K. Matson, P. Garrett-Engele, C. Armour, P. Y. Lum, and D. D. Shoemaker. 2003. Parallel analysis of tagged deletion mutants efficiently identifies genes involved in endoplasmic reticulum biogenesis. Yeast 20:881-892. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, S., Y. Skalsky, and D. J. Garfinkel. 1999. MGA2 or SPT23 is required for transcription of the Δ9 fatty acid desaturase gene, OLE1, and nuclear membrane integrity in Saccharomyces cerevisiae. Genetics 151:473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]