Abstract
The catabolism of fatty acids is important in the lifestyle of many fungi, including plant and animal pathogens. This has been investigated in Aspergillus nidulans, which can grow on acetate and fatty acids as sources of carbon, resulting in the production of acetyl coenzyme A (CoA). Acetyl-CoA is metabolized via the glyoxalate bypass, located in peroxisomes, enabling gluconeogenesis. Acetate induction of enzymes specific for acetate utilization as well as glyoxalate bypass enzymes is via the Zn2-Cys6 binuclear cluster activator FacB. However, enzymes of the glyoxalate bypass as well as fatty acid beta-oxidation and peroxisomal proteins are also inducible by fatty acids. We have isolated mutants that cannot grow on fatty acids. Two of the corresponding genes, farA and farB, encode two highly conserved families of related Zn2-Cys6 binuclear proteins present in filamentous ascomycetes, including plant pathogens. A single ortholog is found in the yeasts Candida albicans, Debaryomyces hansenii, and Yarrowia lipolytica, but not in the Ashbya, Kluyveromyces, Saccharomyces lineage. Northern blot analysis has shown that deletion of the farA gene eliminates induction of a number of genes by both short- and long-chain fatty acids, while deletion of the farB gene eliminates short-chain induction. An identical core 6-bp in vitro binding site for each protein has been identified in genes encoding glyoxalate bypass, beta-oxidation, and peroxisomal functions. This sequence is overrepresented in the 5′ region of genes predicted to be fatty acid induced in other filamentous ascomycetes, C. albicans, D. hansenii, and Y. lipolytica, but not in the corresponding genes in Saccharomyces cerevisiae.
It has become increasingly clear that the breakdown of fatty acids is important in the metabolism, development, and pathogenicity of many fungi. Catabolism occurs via the beta-oxidation pathway, in which fatty acids are activated to the corresponding acyl coenzyme A (CoA) and then oxidation by a series of enzyme steps releases acetyl-CoA and an acyl-CoA shortened by two carbons, which can undergo additional cycles of beta-oxidation. In mammals, beta-oxidation of long-chain fatty acids occurs in peroxisomes, while medium- and short-chain fatty acids undergo beta-oxidation in the mitochondria (reviewed in references 16 and 84). In contrast, in Saccharomyces cerevisiae fatty acids are metabolized entirely in peroxisomes (reviewed in reference 29). In fungi, where fatty acids can serve as sole sources of carbon and energy, the acetyl-CoA must be converted to C4 compounds via the glyoxalate bypass, comprising the enzymes isocitrate lyase and malate synthase, allowing gluconeogenesis (40, 64). Isocitrate lyase and malate synthase are usually, but not always, located in peroxisomes. It has been found that mutations affecting isocitrate lyase, malate synthase, and peroxisomal functions can affect the pathogenicity of both plant and animal pathogens (33, 37, 45, 46, 66). Furthermore it has been found that genes encoding enzymes for fatty acid catabolism are up regulated during infection. For example, microarray analysis of genes during infection of macrophages by Candida albicans showed increased expression of a number of genes involved in fatty acid utilization (47). In the plant pathogen Magnaporthe grisea, the generation of turgor pressure required for penetration of the appressorium has been proposed to depend on the conversion of lipids to glycerol via beta-oxidation and the glyoxalate bypass (69, 85). In addition, fatty acid metabolism has been implicated in secondary metabolism and development in fungi (7, 59, 72). Therefore, the understanding of the regulation of these metabolic pathways in fungi is of considerable significance.
In S. cerevisiae, oleate utilization depends on peroxisomal beta-oxidation. A wide range of peroxisomal proteins and the enzymes necessary for complete conversion of oleate to acetyl-CoA are induced by oleate (29). Acetyl-CoA produced in the peroxisome is converted to acetyl-carnitine for export to the cytosol and the mitochondrion (18, 39, 63, 79, 80). The related transcriptional activators Oaf1 and Pip2 containing DNA binding domains of the Zn2-Cys6 binuclear class together with Adr1, a C2H2 zinc finger protein, are responsible for this induction via the cis-acting elements (ORE) and UAS1, respectively (4, 25, 34, 35, 48, 57, 58). The genes for metabolism of the resulting acetyl-CoA by the glyoxalate bypass and gluconeogenesis is controlled by the Zn2-Cys6 binuclear proteins Cat8 and Sip4 acting at carbon source-responsive elements (CSREs) in the 5′ region of the relevant genes (26, 27, 54, 55, 56, 81). Some of these genes are also regulated by Adr1 (86). Growth on ethanol or acetate as sole carbon sources is also dependent on the Cat8, Sip4, and Adr1 activators as well as the Snf1 kinase (82, 86). In the presence of glucose the Mig1 repressor represses the expression of these genes (23). Growth on acetate depends on acetyl-CoA synthetase-dependent formation of acetyl-CoA in the cytosol. This is converted to acetyl-carnitine by a cytosolic acetyl-carnitine transferase and transported into the mitochondrion, where it is converted back to acetyl-CoA by a mitochondrial acetyl-carnitine transferase for metabolism by the tricarboxylic acid cycle (18, 62, 80).
In the filamentous fungus Aspergillus nidulans, mutations in the facA gene, encoding acetyl-CoA synthetase, and in facC, encoding cytosolic acetyl-carnitine transferase, result in an inability to use acetate as a sole carbon source. These genes are induced by acetate via the FacB activator, which is related to the Cat8 and Sip4 proteins of S. cerevisiae (10, 31, 61, 67, 74, 76, 77). Mutations in acuD and acuE, which encode the glyoxalate bypass enzymes isocitrate lyase and malate synthase, respectively, are also unable to use acetate, but in addition are unable to use fatty acids as sole carbon sources (2, 3, 21, 36, 61). These genes are also regulated by acetate induction via FacB, but facB mutations do not prevent growth on fatty acids, and these genes are, in addition, regulated by fatty acid induction (2, 5). Similarly, acuJ encoding an acetyl-carnitine transferase located in both mitochondria and peroxisomes is required for growth on both acetate and fatty acids because it is necessary for the shuttling of acetyl-CoA via the transporter AcuH (14, 50, 67). The acuJ gene is also subject to FacB-mediated acetate induction as well as fatty acid induction (67; M. J. Hynes, A. Andrianopoulos, S. Delimitrou, S. L. Murray, H. Sealy-Lewis, and M. A. Davis, unpublished data). This is also the case for idpA, the gene for NADP-isocitrate dehydrogenase located in peroxisomes and in mitochondria (68).
A. nidulans is able to utilize short-chain and long-chain fatty acids, and recently it has been shown that beta-oxidation pathways exist in both mitochondria and peroxisomes, as is the case for mammals and plants. There is evidence for fatty acid induction of some of the relevant enzymes in A. nidulans (49, 78) and in Neurospora crassa (28, 38). Nothing is known about the mechanism or the regulatory genes involved. It is therefore predicted that there are three classes of genes: (i) acetate specific, regulated by FacB; (ii) acetate and fatty acid induced and controlled by FacB and unknown fatty acid regulators, and (iii) fatty acid specific induced only by the fatty acid regulators. This latter class is predicted to include enzymes required for fatty acid breakdown via beta-oxidation as well as proteins involved in peroxisome biogenesis and function.
We have sought to investigate this regulatory circuit by isolating mutants unable to use fatty acids as carbon sources but still capable of growth on acetate and by cloning the corresponding genes. Among the genes identified are ones predicted to encode Zn2-Cys6 binuclear proteins. These have been shown to control the expression of genes involved in fatty acid catabolism but not to affect expression of genes involved specifically in acetate utilization. One of these genes affects induction by short-chain (C2 to C6) fatty acids and is not highly conserved in other fungi. Two other genes, one affecting short-chain induction and one affecting induction by all chain lengths, encode related proteins with highly conserved orthologs found in other euascomycetes and a single ortholog in the hemiascomycetes Candida albicans, Debaryomyces hansenii, and Yarrowia lipolytica but not in other hemiascomycete lineages. These proteins have been found to bind in vitro to sequences found to be overrepresented in the 5′ region of a large number of genes predicted to be involved in fatty acid breakdown in the species which contain predicted orthologs but not in the 5′ region of the corresponding genes in S. cerevisiae. We have therefore identified a highly conserved regulatory circuit controlling the use of fatty acids in ascomycete fungi.
MATERIALS AND METHODS
A. nidulans strains, media, and transformation.
Media and conditions for growth of A. nidulans were as described by Cove (11). Carbon and nitrogen sources were added as appropriate to minimal salts. The pH of these was adjusted to 6.5 where necessary, and long-chain fatty acids added were usually dispersed in the medium by the addition of 0.5 to 1% Tergitol (NP-40; Sigma) before melting or autoclaving. All strains were derived from the original Glasgow strain and contained the velA1 mutation to promote uniform flat conidiation, and standard genetic manipulations were as previously described (8, 9). Preparation of protoplasts and transformation were as described elsewhere (1).
Molecular techniques.
Standard methods for DNA manipulations, RNA isolation, nucleic acid blotting, and hybridization have been described previously (12, 60, 75). The sequences of oligonucleotides used are given in Table 1.
TABLE 1.
Primers used in this study
| Primer name | Sequence |
|---|---|
| acuJ upper | GACACTAGTATATGATCGGAC |
| acuJ lower | AACTGCAGCGGAGGGACGA |
| LT1:RT5′ | AACCTTCTCTGGATGGATGC |
| LT1:RT3′ | AGAGCACTGAAACAAATCGG |
| BUZ7:RT5′ | ATGACACGCAGTCGATTTGG |
| BUZ7:RT3′ | TTTTCTGACCTGCCCCAAGC |
| BUX75′-RT | GCATTCGAAAGTATTGTGCGG |
| BUX7RTPCR3′ | GAAGATCTACTTTCACGCAGCGTCTCTT |
Isolation and assays of acuJ-lacZ reporter strains.
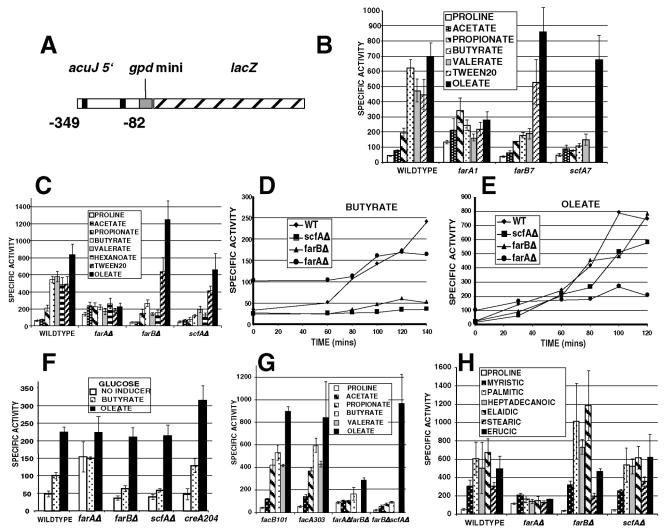
A PCR fragment from the 5′ region of the acuJ gene (corresponding to the predicted gene AN6279.2 in the genome database [http://www.broad.mit.edu/annotation/fungi/aspergillus/]), which has been shown to encode a peroxisomal-mitochondrial carnitine acetyltransferase (Hynes et al., unpublished), was generated using the primers acuJ upper (containing a Spe1 site) and acuJ lower (containing a Pst1 site). This fragment contained sequences from −82 to −349 of the 5′ region (relative to the ATG) and was cloned into Spe1-Pst1-digested pANXKSK+, a vector containing a minimal promoter (gpd-mini) driving expression of the Escherichia coli lacZ gene as well as a mutated A. nidulans argB gene that allows site-specific integration at the argB locus when transformed into argB2-containing strains (53). Transformation of the vector alone has resulted in a strain with a single plasmid copy integrated, and assays of β-galactosidase showed low levels of expression under the conditions used in this paper (M. J. Hynes, unpublished data). Transformation of the acuJ-lacZ plasmid into an argB2 strain yielded a transformant, MH9975, shown by Southern blotting to have a single plasmid integrated, in which β-galactosidase activities were regulated by fatty acids. Crosses generated acuJ-lacZ strains in various genetic backgrounds. Extracts of mycelium were prepared and assayed for β-galactosidase as described elsewhere (13).
Isolation of mutants.
A conidial suspension of strain MH9975 was treated with diepoxyoctane as described previously (32), and dilutions plated on glucose-minimal ammonium tartrate medium with 0.08% sodium deoxycholate were added to restrict the colony size. Colonies were velvet replicated to minimal medium containing 20 mM sodium butyrate as the sole carbon source with 10 mM ammonium chloride as the nitrogen source. Potential mutants affected in butyrate utilization were isolated and tested for their ability to use l-glutamate (50 mM) and acetate (50 mM) as carbon sources. Mutants specifically affected in butyrate utilization were isolated for further study. This yielded strains containing the scfA7 and farB7 mutations. One of the butyrate-nonutilizing mutants (like a number of others) was completely inhibited in growth on medium containing fatty acids. This mutant subsequently was shown to have a mutation in a gene (pexF) encoding an ortholog of the peroxin Pex6 (42, 72, 83). This strain was inoculated onto minimal medium containing 0.5% lactose and 0.2% Tween 80, as a source of the fatty acid oleate, and ammonium chloride. Spontaneous resistant sectors appearing after incubation for 3 to 5 days were isolated. Outcrossing of these yielded isolates separated from the original pexF mutation that were unable to use fatty acids as sole carbon sources but did not show the extreme pexF phenotype. This yielded a strain with the farA1 mutation.
Molecular cloning and sequencing of genes.
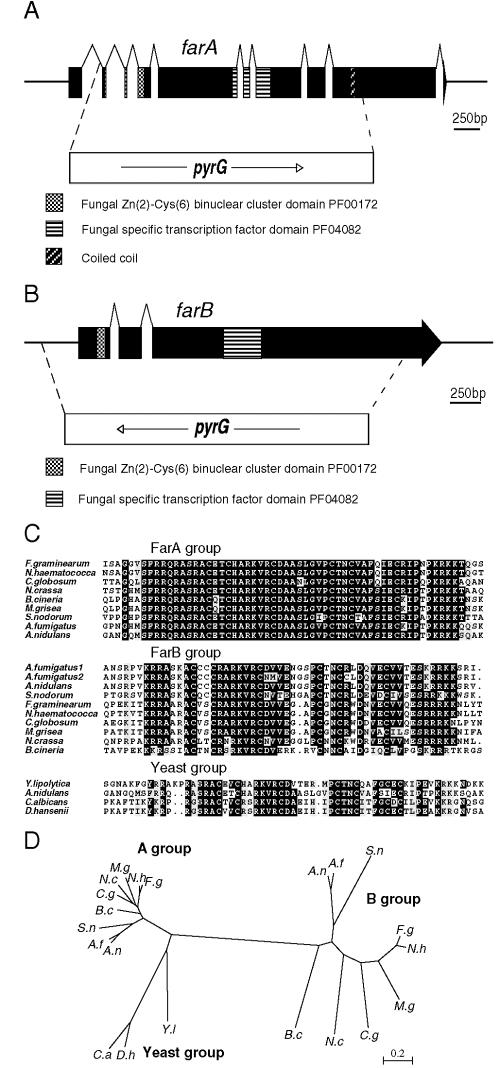
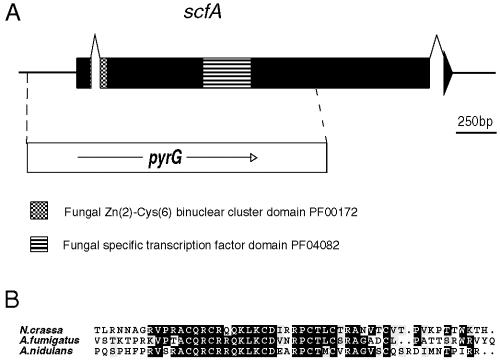
Strains containing the proposed regulatory mutations were crossed to a strain containing the pyrG89 mutation to generate PyrG− double mutants. These strains were used for cloning the genes by functional complementation by transforming with a genomic library in the autonomously replicating vector pRG3AMA1 (51). Transformants selected for uracil-uridine prototrophy were velvet replicated to medium containing butyrate as the sole carbon source, complementing transformants were selected and purified, and genomic DNA was prepared. Plasmid DNA was recovered by transforming this DNA into E. coli, selecting for ampicillin resistance. Alternatively, direct PCR on genomic DNA was used to recover insert DNA from complementing plasmids. Subcloning, sequencing, and comparison with the A. nidulans database (http://www.broad.mit.edu/annotation/fungi/aspergillus/) showed that scfA corresponded to the annotated gene AN1303.2, farA corresponded to AN7050.2, and farB corresponded to AN1425.2.
cDNA corresponding to the genes was synthesized from total RNA using gene-specific primer pairs LT1:RT5′ and LT1:RT3′, BUZ7RT5′ and BUZ7RT3′, and BUX75′RT and BUX7RTPCR3′ for farA, farB, and scfA, respectively, using the Superscript II (Invitrogen) one-step reverse transcriptase PCR kit. Products were cloned into the plasmid pGEMTEasy (Promega Corp.) and sequenced. This led to a reannotation of intron positions in the genes (see Fig. 2 and 7, below).
FIG. 2.
Structures of farA and farB genes and comparisons with orthologs in other fungi. (A) The farA gene, showing intron positions and the sequences coding for the indicated domains. farAΔ was generated by replacing sequences from +225 to +2670 (relative to the start codon) with the A. nidulans pyrG gene followed by gene replacement. (B) Structure of the farB gene, showing intron positions and the sequences coding for the indicated domains. farBΔ was generated by replacing sequences from −227 to +2368 (relative to the start codon) with the A. nidulans pyrG gene followed by gene replacement. (C) Comparison of the Zn2-Cys6 binuclear cluster domains of the FarA and FarB proteins in filamentous fungi and the orthologs in hemiascomycetes compared to A. nidulans FarA (Yeast group). Identical residues present in at least 60% of the sequences are indicated by black boxes, whereas gray shading represents similar residues. Overall comparisons of the proteins, database accession numbers, and intron positions in the genes are presented in the Fig. S1 to S3 in the supplemental material. (D) Unrooted neighbor joining distance tree showing the relationships of the FarA, FarB, and yeast proteins to each other. Sequences were aligned using ClustalW (70) and distance matrices were calculated (19), which were used to create the tree in NJPlot (52). Species abbreviations: A.n, Aspergillus nidulans; A.f; Aspergillus fumigatus; S.n, Stagonospora nodorum; F.g, Fusarium graminearium; N.h, Nectria hematococca; M.g, Magnaporthe grisea; N.c, Neurospora crassa; C.g, Chaetomium globosum; B.c, Botrytis cineria; C.a, Candida albicans; D.h, Debaryomyces hansenii; Y.l, Yarrowia lipolytica.
FIG. 7.
Structure of the scfA gene. (A) The scfA gene, showing intron positions and the sequences coding for the indicated domains. scfAΔ was generated by replacing sequences from −295 to +1470 (relative to the start codon) with the A. nidulans pyrG gene followed by gene replacement. (B) Comparison of the Zn2-Cys6 binuclear cluster domains of scfA with proposed orthologs in N. crassa and A. fumigatus. Identical residues present in at least 60% of the sequences are indicated by black boxes, whereas gray shading represents similar residues. Overall comparisons of the proteins and database accession numbers are presented in Fig. S5 in the supplemental material.
Generation of gene deletions.
Restriction fragments containing the A. nidulans pyrG gene were inserted into the plasmids pSM5953 (farA), pAD5875 (farB), and pSM5831 (scfA), replacing sequences with coordinates relative to the genes +225 to +2760, −277 to +2368, and −295 to +1470, respectively. Linear fragments generated by restriction digests were transformed into the pyrG89 strain MH10076, selecting for uracil-uridine prototrophy. Transformants showing the phenotypes of the corresponding original mutants were analyzed by Southern blot analysis of genomic DNA to confirm precise gene replacement events and further confirmed by complementation of phenotypes by transforming deletion mutants with plasmids containing wild-type genes.
Expression of MBP fusion proteins.
Fragments of farA, farB, and scfA genes were expressed as maltose binding protein (MBP) fusion proteins using the pMAL system (New England BioLabs). The cDNA clones of farA, farB, and scfA, pSM6128, pSM6123, and pSM5777, respectively, were used as templates for PCRs using primers to introduce a restriction site enabling the cloning of complete cDNAs of farA, farB, and scfA into the pMALC2 polylinker in the same reading frame as malE. The resulting plasmids, pSM6350 (encoding MBP fused to amino acids 1 to 399 of FarA), pSM6351 (encoding MBP fused to amino acids 1 to 514 of FarB), and pSM6229 (encoding MBP fused to amino acids 1 to 410 of ScfA), together with the previously described pRT2013 (encoding MBP fused to amino acids 4 to 417 of FacB [74]) were transformed into E. coli strain BL21-DE3 or Rosetta (Novagen) for expression. An overnight culture was diluted 1:100 in M9ZB medium, with the addition of 1 mM MgSO4, 0.4% glucose, and grown at 37°C with aeration to an optical density at 600 nm of 0.6. Expression of fusion plasmids was induced by the addition of isopropyl-β-d-thiogalactopyranoside to a 1 mM final concentration for 2 h. Cells were harvested, and crude extracts were produced as described in the pilot experiment of the pMAL protein fusion and purification system manual (New England BioLabs). Protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad). Expression of fusion proteins of the predicted sizes was visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Electrophoretic mobility shift assays (EMSAs).
Oligonucleotide probes were prepared, and binding reactions were carried out as previously described except that no poly(dI-dC) or Mg2+ was added (74). Electrophoresis of samples occurred on a nondenaturing 5% polyacrylamide gel in 0.5× Tris-borate-EDTA at 100 to 150 V. Gels were blotted onto 3MM paper, dried in a vacuum dryer, and exposed to X-ray film.
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank and assigned the accession numbers DQ386636 (scfA), DQ386637 (farA), and DQ386638 (farB).
RESULTS
Isolation of farA and farB mutants and cloning of the corresponding genes.
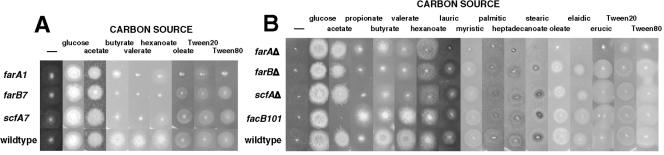
Mutants unable to grow on medium containing butyrate as a sole carbon source were isolated by replica plating from glucose plates containing colonies derived from diluted mutagenized conidiospores. Those specifically unable to grow on butyrate but capable of growth on glutamate and acetate as sole carbon sources were isolated for further analysis. Studies on mutants found to be affected in a variety of beta-oxidation and peroxisomal functions will be reported elsewhere. One of these mutants was completely inhibited on medium containing fatty acids but not acetate. This mutant was found to contain a mutation in the ortholog of the PEX6 gene of S. cerevisiae, which encodes a peroxin required for the import of proteins into the peroxisomal matrix (42, 72, 83). It was thought that this toxicity resulted from mislocalization of peroxisomal beta-oxidation enzymes. Therefore, it was proposed that mutations affecting fatty acid induction would result in resistance. This mutant was used to isolate revertants capable of growth on medium containing 0.5% lactose (to provide a nonrepressing carbon source) and 0.1% Tween 80, which contains principally oleate. The revertants showed weak growth on this medium compared to the parent strain, which was completely inhibited, consistent with growth on lactose as the carbon source and resistance to the effects of oleate catabolism. Outcrossing of one of these mutants allowed the separation of the new mutation from the original peroxisome defect mutant. Strains containing the new mutation were unable to use all fatty acids tested as sole carbon sources, and this was shown to be due to a mutation in a single gene, designated farA (Fig. 1). A further two alleles of this gene were isolated in this revertant screen.
FIG. 1.
Growth of regulatory gene mutants on fatty acids as sole carbon sources. The following carbon sources were added to minimal medium with 10 mM ammonium chloride as the nitrogen source: glucose (1%); acetate (50 mM); butyrate and valerate (10 mM); hexanoate (5 mM), propionate, Tween 20, and Tween 80 (0.1%); lauric, myristic, palmitic, heptadecanoic, stearic, oleate, elaidic, and erucic acids (2.5 mM). Growth was for 2 to 3 days at 37°C.
A second mutant was found to contain a mutation in a single gene, designated farB, resulting in loss of the ability to use the short-chain fatty acids butyrate, valerate, and hexanoate as sole carbon sources but capable of growth on longer-chain-length fatty acids (lauric acid, C12 and above) (Fig. 1A). As reported elsewhere, C7 to C10 fatty acids were found to be toxic to all strains (49).
The farA and farB genes were cloned by complementation by the isolation of double mutants containing the pyrG89 mutation, transforming with a genomic library in the autonomously replicating vector pRG3AMA1 (51), selecting for PyrG+ transformants, and replica plating to medium with butyrate as the sole carbon source. Colonies capable of growth were purified and DNA isolated. The corresponding autonomously replicating plasmids were recovered by transformation into E. coli. Sequencing of insert DNA in the recovered plasmids showed that farA corresponded to the annotated gene AN7050.2, while farB corresponded to AN1425.2 in the genome sequence (http://www.broad.mit.edu/annotation/fungi/aspergillus/). cDNA of each gene, made by reverse transcription-PCR, was cloned and sequenced, leading to a reannotation of intron positions in each gene (Fig. 2A and B). Each gene was deleted by replacement of sequences with the pyrG gene as shown in Fig. 2. The generated mutations segregated as single genes, and each deletion mutant was complemented by subclones of the relevant cloned genes, indicating that no additional events occurred during the isolation of the deletions.
The corresponding phenotypes with respect to fatty acid utilization of each gene deletion were identical to those of the original isolated mutants (Fig. 1B). More extensive testing of the deletion mutants showed that farBΔ resulted in loss of utilization of short-chain fatty acids but not inhibition of growth and did not affect growth on long-chain fatty acids. The farA deletion resulted in loss of the utilization of short-chain fatty acids but also resulted in loss of utilization and inhibition on all fatty acids tested longer than C10 (lauric acid) up to C22 (erucic acid), including the odd-chain-length heptadecanoate (C17) and the unsaturated oleic and elaidic acids (C18). The loss-of-function facB101 mutation (2, 76) did not affect growth on fatty acids apart from a slight effect on butyrate. Growth on propionate (C3) was inhibited in the mutants as well as the wild type and the facB101 mutant was resistant, consistent with previous results showing that propionyl-CoA generated from propionate is inhibitory to growth (6).
The farA and farB genes encode conserved proteins containing fungal-specific transcription factor domains.
Each of the predicted proteins encoded by the two genes were found to contain both a Zn2-Cys6 binuclear cluster domain and a conserved central domain characteristic of fungal transcription factors containing this DNA binding domain (Fig. 2A, B, and C) (62, 73). Database searches revealed predicted conserved orthologs corresponding to each of these genes in filamentous euascomycetes, including a number of plant pathogens (see Fig. S1 and S2 in the supplemental material). In the case of farA the first four introns, including two introns within the sequence encoding the Zn2-Cys6 domain, were found to be conserved in all genes and to have led to extensive misannotation in the databases (see Fig. S4 in the supplemental material). The FarA and FarB proteins showed similarity to each other, and each of the species also had a single copy of each gene, with the exception of Aspergillus fumigatus, which was found to have two predicted copies of the farB gene, apparently arising due to a segmental duplication of approximately 22.6 kbp encoding seven hypothetical annotated genes from chromosome 8 to chromosome 1 (see Fig. S2 in the supplemental material). A single copy of a related gene was found in the hemiascomycete species Candida albicans, Debaryomyces hansenii, and Yarrowia lipolytica, but not in Ashbya (Eremothecium) gossypii nor in S. cerevisiae (see Fig. S3 in the supplemental material). Y. lipolytica had one of the introns conserved in other species, while the C. albicans and D. hansenii genes lacked introns (see Fig. S4 in the supplemental material). The neighbor joining tree constructed showed that the hemiascomycete proteins were most closely related to the FarA group, which were tightly clustered in the euascomycetes. The FarB group was more divergent (Fig. 2D).
In the filamentous ascomycetes, the proposed Zn2-Cys6 binuclear DNA binding domains of these proteins were found to be extraordinarily conserved, particularly within the FarA group, and to be related between groups and with the corresponding domains in the hemiascomycete proteins (Fig. 2C). Of particular note was the finding that the predicted proteins were highly similar to the cutinase transcription factors CTFα and CTFβ of Nectria hematococca. These proteins have been found to bind to sequences in the 5′ region of cutinase genes in this plant pathogen (43, 44). Cutin is a polymer containing hydroxy-oleate, strongly suggesting a connection with fatty acid breakdown.
Effects of the farA and farB genes on induction of an acuJ-lacZ reporter construct.
The acuJ gene has been found to encode a carnitine-acetyltransferase located to peroxisomes and mitochondria and is regulated by FacB-dependent acetate induction and induced by fatty acids (50, 67; Hynes et al., unpublished). A sequence from −82 to −349 from the acuJ gene was inserted upstream of the minimal gpd promoter driving lacZ expression (53), and this was inserted in single copy at the argB locus (Fig. 3A). For studies of induction, proline was used as a noninducing limiting carbon source control. The original farA and farB mutations as well as the deletions were found to affect induction with farB, reducing induction by short-chain fatty acids but not by oleate or Tween 20 (a source of lauric, palmitic, and myristic acids), while the farA mutations led to elevated levels of noninduced expression but reduced induction by all fatty acids tested (Fig. 3B, C, and H). This was also observed for the farAΔ farBΔ double mutant (Fig. 3G). In short time course induction experiments, butyrate and oleate induction was detectable at about 60 min (Fig. 3D and E). Acetate and propionate were weak inducers, and this induction was not affected by the facB101 mutation. In fact, stronger induction in this background as well as in a facA303 background (lacking acetyl-CoA synthetase) was observed (Fig. 3G). This suggested that loss of activation of acetate and propionate to the respective acyl-CoA might enhance the weak induction observed. Induction was observed in the presence of 1% glucose, and a loss of function mutation in the creA gene, encoding the major glucose repressor protein (15), only slightly increased the response to an inducer (Fig. 3F).
FIG. 3.
Effects of regulatory gene mutations on expression of an acuJ-lacZ reporter. (A) A fragment of the acuJ 5′ region was inserted upstream of a minimal promoter (gpd mini) driving lacZ expression. The black bars represent CCGAGG sequences present in this sequence. (B to H) Mycelia of strains of the indicated genotypes were grown for 16 to 18 h in 1% glucose-10 mM ammonium tartrate medium and then transferred to minimal medium with the indicated carbon sources together with 10 mM ammonium chloride for 6 h or for the indicated times (D and E) before harvesting. Mycelia were extracted and assayed for β-galactosidase as previously described (13). The specific activity is expressed in Miller units per minute per milligram of protein. The bars represent standard errors. The concentrations of carbon sources were 10 mM for proline, acetate, butyrate, valerate, and oleate, 5 mM for hexanoate, 0.1% for propionate and Tween 20, and 2.5 mM for myristic, palmitic, heptadecanoic, elaidic, stearic, and erucic acids.
Northern blot analysis of fatty acid induction.
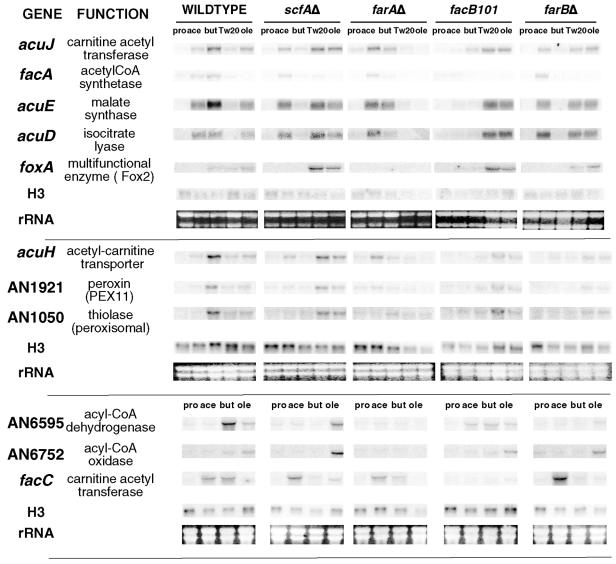
RNA isolated from induced and noninduced (proline) strains was analyzed using probes derived from relevant genes (Fig. 4). Expression of the acetate-specific genes facA and facC was induced by acetate and butyrate, and this was dependent on FacB. Butyrate induction was lost in the farAΔ and farBΔ strains, which we interpret as indicating that butyrate must be metabolized to acetyl-CoA to result in induction via FacB. Long-chain (Tween 20 and oleate) induction was not observed. Similarly, genes required for both acetate and fatty acid utilization, acuD, acuE, acuH, and acuJ, were induced by acetate and butyrate in a FacB-dependent fashion. However, these genes were also long-chain fatty acid inducible, dependent on FarA. The other genes investigated were predicted to be involved specifically in fatty acid utilization, and these were not induced by acetate but were induced by butyrate (FarB and FarA dependent) and Tween 20 and oleate (FarA dependent).
FIG. 4.
Analysis of the effects of regulatory gene mutations on expression of various genes. RNAs were extracted from strains of the indicated genotypes grown for 16 h in 1% glucose-10 mM ammonium tartrate medium and then transferred to medium with the indicated carbon sources for 6 h. Abbreviations: pro, 10 mM l-proline; ace, 10 mM acetate; but, 10 mM n-butyrate; ole, 10 mM oleate; Tw20, 0.1% Tween 20. The results represent three different Northern blot membranes probed multiple times with sequences corresponding to the indicated genes with their proposed functions. Probes were made by labeling either gel-purified restriction fragments or PCR products generated using primers based on genome sequences. H3 represents an EcoRI fragment of the histone H3 clone (17), and rRNA refers to the large rRNA species observed by ethidium bromide staining.
Detection of DNA binding by expressed FarA and FarB fusion proteins.
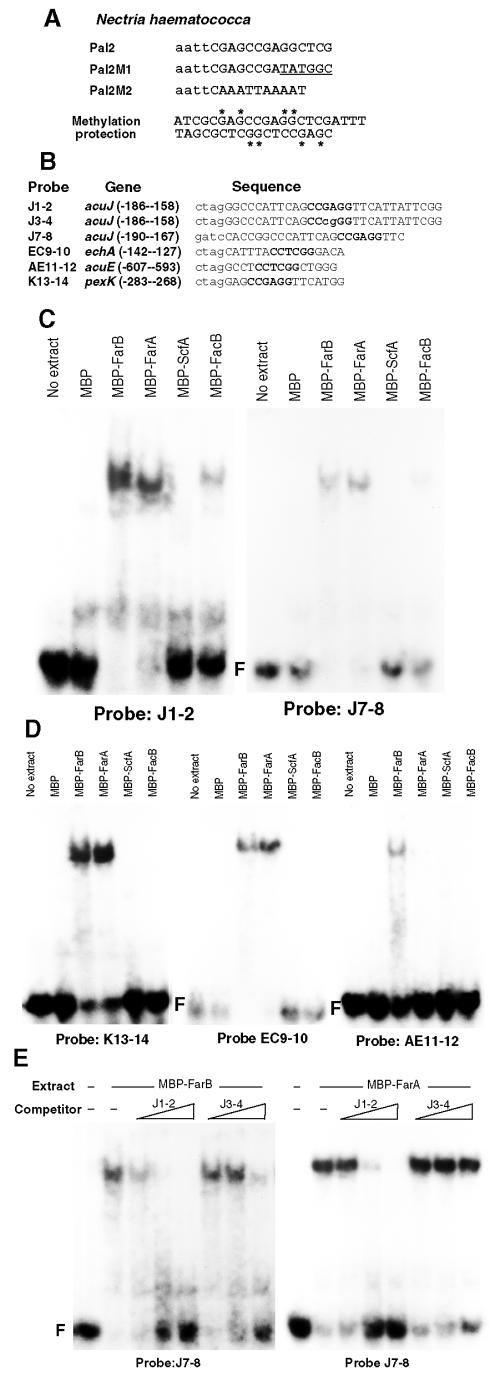
FarA and FarB proteins containing the proposed DNA binding domains were expressed in E. coli as fusion proteins with E. coli MBP (see Materials and Methods) for use in EMSAs. Because of the high degree of similarity between the proposed DNA binding domains of FarA and FarB with the N. hematococca cutinase transcription factors (Fig. 2C), the choice of DNA probes for analysis was guided by studies with these proteins (43, 44). As summarized in Fig. 5A, binding of these proteins to a sequence with a core CCGAGG but not to mutant versions of this sequence were previously observed, and this was consistent with methylation protection experiments (44). Two sequences containing this core were observed in the acuJ 5′ fragment that conferred fatty acid induction on the lacZ reporter (Fig. 3A). Two probes, J1-2 and J7-8, based on the acuJ 5′ sequence (Fig. 5B), were bound by the MBP-FarA and MBP-FarB extracts but not by the MBP extract (Fig. 5C). In addition, J1-2 and J7-8 were weakly bound by MBP-FacB (74). Both of these probes contain the sequence CCAN5CCG (where N = A, G, C, or T), a sequence related to the S. cerevisiae CSRE element bound by Sip4/Cat8 (81). The similarity of the FacB DNA binding domain to that of Cat8/Sip4 (77) presumably accounts for this weak binding. However, this is unlikely to be physiologically significant, as indicated by the lack of effect of the facB101 mutation on expression of the acuJ-lacZ reporter (Fig. 3G).
FIG. 5.
EMSA analysis of DNA binding by expressed MBP fusion proteins. (A) Summary of the key DNA binding studies of cutinase transcription factors in N. hematococca (43, 44). Binding was detected to the probe Pal2 but not Pal2M1 or Pal2M2. Methylation protection studies showed the protection of the G residues, indicated by asterisks. (B) Probes used in EMSAs in this study. Complementary pairs of oligonucleotides were designed based on sequences from the 5′ regions of the genes acuJ, echA, acuE, and pexK, with Spe1/Xba1- or BamH1/BglΙΙ-compatible ends added (in lowercase letters). The database designation of these genes is given in Table S1 in the supplemental material. The conserved core CCGAGG sequence is indicated in bold, and only one strand is shown. Probe J3-4 is identical to J1-2 except for the modification of the core CCGAGG to CCcgGG. (C and D) EMSA of extracts (20 μg of protein added to binding reaction mixtures) to the indicated probes together with no-extract controls. Free probe is designated as F. Binding is represented by the decreased mobility of the probe in the presence of protein, compared to the free probe. (E) Specific competition of binding of FarB and FarA to probe J7-8. Binding was performed with MBP-FarA and MBP-FarB extracts (20 μg of protein) in the presence of increasing amounts of unlabeled competitor DNA. Competitors were the unlabeled probes J1-2 and J3-4, increasing from 10 to 100 to 500 times the concentration of labeled probe.
Additional probes for the EMSA were based on the presence of the core CCGAGG (complement, CCTCGG) in the 5′ region of other genes predicted to be regulated by fatty acids. The gene designated as pexK (corresponding to the annotated gene AN1921) is predicted to encode the peroxin Pex11 involved in peroxisome biogenesis (42, 72, 83). This was found to be induced by fatty acids (Fig. 4). The probe K13-14 containing the core sequence was found to be bound by MBP-FarA and MBP-FarB (Fig. 5D). Similarly, the probe EC9-10, based on a 5′ sequence upstream of the echA gene encoding a mitochondrial enoyl-CoA hydratase (49), also bound MBP-FarA and MBP-FarB (Fig. 5D). However, probe AE11-12, based on a sequence from the acuE 5′ region, was found to only weakly bind to MBP-FarB and not MBP-FarA (Fig. 5D). The biological significance of this result remains to be determined.
Unlabeled DNA (J3-4) containing the mutant sequence CCcgGG replacing the core sequence showed a loss of competition for binding of MBP-FarA and MBP-FarB with the J7-8 probe, although some competition for MBP-FarB was observed (Fig. 5E). Overall the results showed, in agreement with previous observations for the N. hematococca CTF proteins, that the sequence CCGAGG (complement, CCTCGG) is the core binding sequence for these conserved proteins. It was therefore predicted that this sequence would be found in the 5′ regions of genes induced by fatty acids.
Analysis of 5′ sequences of fungal genes for predicted binding sites.
Sequences from the 5′ region (1 kb upstream of the start codon) of a large number of genes from the A. nidulans genome were scanned for the CCTCGG sequence. One or more sequences were found in nearly all genes predicted to be involved in fatty acid metabolism (see Table S1 in the supplemental material). These included genes determining proteins involved in peroxisome biogenesis or function, enzymes of beta-oxidation, including those predicted to be in mitochondria or peroxisomes, metabolite transporters, and glutathione peroxidases (but not catalases) involved in dealing with oxidative stress. The motif was found to occur in either orientation, and at least one copy was most commonly found within 300 bp of the start codon.
As part of the comparative analysis of the genomes of A. nidulans, Aspergillus oryzae, and A. fumigatus (22), a three-way alignment of 1 kb upstream of orthologous genes using Mlagan was performed. The CCTCGG motif was found to commonly occur in high-scoring conserved sequences upstream of relevant genes (these are indicated in Table S1 in the supplemental material). Furthermore, analysis of the patterns of occurrence of these conserved sequences (“conpat” analysis) identified the CCTCGG sequence to be enriched in genes for lipid metabolism and peroxisome localization (see Table 3 in reference 22).
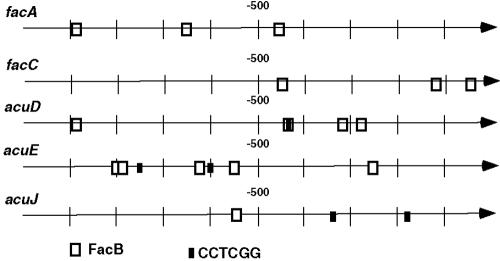
Analysis of the 5′ regions of genes required for acetate utilization showed the presence of FacB sites in all genes, while the CCTCGG motif was present only in genes also regulated by fatty acids (Fig. 6). A more limited analysis of 1 kb upstream of genes from other species containing farA and farB orthologs showed that the CCTCGG motif was overrepresented in those genes that might be expected to be regulated by fatty acids but not in acetate-specific genes (Table 2; see also Table S1 in the supplemental material). In the case of the plant pathogen M. grisea, this included members of the cutinase gene family, and the motif was also found upstream of cutinase genes as well as a predicted lipase in A. nidulans. S. cerevisiae lacks farA and farB orthologs, and fatty acid induction is dependent on the Oaf1/Pip2 heterodimer (29, 34, 35, 58). Consistent with this, the CCTCGG motif was not overrepresented in the 5′ region of relevant genes from S. cerevisiae (Table 2).
FIG. 6.
Positions of FacB binding sites and CCTCGG motifs in the 5′ regions of genes involved in acetate utilization in A. nidulans. FacB binding sites are based on those previously proposed (67, 74) or by sequence inspection in the case of acuJ. Vertical lines represent 100-bp intervals upstream of the start codon for each gene.
TABLE 2.
Presence of CCTCGG motif in the 5′ region of relevant genes from fungal speciesa
| Protein function | Position relative to start codon in:
|
||||||
|---|---|---|---|---|---|---|---|
| A. nidulans | N. crassa | M. grisea | Y. lipolytica | C. albicans | D. hansenii | S. cerevisiae | |
| Acetyl-CoA synthetase | −796 | −170 | |||||
| Carnitine acetyl transferase (cytosol) | −577 | −420 | −697 | ||||
| −503 | |||||||
| Carnitine acetyl transferase | −175 | −955 | −578 | −138 | −397 | −132 | |
| (peroxisome/mitochondrion) | −338 | −661 | −983 | −167 | |||
| −994 | −806 | ||||||
| Isocitrate lyase | −432 | −291 | −471 | −291 | −338 | −193 | |
| −718 | −639 | −908 | −623 | −236 | |||
| −770 | −793 | −589 | |||||
| Malate synthase | −603 | −164 | —b | −663 | −470 | −402 | −429 |
| −738 | −643 | ||||||
| Peroxin (PEX11) | −280 | −471 | −224 | −115 | −308 | −183 | |
| −364 | |||||||
| −624 | |||||||
Positions of CCTCCG or the complement CGGAGG were obtained by scanning 1 kb upstream of the proposed start codon. Genes for M. grisea, N. crassa, and A. nidulans were from annotated sequences obtained from http://www.broad.mit.edu/annotation/fgi/. Reannotation was carried out as necessary. Genes from Y. lipolytica and D. hansenii were from http://www.ncbi.nlm.nih.gov/; genes from C. albicans were from http://www.candidagenome.org/, and genes from S. cerevisiae were from http://www.yeastgenome.org/.
Only approximately 400 bp was available because of the position at end of the contig.
A third Zn2-Cys6 domain-containing protein involved in induction by short-chain fatty acids.
Another mutant, isolated by virtue of lack of growth on butyrate, was found to contain a mutation in a single gene, designated scfA, and resulted in loss of the ability to use the short-chain fatty acids butyrate, valerate, and hexanoate as sole carbon sources but was able to grow on longer-chain-length fatty acids (lauric acid [C12] and above) (Fig. 1A). The scfA gene was cloned by complementation by the same method used for the other genes. Sequencing showed that this gene corresponded to the annotated gene AN1303.2 (http://www.broad.mit.edu/annotation/fungi/aspergillus/). However, isolation and sequencing of cDNA showed that reassignment of intron positions was required. This resulted in the prediction that scfA encoded a protein containing a Zn2-Cys6 binuclear DNA binding motif as well as a fungal-specific transcription factor central domain (Fig. 7A). In marked contrast to FarA and FarB, ScfA was not highly conserved in other fungi, with two possible orthologs with similar DNA binding domains being found only in A. fumigatus and N. crassa (Fig. 7B), and the overall similarity was not high (see Fig. S5 in the supplemental material). The scfA gene was deleted by replacement with pyrG (Fig. 7A), and the phenotypes of scfAΔ strains were found to be the same as for scfA7 mutant strains and very similar to those for farBΔ strains (Fig. 1B). Furthermore, scfAΔ affected short-chain but not long-chain fatty acid induction of the acuJ-lacZ reporter (Fig. 3) as well as showing a similar pattern to the effects of farBΔ on transcription of the genes studied by Northern blotting (Fig. 4). Expression of ScfA as an MBP fusion protein followed by EMSA with the probes used to detect FarA and FarB binding failed to give binding (Fig. 5). Additional probes based on sequences present in the acuJ −82 to −349 region have so far also failed to give significant binding (results not shown).
DISCUSSION
The starting point for this study was the finding that genes specific for acetate utilization were only controlled by FacB-dependent acetate induction, while those required for both acetate and fatty acid utilization were regulated both by FacB and independently by fatty acids. This is consistent with the finding that facB mutations lead to loss of the ability to use acetate as a carbon source but do not affect growth on fatty acids requiring beta-oxidation. The approach of isolating mutants able to grow on acetate but not on butyrate has been successful in leading to the discovery of regulatory genes involved in fatty acid induction of genes regulated also by FacB as well as genes only regulated by fatty acids. Like facB, the farA and farB genes encode proteins containing Zn2-Cys6 binuclear DNA binding domains.
The FarA and FarB proteins are highly conserved in filamentous ascomycetes and have related DNA binding domains. The previously described cutinase transcription factors from N. hematococca (43, 44), which were defined by DNA binding studies using 5′ sequences from cutinase genes, are clearly orthologs and, in agreement with these studies, we have shown that expressed fusion proteins of FarA and FarB bind in vitro to sequences containing the core CCTCGG sequence. Analysis of fungal genomes has shown that this sequence is present in one or more copies within 1 kb of the predicted start codon of a large number of genes predicted to encode proteins involved in fatty acid metabolism, providing strong support for these regulatory genes being involved in induction by fatty acids. Furthermore, comparative analyses of the genomes of Aspergillus species indicate a conservation of this sequence in the upstream region of orthologous genes enriched for lipid metabolism and peroxisomal functions (22). Our findings on the in vivo functions of FarA and FarB provide a striking validation of the value of the phylogenetic footprinting methods used to determine potential functional motifs. The ease of detection of this simple sequence makes it possible to predict in many ascomycete species those genes likely to be regulated by fatty acid induction via these proteins.
In addition to the glyoxalate cycle enzymes, we have found direct evidence for FarA and FarB regulating genes involved in the shuttling of acetyl-CoA between peroxisomes and mitochondria (acuJ and acuH) as well as genes encoding both mitochondrial and peroxisomal beta-oxidation enzymes. We have also found that an ortholog of S. cerevisiae PEX11, predicted to encode a peroxin required for peroxisome proliferation (42, 72, 83), is regulated by FarA and FarB, and we have shown that deletion of this gene in A. nidulans results in reduced growth on fatty acids (G. S. Khew and M. J. Hynes, unpublished data). The CCTCGG motif was found upstream of a number of predicted orthologs for key peroxisomal proteins, including Pex3, which is absolutely required for de novo peroxisome formation (30, 41), as well as Pex1, Pex6, and Pex5, which are required for protein import into the peroxisomal matrix. We have isolated strains containing mutations in these genes and found that the resulting loss of peroxisomal functions leads to mild developmental defects (e.g., reduced sporulation) and complete inhibition of growth by both long- and short-chain fatty acids (Khew and Hynes, unpublished). The farAΔ and farBΔ strains do not show these phenotypes. This indicates that the Pex phenotypes result from mislocalization of induced fatty acid metabolic activities, and these effects are not observed when induction is lost. In addition, it is possible that induction of these peroxisomal functions is not absolutely required for expression but rather serves to increase the capacity for peroxisome formation and function.
Filamentous ascomycetes are very versatile in their ability to use diverse carbon sources. The finding that the conserved CCTCGG motif is present in the upstream region of the cutinase gene families of the plant pathogens M. grisea and N. hematococca as well as the saprophyte A. nidulans indicates that regulation of these genes is via fatty acid induction. We have not extensively scanned potential lipase genes, but in one case in A. nidulans we found the 5′ motif. In an expressed sequence tag analysis of the insect pathogen Metarhizium anisopliae grown under conditions of maximal cuticle degradation, lipases and phospholipases were detected and, significantly, an ortholog of the N. hematococca cutinase transcription factor was found (20). This result is now understandable, since it is highly likely that this fungal species uses orthologs of farA and farB to control enzymes involved in lipid degradation. A broad role for these transcription factors in controlling the expression of enzymes for degradation of sources of fatty acids in filamentous fungi is strongly suggested.
At this stage many questions relating to the mechanism of action of these transcription factors remain to be answered. Deletion of farA results in loss of the ability to use all fatty acids tested and loss of induction by fatty acids. However, the noninduced activity of the acuJ-lacZ reporter was found to be elevated, implying a repressing function for FarA in the absence of inducer. Deletion of farB abolishes growth on and induction by short-chain fatty acids but does not affect long-chain induction. Somewhat increased induction by long-chain fatty acids of the acuJ-lacZ reporter was also observed in the farBΔ background (Fig. 3). Given that FarA and FarB recognize the same DNA sequence, this may indicate competition for binding between inducer-activated and nonactivated proteins. Deletion of scfA gives a very similar phenotype to that of the farBΔ mutant in causing loss of growth on and induction by short-chain fatty acids but does not result in increased long-chain fatty acid induction. We have so far been unable to determine an ScfA binding site, and it is puzzling that, in stark contrast to FarA and FarB, highly conserved orthologs of this protein have not been found in other species. The possibilities that ScfA acts upstream of FarB by controlling the production of the short-chain inducing signal or that it is required for FarB expression are currently being examined. The nature of the inducing signal and the discrimination between short-chain and long-chain inducers is of considerable interest. Response of the acuJ-lacZ reporter to inducer is relatively rapid (Fig. 3), perhaps indicating that the fatty acid inducer does not require extensive metabolism to generate the signal. In S. cerevisiae it has been proposed that the activators Oaf1 and Pip2 respond directly to oleate as inducer, but direct evidence is lacking (29). Zn2-Cys6 binuclear cluster proteins have been proposed to commonly respond directly to inducing metabolites (65). It should also be noted that as beta-oxidation of long-chain fatty acids proceeds, the shortening of chain length might result in an increasing level of short-chain induction. Analysis of the A. nidulans genome reveals multiple genes potentially carrying out enzymatic steps in beta-oxidation, and the substrate and induction specificity of these may be complex.
The finding that the hemiascomycete yeasts C. albicans, D. hansenii, and Y. lipolytica contain orthologs of FarA is intriguing. Other hemiascomycetes have orthologs of the well-studied Oaf1 and Pip2 genes of S. cerevisiae. The overrepresentation of the CCTCGG motif in the 5′ region of relevant genes in these species, but not in S. cerevisiae, provides strong circumstantial evidence for the functional role of these FarA orthologs. The phylogenetic analysis presented (Fig. 2D) suggests one scenario in which a FarA orthog involved in long-chain induction was present in a progenitor of the ascomycetes. In filamentous euascomycetes, this was duplicated and diverged, resulting in the FarB genes being involved in a greater metabolic capacity to respond to short-chain substrates. FarA may have been lost and replaced by Oaf1/Pip2 orthologs in the other hemiascomycetes. Peroxisome biogenesis and alkane utilization in Y. lipolytica have been the subject of much study (e.g., references 24, 71, and 72), and investigation of the regulatory role of the FarA ortholog in this species is required.
Recent studies have suggested that peroxisomal functions and lipid metabolism are important for fungal pathogenesis (33, 37, 45-47, 66, 69, 85). Our discovery in plant and animal pathogens of transcription factors that are highly likely to be involved in controlling these processes is therefore significant. Pathogens may use these regulatory proteins to respond to host fatty acid signals to upregulate functions necessary for the establishment of infection. Deletion of the genes in these species and testing pathogenicity will therefore be of considerable interest.
. .
Supplementary Material
Acknowledgments
This work was supported by a grant from the Australian Research Council.
Assistance by Khanh Nguyen, Rosemary Genovese, Sophie Delimitrou, and Oliver Evans is acknowledged.
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Andrianopoulos, A., and M. J. Hynes. 1988. Cloning and analysis of the positively acting regulatory gene amdR from Aspergillus nidulans. Mol. Cell. Biol. 8:3532-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitt, S., W. McCullough, and C. F. Roberts. 1976. Analysis of acetate non-utilizing (acu) mutants in Aspergillus nidulans. J. Gen. Microbiol. 92:263-282. [DOI] [PubMed] [Google Scholar]
- 3.Ballance, D. J., and G. Turner. 1986. Gene cloning in Aspergillus nidulans: isolation of the isocitrate lyase gene (acuD). Mol. Gen. Genet. 202:271-275. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner, U., B. Hamilton, M. Piskacek, H. Ruis, and H. Rottensteiner. 1999. Functional analysis of the Zn2Cys6 transcription factors Oaf1p and Pip2p. Different roles in fatty acid induction of beta-oxidation in Saccharomyces cerevisiae. J. Biol. Chem. 274:22208-22216. [DOI] [PubMed] [Google Scholar]
- 5.Bowyer, P., J. R. De Lucas, and G. Turner. 1994. Regulation of the expression of the isocitrate lyase gene (acuD) of Aspergillus nidulans. Mol. Gen. Genet. 242:484-489. [DOI] [PubMed] [Google Scholar]
- 6.Brock, M., and W. Buckel. 2004. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 271:3227-3241. [DOI] [PubMed] [Google Scholar]
- 7.Calvo, A. M., H. W. Gardner, and N. P. Keller. 2001. Genetic connection between fatty acid metabolism and sporulation in Aspergillus nidulans. J. Biol. Chem. 276:25766-25774. [DOI] [PubMed] [Google Scholar]
- 8.Clutterbuck, A. J. 1974. Aspergillus nidulans genetics, p. 447-510. In R. C. King (ed.), Handbook of genetics, vol. 1. Plenum Publishing Corp., New York, N.Y. [Google Scholar]
- 9.Clutterbuck, A. J. 1994. Linkage map and locus list, p. 791-795. In S. D. Martinelli and J. R. Kinghorn (ed.), Aspergillus: 50 years on. Elsevier, Amsterdam, The Netherlands.
- 10.Connerton, I. F., J. R. S. Fincham, R. A. Sandeman, and M. J. Hynes. 1990. Comparison and cross-species expression of the acetyl-CoA synthetase genes of the ascomycete fungi, Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 4:451-460. [DOI] [PubMed] [Google Scholar]
- 11.Cove, D. J. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51-56. [DOI] [PubMed] [Google Scholar]
- 12.Davis, M. A., M. C. Askin, and M. J. Hynes. 2005. Amino acid catabolism by an areA-regulated gene encoding an l-amino acid oxidase with broad substrate specificity in Aspergillus nidulans. Appl. Environ. Microbiol. 71:3551-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, M. A., C. S. Cobbett, and M. J. Hynes. 1988. An amdS-lacZ fusion for studying gene regulation in Aspergillus. Gene 63:199-212. [DOI] [PubMed] [Google Scholar]
- 14.De Lucas, J. R., A. I. Dominguez, S. Valenciano, G. Turner, and F. Laborda. 1999. The acuH gene of Aspergillus nidulans, required for growth on acetate and long-chain fatty acids, encodes a putative homologue of the mammalian carnitine/acylcarnitine carrier. Arch. Microbiol. 171:386-396. [DOI] [PubMed] [Google Scholar]
- 15.Dowzer, C. E. A., and J. M. Kelly. 1991. Analysis of the creA gene, a regulator of carbon catabolite repression in Aspergillus nidulans. Mol. Cell. Biol. 11:5701-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton, S., K. Bartlett, and M. Pourfarzam. 1996. Mammalian mitochondrial beta-oxidation. Biochem. J. 320:345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehinger, A., S. H. Denison, and G. S. May. 1990. Sequence, organization and expression of the core histone genes of Aspergillus nidulans. Mol. Gen. Genet. 222:416-424. [DOI] [PubMed] [Google Scholar]
- 18.Elgersma, Y., C. W. van Roermund, R. J. Wanders, and H. F. Tabak. 1995. Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. EMBO J. 14:3472-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felsenstein, J. 1989. PHYLIP: phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 20.Freimoser, F. M., S. Screen, S. Bagga, G. Hu, and R. J. St. Leger. 2003. Expressed sequence tag (EST) analysis of two subspecies of Metarhizium anisopliae reveals a plethora of secreted proteins with potential activity in insect hosts. Microbiology 149:239-247. [DOI] [PubMed] [Google Scholar]
- 21.Gainey, L. D., I. F. Connerton, E. H. Lewis, G. Turner, and D. J. Balance. 1992. Characterization of the glyoxysomal isocitrate lyase genes of Aspergillus nidulans (acuD) and Neurospora crassa (acu-3). Curr. Genet. 21:43-47. [DOI] [PubMed] [Google Scholar]
- 22.Galagan, J. E., S. E. Calvo, C. Cuomo, L.-J. Ma, J. Wortman, S. Batzoglou, S.-I. Lee, M. Batürkmen, C. C. Spevak, J. Clutterbuck, V. Kapitonov, J. Jurka, C. Scazzocchio, M. Farman, J. Butler, S. Purcell, S. Harris, G. H. Braus, O. Draht, S. Busch, C. D'Enfert, C. Bouchier, G. H. Goldman, D. Bell-Pedersen, S. Griffiths-Jones, J. H. Doonan, J. Yu, K. Vienken, A. Pain, M. Freitag, E. U. Selker, D. B. Archer, M. Á. Peñalva, B. R. Oakley, M. Momany, T. Tanaka, T. Kumagai, K. Asai, M. Machida, W. C. Nierman, D. W. Denning, M. Caddick, M. Hynes, M. Paoletti, R. Fischer, B. Miller, P. Dyer, M. S. Sachs, S. A. Osmani, and B. Birren. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105-1115. [DOI] [PubMed] [Google Scholar]
- 23.Gancedo, J. M. 1998. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 62:334-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo, T., Y. Y. Kit, J. M. Nicaud, M. T. Le Dall, S. K. Sears, H. Vali, H. Chan, R. A. Rachubinski, and V. I. Titorenko. 2003. Peroxisome division in the yeast Yarrowia lipolytica is regulated by a signal from inside the peroxisome. J. Cell Biol. 162:1255-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurvitz, A., J. K. Hiltunen, R. Erdmann, B. Hamilton, A. Hartig, H. Ruis, and H. Rottensteiner. 2001. Saccharomyces cerevisiae Adr1p governs fatty acid beta-oxidation and peroxisome proliferation by regulating POX1 and PEX11. J. Biol. Chem. 276:31825-31830. [DOI] [PubMed] [Google Scholar]
- 26.Haurie, V., M. Perrot, T. Mini, P. Jeno, F. Sagliocco, and H. Boucherie. 2001. The transcriptional activator Cat8p provides a major contribution to the reprogramming of carbon metabolism during the diauxic shift in Saccharomyces cerevisiae. J. Biol. Chem. 276:76-85. [DOI] [PubMed] [Google Scholar]
- 27.Hedges, D., M. Proft, and K. D. Entian. 1995. CAT8, a new zinc cluster-encoding gene necessary for derepression of gluconeogenic enzymes in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 15:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hii, V., and J. B. Courtright. 1982. Induction of acyl coenzyme A synthetase and hydroxyacyl coenzyme A dehydrogenase during fatty acid degradation in Neurospora crassa. J. Bacteriol. 150:981-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiltunen, J. K., A. M. Mursula, H. Rottensteiner, R. K. Wierenga, A. J. Kastaniotis, and A. Gurvitz. 2003. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 27:35-64. [DOI] [PubMed] [Google Scholar]
- 30.Hoepfner, D., D. Schildknegt, I. Braakman, P. Philippsen, and H. F. Tabak. 2005. Contribution of the endoplasmic reticulum to peroxisome formation. Cell 122:85-95. [DOI] [PubMed] [Google Scholar]
- 31.Hynes, M. J. 1977. Induction of the acetamidase of Aspergillus nidulans by acetate metabolism. J. Bacteriol. 131:770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hynes, M. J. 1979. Fine-structure mapping of the acetamidase structural gene and its controlling region in Aspergillus nidulans. Genetics 91:381-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Idnurm, A., and B. J. Howlett. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot. Cell 1:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karpichev, I. V., Y. Luo, R. C. Marians, and G. M. Small. 1997. A complex containing two transcription factors regulates peroxisome proliferation and the coordinate induction of β-oxidation enzymes in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:69-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpichev, I. V., and G. M. Small. 1998. Global regulatory functions of Oaf1p and Pip2p (Oaf2p), transcription factors that regulate genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:6560-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz, M. E., and M. J. Hynes. 1989. Isolation and analysis of the acetate regulatory gene, facB, from Aspergillus nidulans. Mol. Cell. Biol. 9:5696-5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura, A., Y. Takano, I. Furusawa, and T. Okuno. 2001. Peroxisomal metabolic function is required for appressorium-mediated plant infection by Colletotrichum lagenarium. Plant Cell 13:1945-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kionka, C., and W. H. Kunau. 1985. Inducible beta-oxidation pathway in Neurospora crassa. J. Bacteriol. 161:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kispal, G., B. Sumegi, K. Kietmeier, I. Bock, G. Gajdos, T. Tomcsanyi, and A. Sandor. 1993. Cloning and sequencing of a cDNA encoding Saccharomyces cerevisiae carnitine acetyltransferase. J. Biol. Chem. 268:1824-1829. [PubMed] [Google Scholar]
- 40.Kornberg, H. L. 1966. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 99:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kragt, A., T. Voorn-Brouwer, M. van den Berg, and B. Distel. 2005. Endoplasmic reticulum-directed Pex3p routes to peroxisomes and restores peroxisome formation in a Saccharomyces cerevisiae pex3Δ strain. J. Biol. Chem. 280:34350-34357. [DOI] [PubMed] [Google Scholar]
- 42.Lazarow, P. B. 2003. Peroxisome biogenesis: advances and conundrums. Curr. Opin. Cell Biol. 15:489-497. [DOI] [PubMed] [Google Scholar]
- 43.Li, D., and P. E. Kolattukudy. 1997. Cloning of cutinase transcription factor 1, a transactivating protein containing Cys6Zn2 binuclear cluster DNA-binding motif. J. Biol. Chem. 272:12462-12467. [DOI] [PubMed] [Google Scholar]
- 44.Li, D., T. Sirakova, L. Rogers, W. F. Ettinger, and P. E. Kolattukudy. 2002. Regulation of constitutively expressed and induced cutinase genes by different zinc finger transcription factors in Fusarium solani f. sp. pisi (Nectria haematococca). J. Biol. Chem. 277:7905-7912. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz, M. C., and G. R. Fink. 2002. Life and death in a macrophage: role of the glyoxylate cycle in virulence. Eukaryot. Cell 1:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorenz, M. C., J. A. Bender, and G. R. Fink. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo, Y., L. V. Karpichev, R. A. Kohanski, and G. M. Small. 1996. Purification, identification and properties of a Saccharomyces cerevisiae oleate-activated upstream activating sequence-binding protein that is involved in the activation of POX1. J. Biol. Chem. 271:12068-12075. [DOI] [PubMed] [Google Scholar]
- 49.Maggio-Hall, L. A., and N. P. Keller. 2004. Mitochondrial beta-oxidation in Aspergillus nidulans. Mol. Microbiol. 54:1173-1185. [DOI] [PubMed] [Google Scholar]
- 50.Midgley, M. 1993. Carnitine acetyltransferase is absent from acuJ mutants of Aspergillus nidulans. FEMS Microbiol. Lett. 108:7-10. [DOI] [PubMed] [Google Scholar]
- 51.Osherov, N., and G. May. 2000. Conidial germination in Aspergillus nidulans requires RAS signaling and protein synthesis. Genetics 155:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perrière, G., and M. Gouy. 1996. WWW-Query: an on-line retrieval system for biological sequence banks. Biochimie 78:364-369. [DOI] [PubMed] [Google Scholar]
- 53.Punt, P. J., A. Kuyvenhoven, and C. A. van den Hondel. 1995. A mini-promoter lacZ gene fusion for the analysis of fungal transcription control sequences. Gene 158:119-123. [DOI] [PubMed] [Google Scholar]
- 54.Rahner, A., A. Scholer, E. Martens, B. Gollwitzer, and H. J. Schuller. 1996. Dual influence of the yeast Cat1p (Snf1p) protein kinase on carbon source-dependent transcriptional activation of gluconeogenic genes by the regulatory gene CAT8. Nucleic Acids Res. 24:2331-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roth, S., and H.-J. Schuller. 2001. Cat8 and Sip4 mediate regulated transcriptional activation of the yeast malate dehydrogenase gene MDH2 by three carbon source-responsive promoter elements. Yeast 18:151-162. [DOI] [PubMed] [Google Scholar]
- 56.Roth, S., J. Kumme, and H.-J. Schüller. 2004. Transcriptional activators Cat8 and Sip4 discriminate between sequence variants of the carbon source-responsive promoter element in the yeast Saccharomyces cerevisiae. Curr. Genet. 45:121-128. [DOI] [PubMed] [Google Scholar]
- 57.Rottensteiner, H., A. J. Kal, B. Hamilton, H. Ruis, and H. F. Tabak. 1997. A heterodimer of the Zn2Cys6 transcription factors Pip2p and Oaf1p controls induction of genes encoding peroxisomal proteins in Saccharomyces cerevisiae. Eur. J. Biochem. 247:776-783. [DOI] [PubMed] [Google Scholar]
- 58.Rottensteiner, H., L. Wabnegger, R. Erdmann, B. Hamilton, H. Ruis, A. Hartig, and A. Gurvitz. 2003. Saccharomyces cerevisiae PIP2 mediating oleic acid induction and peroxisome proliferation is regulated by Adr1p and Pip2p-Oaf1p. J. Biol. Chem. 278:27605-27611. [DOI] [PubMed] [Google Scholar]
- 59.Ruprich-Robert, G., V. Berteaux-Lecellier, D. Zickler, A. Panvier-Adoutte, and M. Picard. 2002. Identification of six loci in which mutations partially restore peroxisome biogenesis and/or alleviate the metabolic defect of pex2 mutants in Podospora. Genetics 161:1089-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 61.Sandeman, R. A., and M. J. Hynes. 1989. Isolation of the facA (acetyl-coenzyme A synthetase) and acuE (malate synthase) genes of Aspergillus nidulans. Mol. Gen. Genet. 218:87-92. [DOI] [PubMed] [Google Scholar]
- 62.Schjerling, P., and S. Holmberg. 1996. Comparative amino acid sequence analysis of the C6 zinc cluster family of transcriptional regulators. Nucleic Acids Res. 24:4599-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmalix, W., and W. Bandlow. 1993. The ethanol-inducible YAT1 gene from yeast encodes a presumptive mitochondrial outer carnitine acetyltransferase. J. Biol. Chem. 268:27428-27439. [PubMed] [Google Scholar]
- 64.Schuller, H. J. 2003. Transcriptional control of nonfermentative metabolism in the yeast Saccharomyces cerevisiae. Curr. Genet. 43:139-160. [DOI] [PubMed] [Google Scholar]
- 65.Sellick, C. A., and R. J. Reece. 2005. Eukaryotic transcription factors as direct nutrient sensors. Trends Biochem. Sci. 30:405-412. [DOI] [PubMed] [Google Scholar]
- 66.Solomon, P. S., R. C. Lee, T. J. Wilson, and R. P. Oliver. 2004. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol. Microbiol. 53:1065-1073. [DOI] [PubMed] [Google Scholar]
- 67.Stemple, C. J., M. A. Davis, and M. J. Hynes. 1998. The facC gene of Aspergillus nidulans encodes an acetate-inducible carnitine acetyltransferase. J. Bacteriol. 180:6242-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szewczyk, E., A. Andrianopoulos, M. A. Davis, and M. J. Hynes. 2001. A single gene produces mitochondrial, cytoplasmic, and peroxisomal NADP-dependent isocitrate dehydrogenase in Aspergillus nidulans. J. Biol. Chem. 276:37722-37729. [DOI] [PubMed] [Google Scholar]
- 69.Thines, E., R. W. Weber, and N. J. Talbot. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell 12:1703-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Titorenko, V. I., J. M. Nicaud, H. Wang, H. Chan, and R. A. Rachubinski. 2002. Acyl-CoA oxidase is imported as a heteropentameric, cofactor-containing complex into peroxisomes of Yarrowia lipolytica. J. Cell Biol. 156:481-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Titorenko, V. I., and R. A. Rachubinski. 2001. The life cycle of the peroxisome. Nat. Rev. Mol. Cell Biol. 2:357-368. [DOI] [PubMed] [Google Scholar]
- 73.Todd, R. B., and A. Andrianopoulos. 1997. Evolution of a fungal regulatory gene family: the Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet. Biol. 21:388-405. [DOI] [PubMed] [Google Scholar]
- 74.Todd, R. B., A. Andrianopoulos, M. A. Davis, and M. J. Hynes. 1998. FacB, the Aspergillus nidulans activator of acetate utilization genes, binds dissimilar DNA sequences. EMBO J. 17:2042-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Todd, R. B., J. A. Fraser, K.-H. Wong, M. A. Davis, and M. J. Hynes. 2005. Nuclear accumulation of the GATA factor AreA in response to complete nitrogen starvation by regulation of nuclear export. Eukaryot. Cell 4:1646-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Todd, R. B., J. M. Kelly, M. A. Davis, and M. J. Hynes. 1997. Molecular characterization of mutants of the acetate regulatory gene facB of Aspergillus nidulans. Fungal Genet. Biol. 22:92-102. [DOI] [PubMed] [Google Scholar]
- 77.Todd, R. B., R. L. Murphy, H. M. Martin, J. A. Sharp, M. A. Davis, M. E. Katz, and M. J. Hynes. 1997. The acetate regulatory gene facB of Aspergillus nidulans encodes a Zn(II)Cys6 transcriptional activator. Mol. Gen. Genet. 254:495-504. [DOI] [PubMed] [Google Scholar]
- 78.Valenciano, S., J. R. Lucas, A. Pedregosa, I. F. Monistrol, and F. Laborda. 1996. Induction of beta-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch. Microbiol. 166:336-341. [DOI] [PubMed] [Google Scholar]
- 79.van Roermund, C. W. T., Y. Elgersma, N. Singh, R. J. A. Wanders, and H. Tabak. 1995. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 14:3480-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Roermund, C. W., E. H. Hettema, M. van den Berg, H. F. Tabak, and R. J. Wanders. 1999. Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 18:5843-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vincent, O., and M. Carlson. 1998. Sip4, a Snf1 kinase-dependent transcriptional activator, binds to the carbon source-responsive element of gluconeogenic genes. EMBO J. 17:7002-7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vincent, O., and M. Carlson. 1999. Gal83 mediates the interaction of the Snf1 kinase complex with the transcription activator Sip4. EMBO J. 18:6672-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voorn-Brouwer, T., I. van der Leij, W. Hemrika, B. Distel, and H. F. Tabak. 1993. Sequence of the PAS8 gene, the product of which is essential for biogenesis of peroxisomes in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1216:325-328. [DOI] [PubMed] [Google Scholar]
- 84.Wanders, R. J., P. Vreken, S. Ferdinandusse, G. A. Jansen, H. R. Waterham, C. W. van Roermund, and E. G. Van Grunsven. 2001. Peroxisomal fatty acid alpha- and beta-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem. Soc. Trans. 29:250-267. [DOI] [PubMed] [Google Scholar]
- 85.Wang, Z. Y., C. R. Thornton, M. J. Kershaw, L. Debao, and N. J. Talbot. 2003. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 47:1601-1612. [DOI] [PubMed] [Google Scholar]
- 86.Young, E. T., K. M. Dombek, C. Tachibana, and T. Ideker. 2003. Multiple pathways are co-regulated by the protein kinase Snf1 and the transcription factors Adr1 and Cat8. J. Biol. Chem. 278:26146-26158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.