Abstract
Cholera toxin gene-negative Vibrio cholerae non-O1, non-O139 strain PL-21 is the etiologic agent of cholera-like syndrome. Hemagglutinin protease (HAP) is one of the major secretory proteins of PL-21. The mature 45-kDa and processed 35-kDa forms of HAP were purified in the presence and absence of EDTA from culture supernatants of PL-21. Enterotoxigenicities of both forms of HAP were tested in rabbit ileal loop (RIL), Ussing chamber, and tissue culture assays. The 35-kDa HAP showed hemorrhagic fluid response in a dose-dependent manner in the RIL assay. Histopathological examination of 20 μg of purified protease-treated rabbit ileum showed the presence of erythrocytes and neutrophils in the upper part of the villous lamina propria. Treatment with 40 μg of protease resulted in gross damage of the villous epithelium with inflammation, hemorrhage, and necrosis. The 35-kDa form of HAP, when added to the lumenal surface of rat ileum loaded in an Ussing chamber, showed a decrease in the intestinal short-circuit current and a cell rounding effect on HeLa cells. The mature 45-kDa form of HAP showed an increase in intestinal short-circuit current in an Ussing chamber and a cell distending effect on HeLa cells. These results show that HAP may play a role in the pathogenesis of PL-21.
Vibrio cholerae non-O1, non-O139 strains are an intriguing group that can cause typical cholera-like disease but do not produce cholera toxin (CT) (23). The virulence factor in V. cholerae non-O1, non-O139 strains that do not produce cholera toxin and yet cause symptoms similar to cholera has not yet been identified (6). It was found that none of the previously described virulence-related genes like the RTX (repeats in toxin) toxin gene cluster, the hlyA gene coding for hemolysin, the mshA gene for mannose-sensitive hemagglutinin pilus, and the stn gene encoding a heat-stable enterotoxin of non-O1 vibrios (NAG-ST) was specifically associated with the pathogenesis of TCP− CTX− non-O1, non-O139 strains that colonized mice and caused fluid accumulation in rabbits (6). In the above-mentioned study, the role of the hapA gene (coding for hemagglutinin protease [HAP]) in the disease process was not investigated. Studies by Benítez et al. with vaccine strain 638, which bears a core deletion of the ctx gene and an insertional mutation of the hapA gene, showed fewer symptoms and a decrease in diarrhea in volunteers compared to those in previous studies using only the core deletion strain (2). In addition, strain 638 induced lower levels of interleukin-8 (IL-8) than its parent wild-type strain did in HT29 cells (2, 30). Cholera vaccine strain CVD 110, a mutant of El Tor Ogawa strain E7946, which is deleted of all known virulence genes except hap, gives rise to a highly inflammatory reaction (36). Concentrated proteins derived from the culture supernatant of CVD 110 caused morphological changes in cultured MDCK-1 epithelial cells and altered their arrangement of filamentous actin and zonula occludens-associated protein (ZO-1) (38). The drastic morphological changes were inhibited by Zincov, a specific bacterial metalloprotease inhibitor. After fast-performance liquid chromatography, the cytotoxic fractions of the sample showed two visible bands with molecular masses of approximately 34 and 32 kDa. Microsequencing of these proteins revealed that they were in fact HAP. Culture supernatants prepared from the reactogenic strains of V. cholerae cause a decrease in the transcellular epithelial resistance of T84 intestinal cells (21). This decrease correlates with the presence of HAP but not with the presence of other potential accessory toxins or proteases (21). Microarray studies of the global transcription pattern of V. cholerae grown in vivo in the rabbit ileal loop (RIL) model have shown that the hemagglutinin protease (hap) gene is one of the 12 genes that belong to the pathogenesis functional group and are highly expressed compared to their levels of expression under laboratory conditions (29). V. cholerae O1 classical and El Tor biotypes as well as non-O1 serotypes produce HAP (3). The secreted HAP has been purified, cloned, and sequenced (9, 14). The hap gene consists of 1,827 nucleotides with a predicted molecular mass of 69.3 kDa. This is larger than the molecular mass of the purified HAP (32 kDa) (14). When the protease is purified in the presence of protease inhibitors, a larger form of HAP (approximately 45 kDa) is isolated (14). The protease undergoes several steps of processing, including cleavage of the signal peptide of the proprotein (69.3 kDa) to generate the mature N-terminal form (45 kDa) and a further proteolytic processing of its C-terminal region, generating the 32-kDa form (14). Our study is the first study where both the mature 45-kDa form and the C-terminally processed 35-kDa form of HAP have been purified from a ctx-negative V. cholerae non-O1, non-O139 strain, PL-21. We have shown that the processed 35-kDa form of HAP results in a dose-dependent hemorrhagic response in the RIL assay, a decrease in the intestinal short-circuit current (Isc) in an Ussing chamber, and a cell rounding effect on HeLa cells. The mature 45-kDa form shows an increase in intestinal short-circuit current in an Ussing chamber and a cell distending effect on HeLa cells. Histopathological examination of the ileal section from the RIL assays showed infiltration of inflammatory cells into the gut mucosa. Our results suggest that HAP may play a role in the pathogenesis of the disease caused by PL-21.
MATERIALS AND METHODS
Bacterial strains.
Vibrio cholerae O6 strain PL-21 was used to purify the hemagglutinin (HA) protease. The PL-21 strain was isolated from hospitalized patients admitted to the Infectious Diseases Hospital, Calcutta, India, with cholera-like disease. The strain was biochemically characterized by the API 20E system (bioMérieux Science, Montalieu-Vercieu, France) and serotyped at the National Institute of Health, Tokyo, Japan. With specific DNA probes, the above strain was found to be devoid of ctx (cholera toxin), zot (zonula occludens toxin), ace (accessory cholera enterotoxin), tcp (toxin-coregulated pilus), and sto (heat-stable enterotoxin) genes but possessed the structural gene (hlyA) for El Tor hemolysin. Escherichia coli strain DH5α was used as a negative control for Ussing chamber studies.
Purification of HAP in the absence and presence of EDTA.
V. cholerae non-O1, non-O139 strain PL-21 was grown in 2 liters of tryptic soy broth (TSB) or AKI medium (23) for 24 h at 37°C under agitation at 120 rpm in an orbital shaker (OSI503; Firstek Scientific). Cells were harvested by centrifugation at 10,000 rpm for 20 min at 4°C in an RA6 rotor (Kubota, Tokyo, Japan). The cell-free culture supernatant was salted out with 60% saturated ammonium sulfate. After centrifugation at 10,000 rpm for 20 min at 4°C, the pellet was resuspended in 25 mM Tris-HCl buffer, pH 7.4. Resuspended proteins were dialyzed against the same buffer, concentrated with an Amicon filtration unit (Millipore), and loaded onto a gel filtration chromatography column (G-200; Pharmacia Biotech). Fractions showing HAP activity were pooled and concentrated by using an Amicon filtration unit. The concentrated G-200 samples were subjected to ion-exchange chromatography (DE-52; Whatman, Kent, United Kingdom) using a column preequilibrated with 25 mM Tris-HCl buffer, pH 7.4. Most of the proteins eluted in the unbound fractions. Fractions constituting the peak were pooled, dialyzed, concentrated, and examined for hemagglutinin, protease, and aminopeptidase activity. Further purification of the unbound fraction was done using another gel filtration chromatography column (G-75; Pharmacia Biotech). The proteins bound to the DE-52 column were eluted in the presence of NaCl (0 to 1 M). Purification in the presence of EDTA was done using EDTA at all stages of purification. In the presence of EDTA, the HA and protease activity was inhibited. Dialysis in EDTA-free buffer for 4 h resulted in recovery of both HA and protease activity of the proteins. The columns were run in a BioLogic DuoFlow chromatographic system (Bio-Rad).
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (18). Protein samples after trichloroacetic acid precipitation were denatured in sample buffer containing 10% glycerol, 0.05% bromophenol blue, 2% SDS, 5% 2-mercaptoethanol, and 10 mM Tris-HCl, pH 6.8, and resolved by 12.5% SDS-PAGE with a discontinuous buffer system at a constant voltage of 60 V for the stacking gel and 120 V for the resolving gel. All chemicals used for this purpose were from Sigma Chemical Co. Proteins with known molecular masses (Bio-Rad) were used as molecular mass markers. Gels were fixed with methanol and glacial acetic acid and stained with Coomassie blue.
Detection of LPS.
Lipopolysaccharide (LPS) in protein samples recovered from the G-200 column was determined by the phenol-sulfuric acid method (5).
Amino acid sequencing.
The purified 45- and 35-kDa proteins were electrophoresed on a 10% SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride membrane. The N-terminal sequences of the blotted proteins were analyzed by using an Applied Biosystems model 491 automated protein sequencer (Applied Biosystems, Tokyo, Japan). Homology search was performed using the BLAST and FASTA programs.
Protease assay.
Casein was chosen as the substrate to assay proteolytic activity. The substrate-enzyme (azocasein-HAP) mixture was incubated at 37°C, and the reaction was terminated with 10% (wt/vol) trichloroacetic acid after 1 h. The precipitated protein was removed by centrifugation (12,000 rpm for 4 min), and the supernatant was transferred to a clean tube containing 525 mM NaOH. Absorbance was measured at 440 nm using a Shimadzu spectrophotometer. Substrate with buffer and substrate with the heat-inactivated enzyme were used as negative controls.
Hemagglutinin assay.
The technique for quantification of hemagglutinin activity was adopted from Jones et al. (16). Protein samples were serially diluted in a microtiter plate. An equal volume of 1% red blood cells was added to each well and incubated at room temperature for 60 min. Purified HA from a V. cholerae O2 strain obtained from K. Banerjee, National Institute of Cholera and Enteric Diseases, Calcutta, India, was used as a positive control (1), and 25 mM Tris-HCl buffer, pH 7.4, was used as a negative control.
Leucyl-p-nitroanilide assay.
The aminopeptidase activity was measured to detect the presence of leucine aminopeptidase (LAP) by a leucyl-p-nitroanilide assay as described by Prescott and Wilkes (26). The increase in A405 due to liberation of p-nitroaniline was measured. Eighty microliters of the sample was added to 2 ml of the substrate (0.2 mM leucyl-p-nitroanilide; Nacalai Tesque, Kyoto, Japan), and the increase in absorbance at 25°C within a period of 1 min was observed. EDTA (10 mM) or bestatin (100 μM; Nacalai Tesque, Kyoto, Japan) was preincubated for 30 min at 37°C with an equal volume of the enzyme solution to study its effect on the enzyme activity.
Hemolysin assay.
Hemolytic activity of V. cholerae strain PL-21 was determined by incubating crude and purified protease derived from the strain with washed rabbit erythrocytes diluted to a final concentration of 1% in 25 mM phosphate buffer, pH 7.4, containing 0.85% NaCl. After incubation at 37°C for 30 min, the mixture was centrifuged at 1,000 rpm for 5 min. The amount of released hemoglobin in the supernatant was measured spectrophotometrically at 540 nm. Purified hemolysin obtained as a generous gift from K. Banerjee was used as a positive control.
Rabbit ileal loop assay.
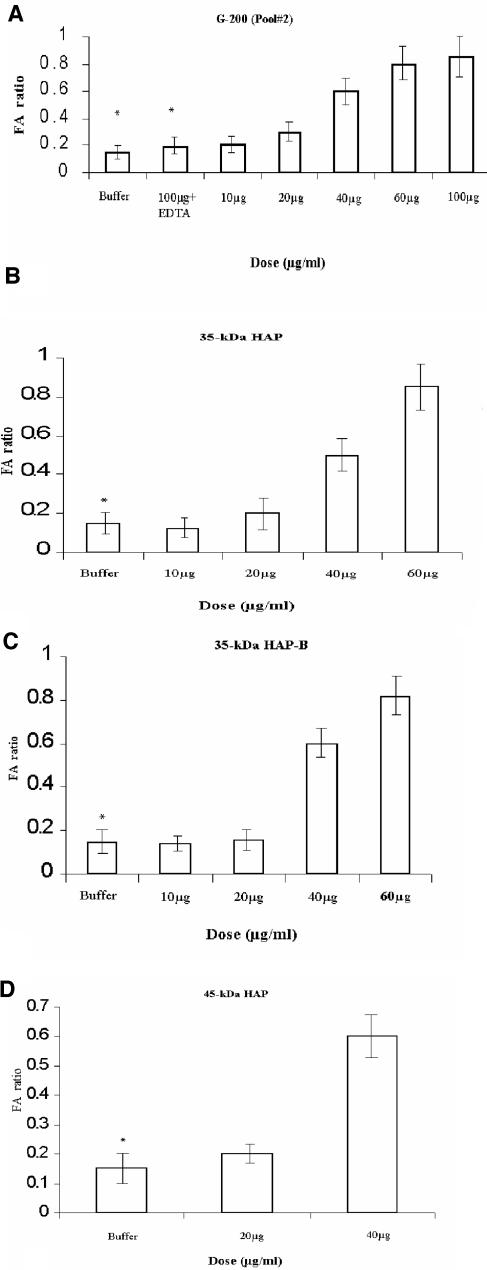
The RIL assay was performed in young New Zealand White rabbits (2 kg) essentially by the method described by De and Chatterjee (4). Rabbits were forced to fast for 24 h prior to the experiment. Animals were anesthetized with ketamine (35 mg/kg of body weight; Sigma). Protease at concentrations ranging from 10 to 100 μg in a volume of 1 ml was injected into each 5-cm loop. Inactivation of HAP by EDTA was studied by preincubating them together for 30 min. Overnight cultures of V. cholerae strain 569B grown in TSB medium, washed, and resuspended in 25 mM phosphate-buffered saline, pH 7.4, were used as a positive control, and 25 mM Tris-HCl buffer, pH 7.4, was used as a negative control. Eighteen hours later, the animals were sacrificed, and the enterotoxic response was determined by measuring the fluid accumulation (FA) ratio, which is the ratio of the volume of fluid accumulation in the intestinal loop to the length of the loop. Results in Fig. 4 show the average of FA ratios done in two experiments. A ratio of greater than 1.0 indicated a strong positive response, while a negative response was defined as an FA ratio of less than 0.2.
FIG. 4.
RIL response of HAP purified from V. cholerae non-O1, non-O139 strain PL-21. Dose-dependent response with G-200 pool 2 (A), 35-kDa HAP (B), 35-kDa HAP-B (C), and 45-kDa HAP (D). Values are means and standard deviations (error bars) for two experiments. The absence of hemorrhagic FA (*) is indicated.
Histopathological analysis.
Tissue samples from the RIL assay, 2 cm in length, were collected and placed in 10% neutral-buffered formalin for histopathological analysis. Tissues were embedded in paraffin and processed following the standard protocol. Sections (3 to 4 μm thick) prepared with a Leica rotary microtome (model RM 2145; Leica, Germany) were embedded on glass slides. Slides were stained with hematoxylin and eosin and examined by light microscopy. Photographs were taken under different magnifications with a Leica DMLB microscope (Germany), equipped with a digital imaging system. Histopathological assessment of inflammation was done using a six-grade classification system (11) and graded as 0 (structural change only), 1 (chronic inflammation), 2 (lamina propria neutrophils), 3 (neutrophil in epithelium), 4 (crypt destruction), or 5 (erosion or ulcer).
Ussing chamber.
All experiments were performed on segments from the small intestines of adult albino rats weighing 300 to 350 grams. Animals were sacrificed under pentobarbital anesthesia. A 15-cm segment of distal ileum was removed, rinsed free of its lumenal contents, opened along the mesenteric border, and stripped of serosal layers as previously described (12). Four sheets of mucosa thus prepared were mounted in a Lucite Ussing chamber (CHM2; World Precision Instruments) having a 9-mm-diameter aperture and bathed by freshly prepared Ringer's solution containing 115 mM NaCl, 25 mM NaHCO3, 2.4 mM K2HPO4, 0.4 mM KH2PO4, 1.2 mM MgCl2, and 1.2 mM CaCl2. The bathing solution was maintained at 37°C with water-jacketed reservoirs connected to a constant-temperature circulating pump and gassed with 5% CO2. After the instrument was balanced, the potential difference and the intestinal short-circuit current were measured as previously described (7). When the potential difference reached a steady state, different concentrations of the test preparations were added to the lumenal surface, resulting in a 1:10 dilution of the original concentrations (1 ml of test samples added to 10 ml of bathing solution). Equal amounts of the Ringer's solution were added to the serosal side to preserve the osmotic balance. Ammonium sulfate-precipitated proteins (100 μg) from E. coli strain DH5α were used as a negative control. At the end of every experiment, 200 μl of 0.5 M glucose was added to the mucosal side of each chamber. Only those tissues that showed an increase in Isc in response to glucose, indicating tissue viability, were included in the analysis.
Tissue culture assay.
The cell rounding and cell distending effects were assayed on HeLa cells grown in 96-well flat-bottomed tissue culture plates. HeLa cells were cultured in Eagle's modified minimum essential medium (Gibco) supplemented with 10% horse serum along with penicillin G and streptomycin sulfate (Sigma) at 37°C in the presence of 5% CO2. When the cells grew to confluence in a 25-mm2 flask, they were harvested by trypsinization and seeded in a 96-well plate at a concentration of approximately 103 cells per well. Cells were permitted to reach confluence by allowing them to grow for 24 h in a CO2 incubator (Heraeus). Samples to be tested were filter sterilized and serially diluted in tissue culture medium containing 2% horse serum. After aspiration of the medium from the 96-well plate, neat or diluted strain PL-21 culture supernatant or purified protease was added to the cells and incubated for 24 h in a CO2 incubator. Cells were examined for morphological changes with a phase-contrast inverted microscope (CK 40; Olympus).
RESULTS
Purification of HAP.
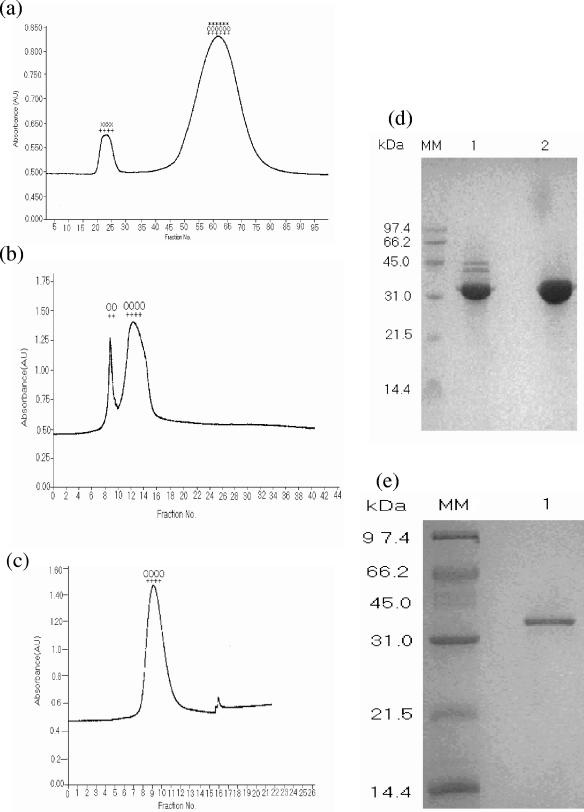
The 35-kDa form of HAP was purified in the absence of EDTA from culture supernatants of V. cholerae PL-21 grown in TSB medium. The proteins in the culture supernatants were precipitated by 60% ammonium sulfate, dissolved in 25 mM Tris-HCl buffer, pH 7.4, and dialyzed in the same buffer before loading onto a gel filtration chromatography column (G-200). LPS and proteins were eluted in the void volume and showed only HA activity (Fig. 1a, first peak). Fractions exhibiting HA, protease, and LAP activities (Fig. 1a, second peak) were pooled, concentrated, and dialyzed before being loaded into an ion-exchange column (DE-52) equilibrated with 25 mM Tris buffer, pH 7.4. The unabsorbed proteins in this column showed two peaks, both exhibiting HA and protease activity (Fig. 1b). The SDS-PAGE profile of the first peak showed 45-, 42-, and 35-kDa bands, while the second peak showed only a 35-kDa band (Fig. 1d, lanes 1 and 2, respectively). The second peak containing the 35-kDa form of HAP was further purified by gel filtration chromatography on G-75 columns. A single peak showing HA and protease activity was eluted (Fig. 1c). The peak fractions were pooled, concentrated, and dialyzed. A single 35-kDa band was observed on an SDS-polyacrylamide gel (Fig. 1e).
FIG. 1.
Chromatographic profiles showing purification of 35-kDa HAP from the culture supernatant of V. cholerae PL-21 strain. (a) Gel filtration chromatography column on G-200 column with ammonium sulfate-precipitated proteins. (b) Anion-exchange chromatography on DE-52 column of pooled fractions showing HA, protease, and LAP activity from G-200 column. (c) Gel filtration chromatography on G-75 column of pooled fractions of the second peak from DE-52 column. The presence of HA (++), protease (00), aminopeptidase (**), and lipopolysaccharide (xx) activity in the peaks is indicated. Absorbance is shown in arbitrary units (AU). (d) SDS-PAGE (12.5%) of the proteins from the two peaks in Fig. 1b. Lanes 1 and 2 contain the proteins from the first and second peaks, respectively. (e) SDS-PAGE (12.5%) of the protein from the single peak in Fig. 1c. The positions (in kilodaltons) of molecular mass markers (MM) are shown to the left of the gels.
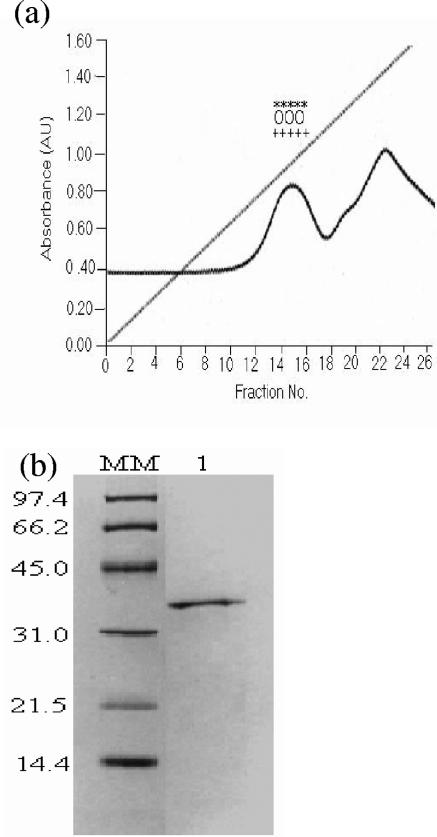
The absorbed proteins in the DE-52 column (run in the absence of EDTA) were eluted by a linear NaCl gradient (0 to 1 M). The first peak showed HA, protease, and LAP activity (Fig. 2a). The fractions of the first peak were pooled, concentrated, dialyzed, and subjected to SDS-PAGE; a single 35-kDa band was visible (Fig. 2b), and the peak protein was termed HAP-B.
FIG. 2.
Chromatographic profiles showing purification of 35-kDa HAP-B from DE-52 column (run in the absence of EDTA) by NaCl gradient (0 to 1 M). (a) The first peak eluted showed HA (++), protease (00), and LAP (**) activity. Absorbance is shown in arbitrary units (AU). (b) SDS-PAGE (12.5%) of the proteins from the first peak showed the presence of a single 35-kDa band. The positions (in kilodaltons) of molecular mass markers (MM) are shown to the left of the gel.
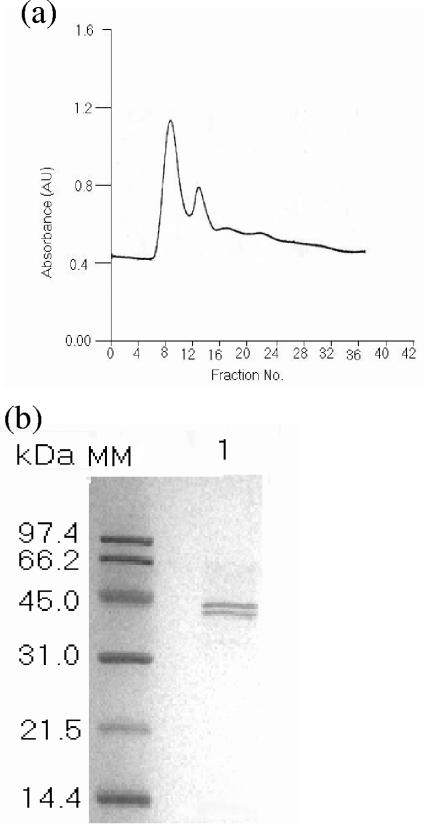
The mature 45-kDa form of HAP was purified in the presence of EDTA by gel filtration (G-200) and ion-exchange (DE-52) and gel filtration (G-75) chromatography. In the presence of EDTA, the 45-kDa form was the predominant form of HAP. The fractions of the first peak in the G-75 column (Fig. 3a) were pooled, concentrated, and dialyzed. The SDS-PAGE profile of this peak proteins showed the presence of 45- and 42-kDa bands (Fig. 3b). The fractions eluted in this column did not show HA and protease activity.
FIG. 3.
Chromatographic profiles showing purification of 45-kDa HAP purified in the presence of EDTA. (a) Gel filtration chromatography on G-75 column of the pooled fractions from successively chromatographed proteins by G-200 and DE-52 columns. Absorbance is shown in arbitrary units (AU). (b) SDS-PAGE (12.5%) of the major peak showed the presence of 45- and 42-kDa bands. The positions (in kilodaltons) of molecular mass markers (MM) are shown to the left of the gel.
The first 15 amino acids of the N termini of the 45- and 35-kDa forms of HAP and the 35-kDa HAP-B were determined to be Ala Gln Ala Thr Gly Thr Gly Pro Gly Gly Asn Gln Lys Thr Gly. Analyses with BLAST and FASTA programs revealed complete homology of the initial N-terminal 15-amino-acid sequences of the HAP purified from strain PL-21 and the soluble HAP of V. cholerae O1.
Purified HAP was proteolytically active.
The purified 35-kDa protein showed protease activity, which was inhibited in the presence of EDTA. Incubation of the purified protein at 65°C for 30 min failed to show any protease activity. The 45-kDa form of HAP purified in the presence of EDTA did not show protease activity, but the protease activity was regained after dialysis in EDTA-free buffer for 4 h.
Purified HAP showed hemagglutinin activity but no hemolytic activity.
The 35-kDa protease showed HA activity against rabbit blood but not against chicken blood. HA activity was absent when the protease was purified in the presence of EDTA. The 45-kDa form of HAP after dialysis in EDTA-free buffer showed HA activity. However, HAP did not hemolyze rabbit erythrocytes.
Purified HAP showed a dose-dependent hemorrhagic fluid response in the rabbit ileal loop assay.
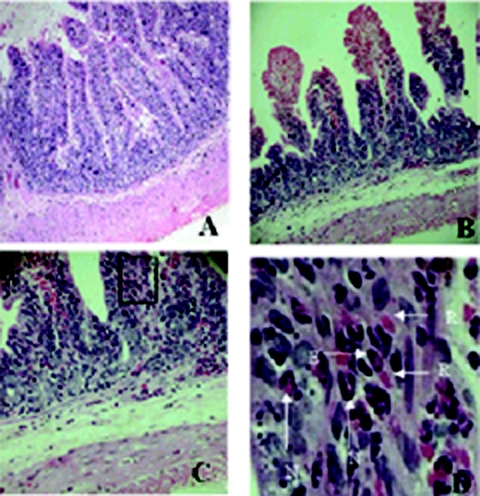
Injection of the purified 35-kDa HAP and HAP-B resulted in a dose-dependent hemorrhagic fluid response in the RIL assay (Fig. 4B and C). At a HAP concentration of 20 μg, a weak hemorrhagic response was elicited. At HAP concentrations increasing up to 60 μg, there was a gradual increase in the fluid accumulation ratio, with hemorrhagic fluid present at all the different concentrations. The protease incubated in the presence of EDTA failed to show any response in the above-described assay (Fig. 4A). The results of the RIL assay were substantiated by histopathological analysis of the ileal loop samples. In all the experiments, the rabbits were sacrificed 18 h postinoculation. The villous architecture and mucosa in the buffer control sections were normal (Fig. 5A). Purified HAP at a concentration of 20 μg resulted in little distortion of the villous pattern. The blood vessels were congested, and inflammatory cells were present in the lamina propia. High-powered photomicrograph showed the presence of erythrocytes in the upper part of the villous lamina propria. After treatment with 40 μg of purified HAP, there were marked alterations of the villous contour with red blood cell and inflammatory cell infiltrates in the villous epithelium and lamina propria and in damaged crypt epithelium (Fig. 5B). Higher magnification of portions of Fig. 5A and B revealed the clear presence of erythrocytes, eosinophils, and neutrophil polymorphs in the lamina propia, and the inflammatory changes with 35-kDa HAP were graded 1, 2, 3, or 4 (Fig. 5C and D). Washed V. cholerae 569B cultures, on the other hand, showed nonhemorrhagic fluid accumulation in the RIL assay. The active dialyzed 45-kDa form tested for entertoxigenicity in the RIL assay at concentrations of 20 μg/ml and 30 μg/ml also showed hemorrhagic fluid response (Fig. 4D).
FIG. 5.
Effect of purified HAP in the RIL assay. Purified HAP-treated ileal tissues were processed for histopathological analysis, and photomicrographs were taken. (A) Villous architecture observed in ileal tissues treated with 25 mM Tris-HCl. This photomicrograph shows normal villous structure. Magnification, ×10. (B) Rabbit ileal tissues treated with 40 μg of purified 35-kDa HAP show disruption of normal villous architecture with shortening of the villi. Magnification, ×20. (C) Magnified view of Fig. 4B shows infiltration of polymorphonuclear neutrophils, eosinophils, and erythrocytes. Magnification, ×40. (D) The black box in Fig. 4C was further magnified to show the presence of red blood cells (R), neutrophils (N), and eosinophils (E). Magnification, ×200.
Purified HAP affects intestinal short-circuit current in a dose-dependent manner.
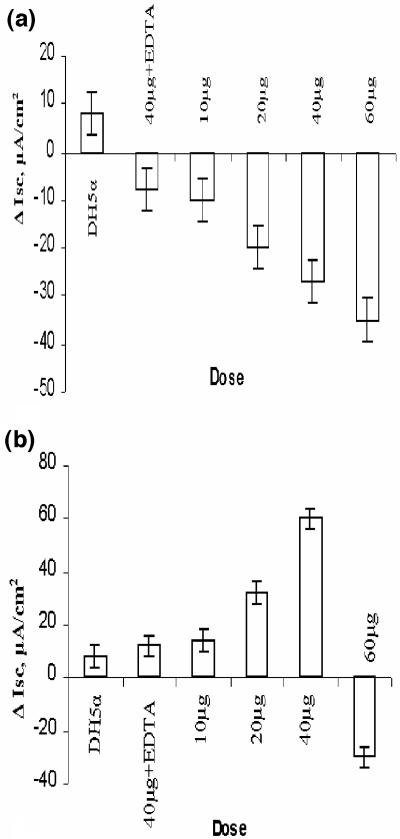
The in vivo activity of 35-kDa HA protease was further substantiated by an in vitro rat intestinal assay (Ussing chamber). The dose-dependent effect of the purified 35-kDa HAP and HAP-B (results not shown) showed a sharp decrease in the intestinal short-circuit current (Fig. 6b). Interestingly, the 45-kDa form showed a significant increase in Isc with 2-μg/ml and 4-μg/ml doses but a sharp decrease at the 6-μg/ml dose (Fig. 6a). The rise in Isc started 45 to 60 min after the addition of the protease to the lumenal side of the rat intestine, and the increase was significantly higher than that caused by proteins precipitated from E. coli DH5α strain. In the presence of EDTA, both forms of HAP failed to show any significant change in Isc (Fig. 6a and b).
FIG. 6.
Dose-dependent response of HAP on the intestinal short-circuit current of rat ileum loaded in a Ussing chamber. HAP was added in the absence or presence of EDTA to the Ringer's solution in an Ussing chamber, and the change in Isc was recorded for the next 2 h from the time the sample was added. (a) Response of Isc to increasing doses of 35-kDa HAP. (b) Response of Isc to increasing doses of 45-kDa HAP. There is an initial increase, but the Isc falls as the dose reaches beyond 40 μg/ml. Values are the means ± standard deviations (error bars) for triplicate experiments. Proteins purified from E. coli DH5α were used as controls.
HAP alters cell morphology.
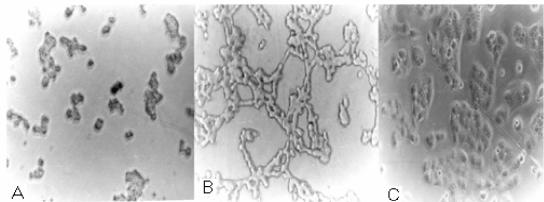
The purified 35-kDa form of HAP also showed cell rounding effect at all doses (Table 1 and Fig. 7A). The active 45-kDa form showed a dose- and time-dependent response. The active 45-kDa form showed a cell rounding effect on HeLa cells at higher concentrations, but at lower concentrations, a cell distending effect was observed (Table 1 and Fig. 7B). The distending effect was present as late as 90 h with 31.25 ng of protease. The culture supernatant of strain PL-21 showed a cell distending effect when grown in TSB medium and a cell rounding effect when grown in AKI medium on HeLa cells after 24 h of incubation. In the presence of EDTA, the cell rounding and cell distending effects were completely inhibited.
TABLE 1.
Effects of 45-kDa and 35-kDa forms of HAP on HeLa cells
| HAP form | Treatment time (h) | Effectb of HAP form at a dose of:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 μg | 2 μg | 1 μg | 500 ng | 250 ng | 125 ng | 62.5 ng | 31.25 ng | 15.6 ng | ||
| 45 kDaa | 24 | CR | CR | CR | CR | |||||
| 48 | CR | CR | CR | CR | CD | |||||
| 72 | CR | CR | CR | CR | CR | CD | CD | |||
| 90 | CR | CR | CR | CR | CR | CR | CR | CD | ||
| 35 kDa | 24 | CR | CR | CR | CR | CR | ||||
| 48 | CR | CR | CR | CR | CR | CR | CR | CR | CR | |
After dialysis in EDTA-free buffer.
CR, cell rounding; CD, cell distension.
FIG. 7.
Effects of HAP on HeLa cells. HeLa cells grown in tissue culture dishes were treated overnight with different concentrations of either the 35-kDa HAP or the 45-kDa HAP. Cells were observed with a phase-contrast microscope. (A) Cell rounding effect of 35-kDa HAP and a higher concentration of 45-kDa HAP. (B) Cell distending effect of a lower concentration of 45-kDa HAP (concentrations given in Table 1). (C) Normal HeLa cells grown in tissue culture plates.
DISCUSSION
Extracellular proteases play important roles in the pathogenesis of diarrhea caused by V. cholerae. Protease-deficient mutants of V. cholerae are reported to be less virulent (35). The most well-studied protease in cholera is hemagglutinin protease. When HAP is purified in the presence of EDTA, a larger 45-kDa form is isolated while in the absence of EDTA, a 32-kDa form is purified (14). The protease undergoes several steps of processing, including cleavage of the signal peptide and further processing of the N terminus and the C terminus to generate the mature protein (14). The 45-, 37-, 32-, and 9-kDa bands are the major bands bound to anti-HAP antibodies, which suggests that they are the proteolytic degradation products of the 45-kDa polypeptide (17). Initial studies by Finkelstein and Hanne had shown that HAP was focused at pH values of 6.3 and 5.3 to 4.7, with the majority of recoverable activity focused at pH 6.3 (9). In this study, we recovered most of the HAP activity in the unbound fraction of the anion-exchange column. Smaller amounts of the bound form of HAP could be recovered along with leucine aminopeptidases after elution by a linear salt gradient (0 to 1 M NaCl). The molecular sizes of HAP and LAP are similar (37). Purified HAP in the absence of EDTA from V. cholerae PL-21 strain in TSB medium showed the presence of both 45- and 35-kDa forms. In the presence of EDTA, the 45-kDa form of HAP was purified in larger amounts but in an inactive state. The N-terminal 15-amino-acid sequences of 45- and 35-kDa HAP were found to be the same in our study as well as in earlier studies (14), showing that the 45-kDa form is processed at the C-terminal end to generate the 35-kDa form. Ours is the first study in which the two major forms of HAP were purified and studied in Ussing chamber, rabbit ileal loop, and tissue culture assays.
The functional activity of HAP is related to its protease activity, and HAP being a metalloprotease, it shows no protease and HA activity in the presence of EDTA. The mature 45-kDa form of HAP purified in the presence of EDTA showed no effects in RIL, Ussing chamber, and tissue culture assays. Functional forms were generated when 45-kDa HAP was dialyzed in EDTA-free buffer. Once the protease is functional, it is processed, as is evident by the presence of 45-, 42-, and 35-kDa bands on SDS-polyacrylamide gels when HAP was purified in the absence of EDTA. Vibrio anguillarum secretes a metalloprotease that also exists in two forms, Pa and Pb (24). The Pb form was converted to the Pa form when the protease was incubated overnight at 4°C. HAP exists in both the 45-kDa and 35-kDa forms but is not completely converted to the smaller processed form at 4°C. Functional studies with only the 45-kDa form are not possible, as one cannot prevent its processing to the 35-kDa form in the absence of EDTA. However, the 45-kDa form can be studied in in vitro models such as Ussing chamber and tissue culture assays. This protein, at lower concentrations of 2 μg/ml and 4 μg/ml, exerts an enterotoxic response in the Ussing chamber in the form of an increase in Isc and a cell distending effect in the HeLa cell assay. At higher concentrations, it shows a decrease in the Isc in the Ussing chamber, suggesting a cytotoxic response and a cell rounding effect on HeLa cells. The most probable explanation for this differential effect is that, at lower concentrations of functional 45-kDa HAP, the amount of 35-kDa form is less abundant, while the amount of the processed 35-kDa form is elevated at higher concentrations of 45-kDa HAP and may be higher than a critical level when it exerts a cytotoxic effect. The Isc changes peaked at 4 μg/ml, while the effect observed at 6 μg/ml revealed that the enterotoxic response of the 45-kDa form was masked by the cytotoxic effect of the 35-kDa form, resulting in a decrease in Isc. The enterotoxic response of the 45-kDa HAP started after a delay of 45 to 60 min and was equivalent to the effect of 1 μg/ml of CT in the rat small intestine (19). Enterotoxicity is caused by an abnormal flux of negatively charged ions from the serosal side to the mucosal side (8). Interestingly, at a higher concentration (6 μg/ml), there was a significant decrease in Isc. It was earlier reported that culture supernatants prepared from the reactogenic strains of V. cholerae cause a decrease in the transcellular epithelial resistance of cultured T84 cells (21). This decrease correlated with the presence of HAP but not with the presence of other potential accessory toxins or proteases like LAP. The decrease persisted in spite of insertional disruption of the lapA gene, suggesting that the lapA gene product does not specifically contribute to the decrease in transcellular epithelial resistance caused by the reactogenic strains of V. cholerae (21).
Culture supernatants of strain PL-21 grown in TSB and AKI media, when applied to HeLa cells, showed cell distending and cell rounding effects, respectively. The active 45-kDa form also showed a cell distending effect, while the 35-kDa form showed a cell rounding effect on HeLa cells. Sterile culture filtrates of 68 nontoxigenic V. cholerae non-O1, non-O139 strains grown in two different media, namely, TSB and AKI, produced different effects on HeLa cells (23). Strains grown in TSB medium more often produced morphological changes consistent with those of cell distention. In contrast, when the same strains were grown in AKI medium, a cell rounding response was observed. The cell rounding toxin purified from V. cholerae O6 and a V. cholerae O1 strain (WO-5, a CT-negative strain) was found to be a 35-kDa protease without hemagglutinating activity and was termed non-membrane-damaging cytotoxin (NMDC) (32, 33). The N-terminal 15-amino-acid sequence of NMDC showed complete homology to that of HAP.
Compared to its growth in vitro, the global transcription pattern of V. cholerae grown in vivo under mid-exponential conditions in the small intestine environment showed that the hapA gene was one of the 12 highly expressed genes that belong to the pathogenesis functional group (29). Although the hap gene is upregulated in the rabbit ileal loop, the actual role of hap in vivo remains to be determined (39). Recent studies have suggested that HAP could be a potential reactogenic factor of genetically attenuated vaccine strains. The vaccine strain 638, in which ctx and hap genes were deleted, was not reactogenic in human volunteers and induced lower levels of IL-8 secretion than its parent wild-type strain in HT29 cells (2, 30). In a similar study in T84 cells, wild-type V. cholerae strains 3038 and C7258 induced levels of IL-8 similar to those of the isogenic hap mutant strains HAP-1 and 638, respectively (39). The supernatants containing HAP did not stimulate IL-8 production at a variety of concentrations tested, suggesting that HAP itself does not induce IL-8 production (39). The difference in results in stimulation of IL-8 secretion by HAP in the above-mentioned studies could be due to the use of two different cell lines, HT29 and T84. Fullner et al. compared the development of inflammation of deletion mutants in V. cholerae El Tor strain P-4 in a murine pulmonary model (10). Of all the deletion mutants tested, ΔhapA, Δhly, ΔrtxA, and ΔhapA hly rtxA strains, the group treated with ΔhapA strain was the only group in which all four mice failed to survive to 24 h (10). These data suggested that the loss of HAP may have altered the cause of infection to be more lethal (10). In the V. cholerae O1 El Tor strain P-4, CT is the major virulence factor, and deletion of hapA may help in further activation of CT by other proteases, or other proteins that are degraded in the presence of HAP may play an additional role in the pathogenesis. V. cholerae non-O1, non-O139 strains such as PL-21 are devoid of the ctxA gene, and the major virulence factor in these strains is still unknown (6). Our results on the effects of HAP in the rabbit ileal loop have shown the presence of inflammatory cells with hemorrhagic response. The other similar protease involved in such a response is fragilysin, a 20-kDa metalloprotease produced by Bacteroides fragilis (25). When purified fragilysin was injected into ligated ileal and colonic loops of rats, rabbits, and lambs, there was significant tissue damage and subsequent fluid accumulation. Histopathological examination revealed mild necrosis of epithelial cells, crypt elongation, villous attenuation, and hyperplasia. Like HAP, the enterotoxic activity of fragilysin was inhibited by the metal chelator EDTA (25). Clostridium difficile toxin A also showed a viscous hemorrhagic fluid response in rabbits and rats (20). Studies on NMDC purified from a nontoxigenic V. cholerae non-O1, non-O139 strain showed that this protein exerts both cytotoxic and enterotoxic responses (33). The authors proposed that these two effects may be due to separate biological active sites for cytotoxicity and enterotoxicity on NMDC. Our results suggested that these kind of bifunctional properties of NMDC might be due to the two forms of HAP, with the 45-kDa form responsible for the enterotoxic response and the 35-kDa form responsible for the cytotoxic response. The enterotoxic response as we observed with 45-kDa HAP in Ussing chamber and tissue culture assays could not be reproduced in the RIL assay. The reasons for such differences may be due to the fact that, in an in vivo system like the RIL assay, the 45-kDa form could be processed into the 35-kDa form by the protease itself as well as by other proteases present in the intestine. The only way the 45-kDa form can show an enterotoxic response in the RIL model is by preventing its C-terminal processing by site-directed mutagenesis of the amino acid residues involved in this processing. However, earlier attempts to express the hap gene in the E. coli system failed (14).
The pathology of cholera has traditionally been considered noninflammatory. However, several reports provide evidence for an inflammatory response. Leukocytes and erythrocytes were detected in the stool samples from cholera patients (31). Cholera vaccine strain CVD 110 that is deleted of all known virulence genes except hap gives rise to a highly inflammatory reaction (36). The morphological changes observed with culture supernatant of CVD 110 were inhibited by Zincov, a specific bacterial metalloprotease inhibitor. After fast-performance liquid chromatography, the cytotoxic fractions of the sample showed two visible bands with molecular masses of approximately 34 and 32 kDa. Microsequencing of these proteins revealed that they were in fact HAP (38). A fluid-accumulating factor (FAF) in the ligated rabbit ileal loop experiment with a non-CT-producing strain of V. cholerae non-O1 was partially purified by ammonium sulfate precipitation and gel filtration with Sephadex G-100 and DEAE cellulose columns (13). The purified protein showed collagenolytic, cytolytic, necrotic, and hemorrhagic activities. Desquamation of epithelial cells, inflammatory edema, and hemorrhage were observed in the rabbit intestine after inoculation of partially purified FAF. The inflammatory effects of FAF are similar to those observed with HAP. Studies have demonstrated that the innate immune cells are activated and inflammatory mediators are upregulated at the mucosal surface following natural infection with V. cholerae O1 and O139 (27). Endothelial cells appeared swollen with margination of neutrophil polymorphs. Elevated numbers of neutrophil polymorphs were found in the lamina propria and infiltrating the surface and crypt epithelium of the duodenum and rectum during the acute stage of cholera in adults. The number of eosinophils in duodenal and rectal tissues increased during convalescence in adult and pediatric cholera patients (28). Our results with purified 35-kDa HAP has shown accumulation of hemorrhagic fluid in the rabbit ileal loop, with histopathological studies demonstrating the presence of red blood cells, neutrophils, and eosinophils. Our data thus suggested that HAP may play an important role in the inflammatory response in cholera.
It was reported that HAP is exported by the same secretory apparatus as CT is, resulting in an indirect increase in its secretion in the absence of CT (34). Thus, HAP may play an important role in the pathogenesis of V. cholerae non-O1, non-O139 strains that are devoid of CT. The same strains were earlier reported as V. cholerae non-O1 (NAG) and were implicated as the etiologic agent for moderate to severe gastroenteritis (15, 22). The severity of infection could depend on the relative presence of different forms of HAP.
Editor: V. J. DiRita
REFERENCES
- 1.Banerjee, K. K., A. N. Ghosh, K. Dutta-Roy, S. C. Pal, and A. C. Ghosh. 1990. Purification and characterization of a novel hemagglutinin from Vibrio cholerae. Infect. Immun. 58:3698-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benítez, J. A., L. Garcia, A. Silva, H. García, R. Fando, B. Cedré, A. Pérez, J. Campos, B. L. Rodríguez, J. L. Pérez, T. Valmaseda, O. Pérez, A. Pérez, M. Ramírez, T. Ledón, M. D. Jidy, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth, B. A., and R. A. Finkelstein. 1986. Presence of the HA/protease and other potential virulence factors in O1 and non-O1 Vibrio cholerae. J. Infect. Dis. 154:183-186. [DOI] [PubMed] [Google Scholar]
- 4.De, S. N., and D. N. Chatterjee. 1953. An experimental study of the mechanism of action of Vibrio cholerae on the intestinal mucous membranes. J. Pathol. Bacteriol. 66:559-562. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugar and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 6.Faruque, S. M., N. Chowdhury, M. Kamruzzaman, M. Dziejman, M. H. Rahman, D. A. Sack, G. B. Nair, and J. J. Mekalanos. 2004. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. USA 101:2123-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field, M., M. C. Rao, and E. B. Chang. 1989. Intestinal electrolyte transport and diarrhoeal disease. N. Engl. J. Med. 21:879-883. [DOI] [PubMed] [Google Scholar]
- 8.Field, M., and I. McCall. 1971. Ion transport in rabbit ileal mucus: I, Na and Cl fluxes and short-circuit current. Am. J. Physiol. 220:1388-1396. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein, R. A., and L. F. Hanne. 1982. Purification and characterization of the soluble hemagglutinin (cholera lectin) produced by Vibrio cholerae. Infect. Immun. 36:1199-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalonos. 2003. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geboes, K., R. Riddell, A. Ost, B. Jensfelt, T. Persson, and R. Lofberg. 2000. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 47:404-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guanalini, S., J. F. Kechur, P. L. Smith, and M. Field. 1980. Somatostatin effects on ion transport in rabbit intestine. Am. J. Physiol. 240:G26-G32. [DOI] [PubMed] [Google Scholar]
- 13.Gyobu, Y., H. Kodama, and H. Uetake. 1988. Production and partial purification of a fluid accumulating factor of non-O1 Vibrio cholerae. Microbiol. Immunol. 32:565-577. [DOI] [PubMed] [Google Scholar]
- 14.Hase, C. H., and R. A. Finkelstein. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J. Bacteriol. 173:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janda, J. M., C. Powers, R. G. Bryant, and S. L. Abbott. 1988. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin. Microbiol. Rev. 1:245-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, G. W., G. D. Abrams, and R. Freter. 1976. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect. Immun. 14:232-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimsey, H. H., and M. K. Waldor. 1998. Vibrio cholerae hemagglutinin/protease inactivates CTXφ. Infect. Immun. 66:4025-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lu, L., M. E. Baldear, T. Savidge, C. Pothoulakis, and W. A. Walker. 2003. Development of microbial-human enterocyte interaction: cholera toxin. Pediatr. Res. 54:211-218. [DOI] [PubMed] [Google Scholar]
- 20.Lyerly, D. M., D. E. Lockwood, S. H. Richardson, and T. D. Wilkins. 1982. Biological activities of toxins A and B of Clostridium difficile. Infect. Immun. 35:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mel, S. F., K. A. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decrease in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris, J. G., Jr. 1994. Non-O group 1 Vibrio cholerae strains not associated with epidemic disease, p. 103-115. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. American Society for Microbiology, Washington, D.C.
- 23.Mukhopadhyay, A. K., P. K. Saha, S. Garg, S. K. Bhattacharya, T. Shimada, T. Takeda, Y. Takeda, and G. B. Nair. 1995. Distribution and virulence of Vibrio cholerae belonging to serogroups other than O1 and O139: a nationwide survey. Epidemiol. Infect. 114:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norqvist, A., B. Norman, and H. Wolf-Watz. 1990. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect. Immun. 58:3731-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osibo, R. J., D. M. Lyerly, R. L. Van Tassell, and T. D. Wilkins. 1995. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vitro. Infect. Immun. 63:3820-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prescott, J. M., and S. H. Wilkes. 1976. Aeromonas aminopeptidase. Methods Enzymol. 45:530-543. [DOI] [PubMed] [Google Scholar]
- 27.Qadri, F., T. R. Bhuiyan, K. K. Dutta, R. Raqib, M. S. Alam, N. H. Alam, A.-M. Svennerholm, and M. M. Mathan. 2004. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surfaces of the gut. Gut 53:62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qadri, F., R. Raquib, F. Ahmed, T. Rahman, C. Wennerae, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qing, X., M. Dziejman, and J. J. Mekalanos. 2003. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc. Natl. Acad. Sci. USA 100:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez, B. L., A. Rojas, J. Campus, T. Ledon, E. Valle, W. Toledo, and R. Fando. 2001. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect. Immun. 69:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha, D. R., S. K. Niyogi, G. B. Nair, B. Manna, and S. K. Bhattacharya. 2000. Detection of faecal leucocytes and erythrocytes from stools of cholera patients suggesting an evidence of an inflammatory response in cholera. Indian J. Med. Res. 112:5-8. [PubMed] [Google Scholar]
- 32.Saha, P. K., H. Koley, A. K. Mukhopadhyay, S. K. Bhattacharya, G. B. Nair, B. S. Ramakrishnan, S. Krishnan, T. Takeda, and Y. Takeda. 1996. Nontoxigenic Vibrio cholerae O1 serotype Inaba biotype El Tor associated with a cluster of cases of cholera in southern India. J. Clin. Microbiol. 34:1114-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saha, P. K., H. Koley, and G. B. Nair. 1996. Purification and characterization of an extracellular secretogenic non-membrane-damaging cytotoxin production by clinical strains of Vibrio cholerae non-O1. Infect. Immun. 64:3101-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, V. J. DiRita, and M. Bagdasarian. 1997. General secretion pathway (esp) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider, D. R., and C. D. Parker. 1978. Isolation and characterization of protease-deficient mutant of Vibrio cholerae. J. Infect. Dis. 138:143-151. [DOI] [PubMed] [Google Scholar]
- 36.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. L. Gurrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 64:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toma, C., and Y. Honma. 1996. Cloning and genetic analysis of the Vibrio cholerae aminopeptidase gene. Infect. Immun. 64:4495-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, Z., D. Milton, P. Nybom, A. Sjo, and K.-E. Magnusson. 1996. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb. Pathog. 21:111-123. [DOI] [PubMed] [Google Scholar]
- 39.Zhou, X., D. Q. Gas, J. Michalski, J. A. Benitez, and J. B. Kaper. 2004. Induction of interleukin-8 in T84 cells by Vibrio cholerae. Infect. Immun. 72:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]