Listeria monocytogenes is a gram-positive bacterium with a Jekyll and Hyde personality (108): it is well adapted as a saprophyte for peaceful survival in soil and decaying vegetation (Dr. Jekyll) (36), but it has a second life as an intracellular bacterial pathogen capable of causing serious infection in humans and in many animal species (Mr. Hyde) (28, 96, 115). In its Mr. Hyde phase, the bacterium is a significant public health hazard, responsible for an estimated 28% of deaths attributable to known food-borne pathogens in the United States (75). How does L. monocytogenes manage the switch between mild-mannered environmental bacterium and potentially deadly human pathogen? The transformation appears to be mediated through complex regulatory pathways that modulate the expression of virulence factors in response to environmental cues. This review will summarize the current understanding of L. monocytogenes virulence gene regulation and will put forth a model that depicts how a humble soil-grown bacterium might transform into a deadly invader.
LIFE IN THE SOIL: THE PEACEFUL EXISTENCE OF A BACTERIAL DR. JEKYLL
L. monocytogenes is a ubiquitous bacterium that sets up home in a variety of environmental locations. L. monocytogenes has been isolated from soil, ground water, silage, and decaying vegetation (reviewed in reference 36); however, relatively little is known about the bacterium's potentially peaceful Dr. Jekyll existence. Genome sequencing indicates the presence of multiple gene products that may facilitate the utilization by L. monocytogenes of a variety of carbon sources, including plant sugars (48, 84). To access nutrient sources, L. monocytogenes expresses flagella and exhibits swimming motility at temperatures below 30°C; in many strains (but not all) swimming motility is repressed at 37°C (51, 87, 117). Although L. monocytogenes does not form spores, the bacterium is well known for its ability to withstand a variety of environmental stresses, including low temperature and high osmolarity (99), thus making it a hardy environmental organism.
It is possible and, perhaps, probable that the existence of L. monocytogenes outside of mammalian host cells is not entirely a quiet and sedate country life but, rather, a constant territorial battle with other single-cell and multicellular organisms that are lurking nearby. Although it is commonly isolated from environmental sources (36), L. monocytogenes maintains an arsenal of gene products that appear to be designed to facilitate survival within mammalian host cells. Maintenance of this arsenal in an organism that is broadly present in the environment suggests the possibility that these gene products may be utilized not only in mammals but also against other eukaryotic organisms in the environment. For example, while protozoa have not been reported as a reservoir for L. monocytogenes, as is the case with Legionella pneumophila, L. monocytogenes does survive and replicate within amoebae (49, 70, 111). L. monocytogenes is an efficient pathogen of at least one insect species (Drosophila melanogaster), although infections must be systemically induced (73). It is likely that further studies will identify additional nonmammalian organisms that serve as hosts for L. monocytogenes.
L. MONOCYTOGENES WITHIN MAMMALIAN HOSTS: A BACTERIAL MR. HYDE
Although L. monocytogenes is well adapted to persistence in the environment (36), the majority of studies focused on L. monocytogenes have investigated infection of mammalian hosts, or the Mr. Hyde phase of the organism: the invasion and survival within mammalian host cells and the immune response to bacterial infection (reviewed in references 28, 66, and 86). L. monocytogenes is capable of invading and replicating within a wide range of animal cell types, including macrophages and nonprofessional phagocytes (45, 64, 71, 89). A number of bacterial gene products have been identified that facilitate the intracellular growth and spread of the bacterium to adjacent host cells (88, 90), and the functions of these gene products have been discussed in several excellent recent reviews (20, 28, 57, 63, 115). Briefly, these gene products include the invasion-associated surface proteins internalin A and B (InlA and InlB), gene products associated with escape from the host cell vacuole (the hly-encoded cholesterol-dependent cytolysin listeriolysin O [LLO] as well as phospholipases encoded by plcA and plcB), and ActA, a protein required for actin-based intracellular bacterial motility and cell-to-cell spread. Additional gene products, such as Mpl, a zinc-dependent metalloprotease that processes PlcB to its mature form, and Hpt, a hexose phosphate transporter that allows bacteria to utilize phosphorylated sugars such as glucose-1-phosphate within the host cell cytosol, also contribute to bacterial life within the mammalian host cell. Other gene products, such as the bile salt hydrolase encoded by bsh (4, 27) and the bile exclusion locus bilE (104), may function to promote bacterial survival in the liver or in extracellular environments within the mammalian host, such as the small intestine or within the gall bladder (52). For nearly every gene product identified thus far as contributing to L. monocytogenes survival within the host, gene expression is regulated by a transcriptional activator known as positive regulatory factor A (PrfA) (12, 40, 76, 78). Strains lacking functional PrfA are highly attenuated in animal models of infection and are forever locked into a docile and nonthreatening state.
As L. monocytogenes is clearly capable of adapting to multiple environments, including those outside as well as inside host cells, it is important to ask what the mechanisms are that control the switch that changes L. monocytogenes from a quiet soil bacterium to a ruthless invader. At least some of the answers appear to lie in the regulation of the key regulatory protein PrfA and include both the regulation of prfA transcription and PrfA protein activity.
INITIAL CONTROL OF THE BACTERIAL BEAST: REGULATION OF prfA EXPRESSION
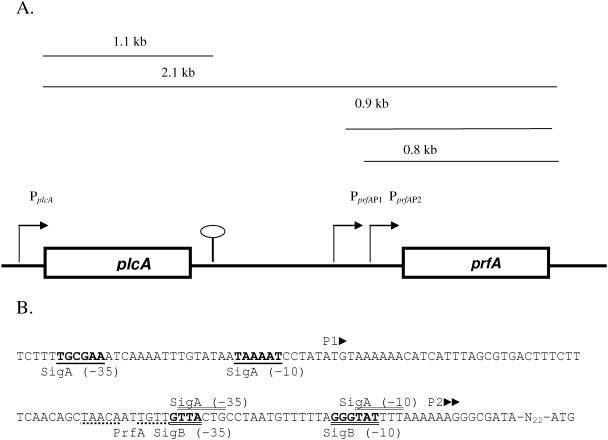
Transcriptional control of prfA is the first mechanism used by L. monocytogenes to regulate the expression of its virulence gene products. Three promoter regions have been identified that contribute to the regulation of prfA expression (Fig. 1A). Two promoter regions, PprfAP1 and PprfAP2, are located just upstream of the prfA coding sequence and direct the expression of monocistronic prfA transcripts. The upstream plcA promoter (PplcA) directs both a monocistronic plcA transcript and a bicistronic transcript encoding plcA and prfA (11).
FIG. 1.
(A) Map of the L. monocytogenes plcA-prfA region, not drawn to scale. The heavy line at the bottom of the panel represents the DNA sequence, with gene coding regions indicated as boxes; the four lines above the gene coding region represent possible mRNA transcripts, including a 1.1-kb plcA transcript, a 2.1-kb plcA-prfA bicistronic transcript, and 0.9- and 0.8-kb prfA transcripts (11, 13, 42, 67, 73, 91). Transcriptional start sites are indicated by bent arrows, and the plcA transcription terminator is indicated by a stem-loop. (B) DNA sequence of the prfA promoter region (46, 91). Triangles indicate transcriptional start sites identified for P1prfA and P2prfA. The σA-dependent P1prfA promoter is in boldface and underlined once. Two adjacent transcriptional start sites, possibly reflecting transcription from either the σA-dependent or σB-dependent promoter comprising the P2prfA region, are marked by triangles (46). In the P2prfA region, the σB-dependent promoter is in boldface and underlined twice; the proposed σA-dependent promoter is marked by double lines above the sequence, and the PrfA binding box (100), which is immediately upstream of the P2 promoter region, is marked by a dotted line beneath the sequence.
Transcription of DNA in bacteria is driven by RNA polymerase, whose specificity is determined by regulatory proteins known as sigma (σ) factors (reviewed in reference 50). The primary sigma factor determining RNA polymerase specificity in actively growing, unstressed cells is σA (50, 81). The PprfAP1 promoter has characteristics of a σA-dependent promoter (83). Transcripts are produced from this promoter by actively growing L. monocytogenes in broth culture. The RNA transcript of prfA directed by PprfAP1 contains a thermosensitive structure that inhibits translation of PrfA at temperatures lower than 30°C but melts at higher temperatures, allowing translation (56). The reduced efficiency of PrfA translation at low temperatures may explain the reduced transcription of PrfA-dependent genes observed at low temperatures in broth culture. The production of monocistronic prfA transcript is independent of temperature, while bicistronic plcA-prfA transcript, which is dependent on PrfA activation, is only produced at higher temperatures (67). The presence of a pool of untranslated prfA transcripts may allow rapid synthesis of PrfA following infection of warm-blooded mammalian and avian host organisms, which generally have temperatures higher than the surrounding environment. Temperature regulation of bacterial protein levels is not unique to L. monocytogenes PrfA. To illustrate, the Escherichia coli heat shock response associated with σ32 is dependent upon the presence of a pool of untranslated rpoH mRNA to achieve rapid increases in σ32 under increased temperature conditions (82, 109).
A second prfA promoter region, PprfAP2, also directs monocistronic prfA transcripts (42) (Fig. 1A and B). The P2prfA region contains a putative PrfA binding box, which provides an autoregulatory loop (42, 100). The P2prfA region comprises both a σA- and a σB-dependent promoter (91). σB-Dependence of the PprfAP2 promoter has been demonstrated (83, 91, 98). RNA polymerase complexed with σB recognizes the promoters of a number of genes whose products contribute to the ability of L. monocytogenes to withstand environmental stresses including low pH, high osmolarity, oxidative stress, and carbon starvation (3, 15, 37, 38, 39, 58, 118, 119). The products of a number of stress response genes have been implicated in virulence; these genes include bsh, whose product is important for resisting the stresses imposed by exposure to bile salts (27), the gad system, involved in resisting acid shock (22), and hfq, a general stress response gene involved in resistance to osmotic and ethanol stress (15). Transcription of the invasion-associated internalin genes inlA and inlB is also influenced by σB (59, 60). L. monocytogenes cells exposed to environmental stress conditions (specifically, 0.3 M NaCl or growth to stationary phase) show a relative increase in monocistronic prfA transcripts initiated from PprfAP2 (M. Kazmierczak, M. Wiedmann, and K. J. Boor, submitted for publication). As the PprfAP2--directed message does not contain the thermosensitive RNA secondary structure present in PprfAP1-directed messages (56), translation of the PprfAP2 transcript may thus account for the observed expression of PrfA in some low-temperature environments, such as the cytosol of insect cells, where PrfA-dependent gene products are expressed and functional (13, 26, 73).
Finally, bicistronic plcA-prfA transcripts are produced from the upstream PrfA-dependent PplcA promoter (Fig. 1A) (11, 13, 73). PrfA thereby upregulates its own production, and this autoregulation is required for bacterial cell-to-cell spread within tissue culture cells and for bacterial virulence in animal models of infection (11, 41).
ADDITIONAL CONTROL OF THE BACTERIAL BEAST: REGULATION OF PrfA ACTIVITY
In addition to the existence of transcriptional and posttranscriptional mechanisms that control prfA expression and translation, PrfA activity is controlled on a posttranslational level. PrfA is a member of the Crp/Fnr transcription regulator family (65, 112). As a group, Crp/Fnr regulators respond to a broad array of signals, both intracellular and exogenous, such as the presence of small molecular cofactors (e.g., cyclic AMP for Crp) (53), as well as changes in redox potential, oxygen availability, or temperature (reviewed in reference 62). Mutants of Crp, known as Crp*, have been identified that contain amino acid substitutions that appear to lock the protein into a constitutively active form, even in the absence of the signal molecule, cyclic AMP (53). Ripio et al. (95) were the first to describe a similar mutation in PrfA (PrfA G145S, or PrfA*), which was identified in an L. monocytogenes strain (NCTC 7973) that constitutively expressed high levels of PrfA-dependent gene products. Recent evidence suggests that the PrfA G145S mutation may stabilize the helix-turn-helix motif relative to that of the wild-type PrfA to enhance the protein's DNA-binding affinity (29). Since the identification of PrfA G145S, additional PrfA mutations have been identified that also appear to result in a constitutively activated form of the protein (PrfA I45S, PrfA E77K, PrfA L140F, and PrfA G155S) (54, 103, 116, 121). Interestingly, recent data suggest that strains containing PrfA* mutations may be locked into a Mr. Hyde state that can increase bacterial virulence in animal models (103). For example, strains containing the PrfA G155S mutation were approximately fivefold more virulent than wild-type strains following intravenous injection of mice (103).
It is clear that PrfA exists in high- and low-activity states, with the transitions between activation states occurring in response to environmental signals; however, the nature of the potential small molecule cofactor bound by PrfA (or PrfA posttranslational modification) that triggers PrfA activation is not yet known (65, 92). A number of environmental conditions influence the expression of PrfA-dependent gene products (106). Growth in rich medium or in medium supplemented with readily metabolized carbohydrates (such as glucose, fructose, maltose, or cellobiose) inhibits transcription of PrfA-dependent virulence genes (hly, plcA, plcB, mpl, and actA) without affecting PrfA protein levels (32, 77). Repression of virulence gene expression by cellobiose, a common carbohydrate in plant materials but not in animal hosts, appears to be mediated by at least three different mechanisms (5, 6, 9, 55, 69, 77). In contrast to the repression of virulence gene expression by these readily metabolized sugars, the presence of phosphorylated sugars, such as glucose-1-phosphate, supports bacterial growth with no repression of PrfA-dependent virulence gene expression (94). Phosphorylated sugars present within the cytosol of mammalian host cells are postulated to serve as molecular cues signaling the opportunity for rapid intracellular L. monocytogenes growth (14).
Other environmental signals are known to influence virulence gene expression in L. monocytogenes. PrfA-dependent LLO production and actA expression are both activated in iron-depleted medium (17, 23). As free iron levels are extremely low in mammalian host cells (∼10−18 M) (68), available iron may serve as a cue used by L. monocytogenes to assess its location. It is well established that expression of PrfA-dependent genes increases following treatment of the culture medium with activated charcoal (31, 32, 47, 93). Ermolaeva et al. (33) have presented evidence to suggest that activated charcoal acts by absorbing a small diffusible autorepressor molecule which L. monocytogenes produces during exponential growth. This strategy is reminiscent of quorum sensing mechanisms used in other bacteria to regulate genes in a bacterial cell concentration-dependent fashion (1, 34), but whether this form of virulence gene repression occurs in L. monocytogenes remains undetermined. In summary, several environmental conditions have been shown to influence virulence gene expression, presumably by influencing the state of PrfA activation, but the molecular mechanism responsible for the conversion of PrfA to its fully active state remains unknown.
THE TRANSITION TO MR. HYDE FOLLOWING BACTERIAL INVASION OF THE MAMMALIAN HOST
Animals have a wide array of defense mechanisms specifically designed to prevent pathogenic bacteria from settling in and making themselves at home. Once L. monocytogenes is ingested by a mammalian host organism, its survival within that host depends upon the bacterium's ability to withstand a number of defense mechanisms. Exposure to stresses imposed by host defense mechanisms may actually help prepare L. monocytogenes for its Mr. Hyde existence. Specifically, accumulating evidence suggests that environmental stress conditions encountered during passage through the stomach to the gut contribute to the infectious life cycle of L. monocytogenes (18, 19, 21, 74, 85, 97). For example, one early host defense encountered by L. monocytogenes following ingestion is the low pH environment of the stomach. A clear connection has been established between acid tolerance and virulence in L. monocytogenes, in that mutants with increased acid tolerance show increased virulence in mice (85), and decreased acid tolerance is correlated with decreased virulence (21, 74). The genetic mechanism(s) of this effect is not well understood, but preadaptation of L. monocytogenes (by exposure to pH 4.5 to 5.5, similar to the pH found in the stomach after eating [22]) increases the invasiveness of the bacteria in cell culture (18) as well as bacterial survival following macrophage infection (18, 19, 44) and following intragastric inoculation of mice (97).
The acid tolerance response of L. monocytogenes is at least partially dependent on σB (37, 38, 119), and at low pH, the production of monocistronic prfA transcript is strongly increased; this transcript accumulation may serve to prime the bacterium for its responses to subsequent host environments (19). As expression of a variety of stress response genes and invasion-associated internalins is regulated by the stress-responsive σB (58, 59, 60), the predicted net effect of L. monocytogenes passage through the stomach and intestine may be an increase in the production of a variety of proteins important for invasion and infection. Indeed, recent data indicate that σB plays a critical role during the gastrointestinal stage of listeriosis in guinea pigs (46). While passage of L. monocytogenes through the gut clearly is not essential for virulence, as infections in animals can be established by intraperitoneal or intravenous injection (11, 14, 27, 30, 44, 71, 119), it may increase the efficiency of infection under natural conditions.
THE MAMMALIAN CYTOSOL AND THE FULL UNLEASHING OF MR. HYDE
When L. monocytogenes leaves the lumen of the intestine and enters a host cell, it once again encounters several changes in its immediate environment. In contrast to the relatively high available iron and carbohydrate levels in the intestinal lumen, the phagocytic vacuole is postulated to have low quantities of available iron and carbohydrates. Low iron and low carbohydrate concentrations activate transcription from some PrfA-dependent virulence gene promoters (8, 16, 17, 32, 77). Exposure of L. monocytogenes to H2O2 increases transcription of prfA and hly, suggesting that the presence of reactive oxygen intermediates, such as those generated in activated macrophages, also may up-regulate virulence gene expression (72). The phagocytic vacuole of a macrophage rapidly becomes acidified to a pH of approximately 5.5 to 6 (2). Hence, following engulfment, the L. monocytogenes invader is subjected to multiple rapid environmental changes, including exposure to oxygen radicals, reduced pH, and reduced nutrient density.
Some PrfA-dependent gene products have clearly targeted roles within specific cellular locations and are differentially expressed depending upon their cellular location (10). For example, actA expression is primarily confined to the host cell cytosol, where it directs actin polymerization (10, 43, 80). Differential expression of PrfA-dependent promoters is influenced by sequence variations within a promoter region's PrfA box, with relative activation reflecting the similarity of a given promoter's PrfA box to the PrfA-box consensus sequence (24, 100, 120). The promoters with perfect PrfA-box sequences, Phly and PplcA, are the most efficiently transcribed and produce transcripts at relatively low PrfA concentrations (100).
Activation of transcription from the plcA promoter by PrfA initiates an important regulatory circuit within the host by which PrfA upregulates its own production (11, 76) and produces an increase in PrfA concentrations to enable the bacteria to establish themselves in a host cell and to move to infect new cells. Mutants that produce very small amounts of PrfA are capable of escaping from vacuoles but not of polymerizing actin or spreading between host cells (41). Cell-to-cell spread is mediated by the actin nucleating protein ActA (61, 87, 113), which is transcribed from two promoters, PactA and Pmpl (61, 114). These promoters each have a single mismatched base in their PrfA boxes; therefore, transcription activation from these promoters requires an increased concentration of PrfA, such as that produced by L. monocytogenes present in host cytosol (100). PC-PLC, the product of the plcB gene, is also produced by transcription from PactA and Pmpl (61, 114), and its production is important for efficient bacterial cell-to-cell spread, as it permits bacterial escape from the secondary vacuoles created when an L. monocytogenes cell moves into a neighboring host cell (105, 114). Two additional PrfA-dependent genes that contribute to virulence, inlC and hpt, also have single mismatches in their PrfA boxes (14, 30, 74). inlC encodes a small, secreted protein called internalin C (30). Expression of inlC is enhanced in the cytoplasm of mammalian cells (10, 30), and ΔinlC mutants have reduced virulence in mice (30). The function of internalin C has not yet been fully established, although recent results show that it supports internalin A in stimulating invasion of mammalian cells (7). The hpt gene encodes a hexose phosphate transporter which allows L. monocytogenes to grow using phosphorylated sugars such as glucose-1-phosphate as a carbon source (14). Deletion of the hpt gene results in bacteria with a significantly reduced intracellular growth rate and attenuated virulence in mice (14), suggesting that hexose phosphates serve as important carbon sources for growth of L. monocytogenes in the cytoplasm.
PrfA HELPS MEDIATE THE L. MONOCYTOGENES SWITCH FROM ENVIRONMENTAL DR. JEKYLL TO PATHOGENIC MR. HYDE
Increasing evidence suggests that PrfA is a key part of the potion that transforms Dr. Jekyll into pathogenic Mr. Hyde. Overall, if one were to generate a model (or write a novel) describing the fateful Jekyll-and-Hyde transition of L. monocytogenes, it might be best put forward as follows: in response to environmental signals outside of a host, L. monocytogenes maintains its Dr. Jekyll persona by repressing both PrfA production and activity through transcriptional (promoter expression), posttranscriptional (RNA thermosensor), and posttranslational mechanisms (PrfA activation), thereby cloaking the expression of its primary virulence factors except for the internalins, which appear to be produced in advance of infection (12, 59). Once the bacteria are ingested by a mammalian host, the increase in temperature and exposure to reduced pH in the stomach stimulates increased production of stress response proteins, internalins, and PrfA, thus beginning the transition to virulence. In the intestine, internalin A mediates attachment and invasion of host epithelial cells with the support of other internalin proteins. Once within the cell phagosome, low iron and low carbohydrate concentrations repress internalin production while PrfA-dependent activation of the Phly and PplcA promoters allows production of LLO and PlcA to promote lysis of the phagocytic vacuole, thereby enabling entry of the bacteria into the cytosol. Within the cytosol, the full transformation of the L. monocytogenes Dr. Jekyll into Mr. Hyde is completed when high levels of active PrfA protein activate transcription from the PactA and Pmpl promoters. The resulting production of ActA and PlcB enables spread of the bacteria to adjacent cells.
While this story in progress features PrfA as the protagonist controlling the L. monocytogenes transition from the outside environment to the inside of the host, additional characters are clearly required. Full induction of ActA expression, for example, seems to require additional unknown steps or factors beyond what can be explained by PrfA binding (102, 103). Secondary structure of the 150-bp 5′ untranslated region of the actA mRNA has recently been shown to be important in full ActA expression, but the detailed mechanism is as yet unknown (122). Posttranscriptional mechanisms also contribute to synthesis of internalin A and B (110) and LLO (101). Mutations mapping outside of the PrfA locus that affect virulence gene expression in L. monocytogenes have been identified (69, 103), suggesting the potential presence of other transcription factors, regulatory elements, and signaling molecules required for the regulation of virulence in L. monocytogenes.
THE FINALE: THE GOOD NEWS AND THE BAD NEWS
While the bad news is that L. monocytogenes is capable of undergoing the dangerous transition from an environmental Dr. Jekyll to a pathogenic Mr. Hyde within the host, the good news may be that the Mr. Hyde form seems to suffer a competitive disadvantage outside the host. L. monocytogenes prfA mutants that contain constitutively activated alleles of prfA (and are thus locked into the Mr. Hyde phase) are fully virulent, and in some cases hypervirulent, in mouse models of infection; however, these mutants are severely compromised for flagellum-mediated swimming motility and therefore may be hindered in nutrient acquisition in environments outside the host. It therefore appears that L. monocytogenes must maintain a balance between life in the outside environment and life within the host; thus, bacteria that can undergo the switch back to the humble Dr. Jekyll form may be favored over the evolution of increasingly dangerous Mr. Hydes.
The last decade has seen an enormous expansion in our understanding of how L. monocytogenes regulates the transition from peaceful saprophyte to deadly pathogen. The switch from environmental microbe to pathogen is mediated by a diverse array of microorganisms encompassing both bacteria and fungi. In addition to L. monocytogenes, the organisms able to make the transition from the outside environment to inside a mammalian host include important pathogens such as Vibrio cholerae (107), Bacillus anthracis (25), Cryptosporidium parvum (35), and L. pneumophila (79). In most cases there is limited understanding of what molecular mechanisms serve to mediate the switch from life outside the host to life within a host, and, thus, the more we know of the strategies used by one environmental pathogen, L. monocytogenes, the better we may understand whether similar strategies might exist and be used by other pathogens to mediate deadly transitions.
Acknowledgments
L. monocytogenes research in the authors' laboratories is supported by the National Institutes of Health (grants AI41816 and AI055651 to N.E.F. and AI052151 to K.J.B.) and by the Cooperative State Research, Education, and Extension Service, National Research Initiative Competitive Grants Program (NRI Proposal 2005-35201-15330 to K.J.B.) of the U. S. Department of Agriculture.
Editor: J. B. Kaper
REFERENCES
- 1.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421-424. [DOI] [PubMed] [Google Scholar]
- 2.Beauregard, K. E., K.-D. Lee, R. J. Collier, and J. A. Swanson. 1997. pH-dependent perforation of macrophage phagosomes by listeriolysin O from Listeria monocytogenes. J. Exp. Med. 186:1159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, L. A., M. S. Çetin, R. W. Hutkins, and A. K. Benson. 1998. Identification of the gene encoding the alternative sigma factor σB from Listeria monocytogenes and its role in osmotolerance. J. Bacteriol. 180:4547-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begley, M., R. D. Sleator, C. G. M. Gahan, and C. Hill. 2005. Contribution of three bile-associated loci, bsh, pva, and btlB, to gastrointestinal persistence and bile tolerance of Listeria monocytogenes. Infect. Immun. 73:894-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behari, J., and P. Youngman. 1998. Regulation of hly expression in Listeria monocytogenes by carbon sources and pH occurs through separate mechanisms mediated by PrfA. Infect. Immun. 66:3635-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behari, J., and P. Youngman. 1998. A homolog of CcpA mediates catabolite control in Listeria monocytogenes but not carbon source regulation of virulence genes. J. Bacteriol. 180:6316-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman, B., D. Raffelsbauer, M. Kuhn, M. Goetz, S. Hom, and W. Goebel. 2002. InlA- but not InlB-mediated internalization of Listeria monocytogenes by non-phagocytic mammalian cells needs the support of other internalins. Mol. Microbiol. 43:557-570. [DOI] [PubMed] [Google Scholar]
- 8.Böckmann, R., C. Dickneite, B. Middendorf, W. Goebel, and Z. Sokolovic. 1996. Specific binding of the Listeria monocytogenes transcriptional regulator PrfA to target sequences requires additional factor(s) and is influenced by iron. Mol. Microbiol. 22:643-653. [DOI] [PubMed] [Google Scholar]
- 9.Brehm, K., M-.T. Ripio, J. Kreft, and J.-A. Vázquez-Boland. 1999. The bvr locus of Listeria monocytogenes mediates virulence gene repression by β-glucosides. J. Bacteriol. 181:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubert, A., Z. Sokolovic, S.-K. Chun, L. Papatheodorou, A. Simm, and W. Goebel. 1999. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol. Gen. Genet. 261:323-336. [DOI] [PubMed] [Google Scholar]
- 11.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborty, T., M. Leimeister-Wächter, E. Domann, M. Hartl, W. Goebel, T. Nichterlein, and S. Notermans. 1992. Coordinate regulation of virulence genes in Listeria monocytogenes requires the product of the prfA gene. J. Bacteriol. 174:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng, L. W., and D. A. Portnoy. 2003. Drosophila S2 cells: an alternative infection model for Listeria monocytogenes. Cell Microbiol. 5:875-885. [DOI] [PubMed] [Google Scholar]
- 14.Chico-Calero, I., M. Suárez, B. González-Zorn, M. Scortti, J. Slaghuis, W. Goebel, European Listeria Genome Consortium, and J. A. Vázquez-Boland. 2002. Hpt, a bacterial homologue of the microsomal glucose-6-phosphate translocase, mediates rapid intracellular proliferation in Listeria. Proc. Natl. Acad. Sci. USA. 99:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christiansen, J. K., M. H. Larsen, H. Ingmer, L. Søgaard-Andersen, and B. H. Kallipolitis. 2004. The RNA-binding protein Hfq of Listeria monocytogenes: role in stress tolerance and virulence. J. Bacteriol. 186:3355-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conte, M. P., C. Longhi, M. Polidoro, G. Petrone, V. Buonfiglio, S. Di Santo, E. Papi, L. Seganti, P. Visca, and P. Valenti. 1996. Iron availability affects entry of Listeria monocytogenes into the enterocyte-like cell line Caco-2. Infect. Immun. 64:3925-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conte, M. P., C. Longhi, G. Petrone, M. Polidoro, P. Valenti, and L. Seganti. 2000. Modulation of actA expression in Listeria monocytogenes by iron. J. Med. Microbiol. 49:681-683. [DOI] [PubMed] [Google Scholar]
- 18.Conte, M. P., G. Petrone, A. M. Di Biase, M. G. Ammendolia, F. Superti, and L. Seganti. 2000. Acid tolerance in Listeria monocytogenes influences invasiveness of enterocyte-like cells and macrophage-like cells. Microb. Pathog. 29:137-144. [DOI] [PubMed] [Google Scholar]
- 19.Conte, M. P., G. Petrone, A. M. D. Biase, C. Longhi, M. Penta, A. Tinari, F. Superti, G. Fabozzi, P. Visca, and L. Seganti. 2002. Effect of acid adaptation on the fate of Listeria monocytogenes in THP-1 human macrophages activated by gamma interferon. Infect. Immun. 70:4369-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cossart, P. 2002. Molecular and cellular basis of the infection by Listeria monocytogenes: an overview. Int. J. Med. Microbiol. 291:401-409. [DOI] [PubMed] [Google Scholar]
- 21.Cotter, P. D., N. Emerson, C. G. M. Gahan, and C. Hill. 1999. Identification and disruption of lisRK, a genetic locus encoding a two-component signal transduction system involved in stress tolerance and virulence in Listeria monocytogenes. J. Bacteriol. 181:6840-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotter, P. D., and C. Hill. 2003. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowart, R. E., and B. G. Foster. 1981. The role of iron in the production of haemolysin by Listeria monocytogenes. Curr. Microbiol. 6:287-290. [Google Scholar]
- 24.Dickneite, C., R. Böckmann, A. Spory, W. Goebel, and Z. Sokolovic. 1998. Differential interaction of the transcription factor PrfA and the PrfA-activating factor (Paf) of Listeria monocytogenes with target sequences. Mol. Microbiol. 27:915-928. [DOI] [PubMed] [Google Scholar]
- 25.Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell Microbiol. 2:453-463. [DOI] [PubMed] [Google Scholar]
- 26.Dramsi, S., C. Kocks, C. Forestier, and P. Cossart. 1993. Internalin-mediated invasion of epithelial cells by Listeria monocytogenes is regulated by the bacterial growth state, temperature, and the pleiotropic activator prfA. Mol. Microbiol. 9:931-941. [DOI] [PubMed] [Google Scholar]
- 27.Dussurget, O., D. Cabanes, P. Dehoux, M. Lecuit, European Listeria Genome Consortium, C. Buchrieser, P. Glaser, and P. Cossart. 2002. Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 45:1095-1106. [DOI] [PubMed] [Google Scholar]
- 28.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587-610. [DOI] [PubMed] [Google Scholar]
- 29.Eiting, M., G. Hagelüken, W.-D. Schubert, and D. W. Heinz. 2005. The mutation G145S in PrfA, a key virulence regulator of Listeria monocytogenes, increases DNA-binding affinity by stabilizing the HTH motif. Mol. Microbiol. 56:433-446. [DOI] [PubMed] [Google Scholar]
- 30.Engelbrecht, F., S.-K. Chun, C. Ochs, J. Hess, F. Lottspeich, W. Goebel, and Z. Sokolovic. 1996. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol. Microbiol. 21:823-837. [DOI] [PubMed] [Google Scholar]
- 31.Ermolaeva, S., N. Varfolomeeva, Y. Belyi, and I. Tartakovskii. 1997. Isolation and characterization of a Listeria monocytogenes mutant strain hyperproducing virulence factors. FEMS Microbiol. Lett. 150:189-195. [DOI] [PubMed] [Google Scholar]
- 32.Ermolaeva, S., Y. Belyi, and I. Tartakovskii. 1999. Characteristics of virulence factor expression by activated charcoal in Listeria monocytogenes. FEMS Microbiol. Lett. 174:137-141. [DOI] [PubMed] [Google Scholar]
- 33.Ermolaeva, S., S. Novella, Y. Vega, M.-T. Ripio, M. Scortti, and J. A. Vázquez-Boland. 2004. Negative control of Listeria monocytogenes virulence genes by a diffusible autorepressor. Mol. Microbiol. 52:601-611. [DOI] [PubMed] [Google Scholar]
- 34.Falcão, J. P., F. Sharp, and V. Sperandio. 2004. Cell-to-cell signaling in intestinal pathogens. Curr. Issues Intest. Microbiol. 5:9-17. [PubMed] [Google Scholar]
- 35.Fayer, R., J. P. Dubey, and D. S. Lindsay. 2004. Zoonotic protozoa: from land to sea. Trends Parasitol. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 36.Fenlon, D. R. 1999. Listeria monocytogenes in the natural environment, p. 21-38. In E. T. Ryser and E. H. Marth (ed.), Listeria, listeriosis, and food safety, 2nd ed. Marcel Dekker, New York, N.Y.
- 37.Ferreira, A., C. P. O'Byrne, and K. J. Boor. 2001. Role of σB in heat, ethanol, acid, and oxidative stress resistance and during carbon starvation in Listeria monocytogenes. Appl. Environ. Microbiol. 67:4454-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira, A., D. Sue, C. P. O'Byrne, and K. J. Boor. 2003. Role of Listeria monocytogenes σB in survival of lethal acidic conditions and in the acquired acid tolerance response. Appl. Environ. Microbiol. 69:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser, K. R., D. Sue, M. Wiedmann, K. J. Boor, and C. P. O'Byrne. 2003. Role of σB in regulating the compatible solute uptake systems of Listeria monocytogenes: osmotic induction of opuC is σB dependent. Appl. Environ. Microbiol. 69:2015-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freitag, N. E., P. Youngman, and D. A. Portnoy. 1992. Transcriptional activation of the Listeria monocytogenes hemolysin gene in Bacillus subtilis. J. Bacteriol. 174:1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freitag, N. E., L. Rong, and D. A. Portnoy. 1993. Regulation of the prfA transcriptional activator of Listeria monocytogenes: multiple promoter elements contribute to intracellular growth and cell-to-cell spread. Infect. Immun. 61:2537-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freitag, N. E., and D. A. Portnoy. 1994. Dual promoters of the Listeria monocytogenes prfA transcriptional activator appear essential in vitro but are redundant in vivo. Mol. Microbiol. 12:845-853. [DOI] [PubMed] [Google Scholar]
- 43.Freitag, N. E., and K. E. Jacobs. 1999. Examination of Listeria monocytogenes intracellular gene expression by using the green fluorescent protein of Aequorea victoria. Infect. Immun. 67:1844-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gahan, C. G. M., and C. Hill. 1999. The relationship between acid stress responses and virulence in Salmonella typhimurium and Listeria monocytogenes. Int. J. Food Microbiol. 50:93-100. [DOI] [PubMed] [Google Scholar]
- 45.Gaillard, J.-L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 46.Garner, M. R., B. L. Njaa, M. Wiedmann, and K. J. Boor. 2006. Sigma B contributes to Listeria monocytogenes gastrointestinal infection but not to systemic spread in the guinea pig infection model. Infect. Immun. 74:876-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1989. Production of thiol-dependent haemolysins by Listeria monocytogenes and related species. J. Gen. Microbiol. 135:481-487. [DOI] [PubMed] [Google Scholar]
- 48.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloeker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couvé, A. d. Daruvar, P. Dehoux, E. Domann, G. Domínguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K.-D. Entian, H. Fsihi, F. G.-D. Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gómez-López, T. Hain, J. Hauf, D. Jackson, L.-M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueño, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. d. Pablos, J.-C. Pérez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vázquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 49.Greub, G., and D. Raoult. 2004. Microorganisms resistant to free-living amoebae. Clin. Microbiol. Rev. 17:413-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gruber, T. M., and C. A. Gross. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57:441-466. [DOI] [PubMed] [Google Scholar]
- 51.Gründling, A., L. S. Burrack, H. G. A. Bouwer, and D. E. Higgins. 2004. Listeria monocytogenes regulates flagellar motility gene expression through MogR, a transcriptional repressor required for virulence. Proc. Natl. Acad. Sci. USA. 101:12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardy, J., K. P. Francis, M. DeBoer, P. Chu, K. Gibbs, C. H. Contag. 2004. Extracellular replication of Listeria monocytogenes in the murine gall bladder. Science 303:851-853. [DOI] [PubMed] [Google Scholar]
- 53.Harman, J. G. 2001. Allosteric regulation of the cAMP receptor protein. Biochim. Biophys. Acta 1547:1-17. [DOI] [PubMed] [Google Scholar]
- 54.Herler, M., B. Bubert, M. Goetz, Y. Vega, J. A. Vazquez-Boland, and W. Goebel. 2001. Positive selection of mutations leading to loss or reduction of transcriptional activity of PrfA, the central regulator of Listeria monocytogenes virulence. J. Bacteriol. 183:5562-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huillet, E., S. Larpin, P. Pardon, and P. Berche. 1999. Identification of a new locus in Listeria monocytogenes involved in cellobiose-dependent repression of hly expression. FEMS Microbiol. Lett. 174:265-272. [DOI] [PubMed] [Google Scholar]
- 56.Johansson, J., A. Renzoni, C. Chiaruttini, M. Springer, and P. Cossart. 2002. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell 110:551-561. [DOI] [PubMed] [Google Scholar]
- 57.Kathariou, S. 2002. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 65:1811-1829. [DOI] [PubMed] [Google Scholar]
- 58.Kazmierczak, M., S. Mithoe, K. J. Boor, and M. Wiedmann. 2003. Listeria monocytogenes σB regulates stress response and virulence functions. J. Bacteriol. 185:5722-5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim, H., K. J. Boor, and H. Marquis. 2004. Listeria monocytogenes σB contributes to invasion of human intestinal epithelial cells. Infect. Immun. 72:7374-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim, H., H. Marquis, and K. J. Boor. 2005. σB contributes to Listeria monocytogenes invasion by controlling expression of inlA and inlB. Microbiology 151:3215-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kocks, C., E. Gouin, M. Tabouret, P. Berche, H. Ohayon, and P. Cossart. 1992. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell 68:521-531. [DOI] [PubMed] [Google Scholar]
- 62.Körner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27:559-592. [DOI] [PubMed] [Google Scholar]
- 63.Kreft, J., and J. A. Vazquez-Boland. 2001. Regulation of virulence genes in Listeria. Int. J. Med. Microbiol. 291:145-157. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn, M., S. Kathariou, and W. Goebel. 1988. Hemolysin supports survival but not entry of the intracellular bacterium Listeria monocytogenes. Infect. Immun. 56:79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lampidis, R., R. Gross, Z. Sokolovic, W. Goebel, and J. Kreft. 1994. The virulence regulator protein of Listeria ivanovii is highly homologous to PrfA from Listeria monocytogenes and both belong to the Crp-Fnr family of transcription regulators. Mol. Microbiol. 13:141-151. [DOI] [PubMed] [Google Scholar]
- 66.Lara-Tejero, M., and E. G. Pamer. 2004. T cell responses to Listeria monocytogenes. Curr. Opin. Microbiol. 7:45-50. [DOI] [PubMed] [Google Scholar]
- 67.Leimeister-Wächter, M., E. Domann, and T. Chakraborty. 1992. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J. Bacteriol. 174:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Litwin, C. M., and S. B. Calderwood. 1993. Role of iron in regulation of virulence genes. Clin. Microbiol. Rev. 6:137-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukowiak, A. M., K. J. Mueller, N. E. Freitag, and P. Youngman. 2004. Deregulation of Listeria monocytogenes virulence gene expression by two distinct and semi-independent pathways. Microbiology 150:321-333. [DOI] [PubMed] [Google Scholar]
- 70.Ly, T. M., and H. E. Muller. 1990. Ingested Listeria monocytogenes survive and multiply in protozoa. J. Med. Microbiol. 33:51-54. [DOI] [PubMed] [Google Scholar]
- 71.Mackaness, G. B. 1962. Cellular resistance to infection. J. Exp. Med. 116:381-406. [PubMed] [Google Scholar]
- 72.Makino, M., M. Kawai, I. Kawamura, M. Fujita, F. Gejo, and M. Mitsuyama. 2005. Involvement of reactive oxygen intermediate in the enhanced expression of virulence-associated genes of Listeria monocytogenes inside activated macrophages. Microbiol. Immunol. 49:805-811. [DOI] [PubMed] [Google Scholar]
- 73.Mansfield, B. E., M. S. Dionne, D. S. Schneider, and N. E. Freitag. 2003. Exploration of host-pathogen interactions using Listeria monocytogenes and Drosophila melanogaster. Cell Microbiol. 5:901-911. [DOI] [PubMed] [Google Scholar]
- 74.Marron, L., N. Emerson, C. G. M. Gahan, and C. Hill. 1997. A mutant of Listeria monocytogenes LO28 unable to induce an acid tolerance response displays diminished virulence in a murine model. Appl. Environ. Microbiol. 63:4945-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCraig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mengaud, J., S. Dramsi, E. Gouin, J. A. Vázquez-Boland, G. Milon, and P. Cossart. 1991. Pleiotropic control of Listeria monocytogenes virulence factors by a gene that is autoregulated. Mol. Microbiol. 5:2273-2283. [DOI] [PubMed] [Google Scholar]
- 77.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 78.Milohanic, E., P. Glaser, J.-Y. Coppée, L. Frangeul, Y. Vega, J. A. Vázquez-Boland, F. Kunst, P. Cossart, and C. Buchrieser. 2003. Transcriptome analysis of Listeria monocytogenes identifies three groups of genes differently regulated by PrfA. Mol. Microbiol. 47:1613-1625. [DOI] [PubMed] [Google Scholar]
- 79.Molofsky, A. B., and M. S. Swanson. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53:29-40. [DOI] [PubMed] [Google Scholar]
- 80.Moors, M. A., B. Levitt, P. Youngman, and D. A. Portnoy. 1999. Expression of listerolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect. Immun. 67:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moran, C. P. 1993. RNA polymerase and transcription factors, p. 653-667. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 82.Morita, M. T., Y. Tanaka, T. S. Kodama, Y. Kyogoku, H. Yanagi, and T. Yura. 1999. Translational induction of heat shock transcription factor σ32: evidence for a built-in RNA thermosensor. Gene Dev. 13:655-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nadon, C. A., B. M. Bowen, M. Wiedmann, and K. J. Boor. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson, K. E., D. E. Fouts, E. F. Mongodin, J. Ravel, R. T. DeBoy, J. F. Kolonay, D. A. Rasko, S. V. Angiuoli, S. R. Gill, I. T. Paulsen, J. Peterson, O. White, W. C. Nelson, W. Nierman, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, D. H. Haft, J. Selengut, S. Van Aken, H. Khouri, N. Fedorova, H. Forberger, B. Tran, S. Kathariou, L. D. Wonderling, G. A. Uhlich, D. O. Bayles, J. B. Luchansky, and C. M. Fraser. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 32:2386-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O'Driscoll, B., C. G. M. Gahan, and C. Hill. 1996. Adaptive acid tolerance response in Listeria monocytogenes: isolation of an acid-tolerant mutant which demonstrates increased virulence. Appl. Environ. Microbiol. 62:1693-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pamer, E. G. 2004. Immune responses to Listeria monocytogenes. Nat. Rev. Immunol. 4:812-823. [DOI] [PubMed] [Google Scholar]
- 87.Peel, M., W. Donachie, and A. Shaw. 1988. Temperature-dependent expression of flagella of Listeria monocytogenes studied by electron microscopy, SDS-PAGE and western blotting. J. Gen. Microbiol. 134:2171-2178. [DOI] [PubMed] [Google Scholar]
- 88.Pistor, S., T. Chakraborty, K. Niebuhr, E. Domann, and J. Wehland. 1994. The ActA protein of Listeria monocytogenes acts as a nucleator inducing reorganization of the actin cytoskeleton. EMBO J. 13:758-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Portnoy, D. A., P. S. Jacks, and D. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauch, M., Q. Luo, S. Muller-Altrock, and W. Goebel. 2005. σB-Dependent in vitro transcription of prfA and some newly identified genes of Listeria monocytogenes whose expression is affected by PrfA in vivo. J. Bacteriol. 187:800-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Renzoni, A., A. Klarsfeld, S. Dramsi, and P. Cossart. 1997. Evidence that PrfA, the pleiotropic activator of virulence genes in Listeria monocytogenes, can be present but inactive. Infect. Immun. 65:1515-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ripio, M.-T., G. Domíngez-Bernal, M. Suárez, K. Brehm, P. Berche, and J.-A. Vázquez-Boland. 1996. Transcriptional activation of virulence genes in wild-type strains of Listeria monocytogenes in response to a change in the extracellular medium composition. Res. Microbiol. 147:371-384. [DOI] [PubMed] [Google Scholar]
- 94.Ripio, M.-T., K. Brehm, M. Lara, M. Suárez, and J.-A. Vázquez-Boland. 1997. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J. Bacteriol. 179:7174-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ripio, M.-T., G. Domínguez-Bernal, M. Lara, M. Suárez, and J.-A. Vázquez-Boland. 1997. A Gly145Ser substitution in the transcriptional activator PrfA causes constitutive overexpression of virulence factors in Listeria monocytogenes. J. Bacteriol. 179:1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts, A. J., and M. Wiedmann. 2003. Pathogen, host, and environmental factors contributing to the pathogenesis of listeriosis. Cell Mol. Life Sci. 60:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saklani-Jusforgues, H., E. Fontan, and P. L. Goossens. 2000. Effect of acid-adaptation on Listeria monocytogenes survival and translocation in a murine intragastric infection model. FEMS Microbiol. Lett. 193:155-159. [DOI] [PubMed] [Google Scholar]
- 98.Schwab, U., B. Bowen, C. Nadon, M. Wiedmann, and K. J. Boor. 2005. The Listeria monocytogenes prfAP2 promoter is regulated by σB in a growth phase dependent manner. FEMS Microbiol. Lett. 245:329-336. [DOI] [PubMed] [Google Scholar]
- 99.Seeliger, H. P. R., and D. Jones. 1986. Genus Listeria, p. 1235-1245. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md. [Google Scholar]
- 100.Sheehan, B., A. Klarsfeld, T. Msadek, and P. Cossart. 1995. Differential activation of virulence gene expression by PrfA, the Listeria monocytogenes virulence regulator. J. Bacteriol. 177:6469-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shen, A., and D. E. Higgins. 2005. The 5′ untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity. Mol. Microbiol. 57:1460-1473. [DOI] [PubMed] [Google Scholar]
- 102.Shetron-Rama, L. M., H. Marquis, H. G. A. Bouwer, and N. E. Freitag. 2002. Intracellular induction of Listeria monocytogenes actA expression. Infect. Immun. 70:1087-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shetron-Rama, L. M., K. Mueller, J. M. Bravo, H. G. A. Bouwer, S. S. Way, and N. E. Freitag. 2003. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol. Microbiol. 48:1537-1551. [DOI] [PubMed] [Google Scholar]
- 104.Sleator, R. D., H. H. Wemekamp-Kamphuis, C. G. M. Gahan, T. Abee, and C. Hill. 2005. A PrfA-regulated bile exclusion system (BilE) is a novel virulence factor in Listeria monocytogenes. Mol. Microbiol. 55:1183-1195. [DOI] [PubMed] [Google Scholar]
- 105.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63:4231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sokolovic, Z., J. Riedel, M. Wuenscher, and W. Goebel. 1993. Surface-associated, PrfA-regulated proteins of Listeria monocytogenes synthesized under stress conditions. Mol. Microbiol. 8:219-227. [DOI] [PubMed] [Google Scholar]
- 107.Soomro, A. L., and N. Junejo. 2004. Vibrio cholerae in the environment. J. Coll. Physicians Surg. Pak. 14:509-512. [PubMed] [Google Scholar]
- 108.Stevenson, R. L. 1886. The strange case of Dr. Jekyll and Mr. Hyde. Scribner, New York, N.Y.
- 109.Straus, D. B., W. A. Walter, and C. A. Gross. 1987. The heat shock response of E. coli is regulated by changes in the concentration of σ32. Nature 329:348-351. [DOI] [PubMed] [Google Scholar]
- 110.Stritzker, J., C. Schoen, and W. Goebel. 2005. Enhanced synthesis of internalin A in aro mutants of Listeria monocytogenes indicates posttranscriptional control of the inlAB mRNA. J. Bacteriol. 187:2836-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 112.Thirumuruhan, R., K. Rajashankar, A. A. Fedorov, T. Dodatko, M. R. Chance, P. Cossart, and S. C. Almo. 11 March 2003, posting date. Crystal structure of PrfA, the transcriptional regulator in Listeria monocytogenes. www.rcsb.org/pdb/cgi/explore.cgi?pdbId=1OMI. [Online.]
- 113.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular parasite, Listeria monocytogenes. J. Cell Bio. 109:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vázquez-Boland, J.-A., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60:219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vázquez-Boland, J.-A., M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vega, Y., M. Rauch, M. J. Banfield, S. Ermolaeva, M. Scortti, W. Goebel, and J. A. Vázquez-Boland. 2004. New Listeria monocytogenes prfA* mutants, transcriptional properties of PrfA* proteins and structure-function of the virulence regulator PrfA. Mol. Microbiol. 52:1553-1565. [DOI] [PubMed] [Google Scholar]
- 117.Way, S. S., L. J. Thompson, J. E. Lopes, A. M. Hajjar, T. R. Kollmann, N. E. Freitag, and C. B. Wilson. 2004. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol. 6:235-242. [DOI] [PubMed] [Google Scholar]
- 118.Wemekamp-Kamphuis, H. H., J. A. Wouters, P. P. L. A. de Leeuw, T. Hain, T. Chakraborty, and T. Abee. 2004. Identification of sigma factor σB-controlled genes and their impact on acid stress, high hydrostatic pressure, and freeze survival in Listeria monocytogenes EGD-e. Appl. Environ. Microbiol. 70:3457-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams, J. R., C. Thayyullathil, and N. E. Freitag. 2000. Sequence variations within PrfA DNA binding sites and effects on Listeria monocytogenes virulence gene expression. J. Bacteriol. 182:837-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wong, K. K. Y., and N. E. Freitag. 2004. A novel mutation within the central Listeria monocytogenes regulator PrfA that results in constitutive expression of virulence gene products. J. Bacteriol. 186:6265-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wong, K. K. Y., H. G. A. Bouwer, and N. E. Freitag. 2004. Evidence implicating the 5′ untranslated region of Listeria monocytogenes actA in the regulation of bacterial actin-based motility. Cell Microbiol. 6:155-166. [DOI] [PubMed] [Google Scholar]